Effect of Exercise Training on Metabolic Homeostasis and Some Hemodynamics (Some Hepatic and Cardiovascular Functions) in Experimentally Induced Obesity
Received: 11-Apr-2018 / Accepted Date: 25-Apr-2018 / Published Date: 27-Apr-2018 DOI: 10.4172/2165-7904.1000368
Abstract
Background: Obesity is associated with many chronic disorders such as type-2 diabetes mellitus, essential hypertension and non-alcoholic fatty liver disease (NAFLD). High fat diet (HFD) induced obesity in rats is associated with altered adipocytes release of several adipocytokines. Physical exercise has also been shown to have positive effects in the prevention and attenuation of many of the obesity-related disorders, however; the mechanisms have not been fully elucidated.
Objective: The present study was designed to examine the effect of moderate intensity exercise training on existing cardio metabolic and hepatic complications linked to obesity including dyslipidemia, insulin resistance, hypertension and NAFLD.
Materials and methods: Thirty healthy adult male albino rats of initial body weight 151-190 gm were included. Rats were randomly and equally divided into 3 groups: group (1): normal diet fed group, group (II): HFD induced obesity group in which obesity was induced by HFD for 12 weeks and group (III): HFD induced obesity group fed on high fat diet for 12 weeks then followed by moderate intensity swimming exercise training for 8 weeks. Rats were examined for the body weight, length, abdominal circumference (AC) and body mass index (BMI), Systolic, diastolic & mean arterial blood pressures, heart rate, serum glucose, insulin & HOMA-IR, serum total cholesterol (TC), triglyceride (TG), very low-density lipoprotein (VLDL), low-density lipoprotein (LDL), high-density lipoprotein (HDL) & atherogenic index (AI), serum adiponectin, leptin, irisin, , tumor necrosis factor alpha (TNF- α), Interleukin-6 (IL-6) malondialdehyde (MDA), superoxide dismutase (SOD), alanine aminotransferase (ALT), aspartate aminotransferase (AST), Alkaline Phosphatase (ALP), lactate dehydrogenase (LDH) and albumin. Histopathological examination for hepatic tissue was also evaluated.
Results: The present study revealed that HFD significantly increased final body weight, BMI, AC, Systolic, diastolic & mean arterial blood pressures, heart rate, serum levels of glucose, insulin, TC, TG, LDL, VLDL, ALT, AST, ALP, LDH, TNF- α, IL-6, MDA and leptin levels, in addition to HOMA-IR and atherogenic index in HFD-induced obesity group. However, there were significant decreases in serum levels of HDL, SOD, albumin, adiponectin and irisin levels in the same group. Histopathological changes in hepatic tissue that indicate the development of NASH were also observed in HFD-induced obesity group. On the other hand, chronic moderate intensity swimming exercise training significantly reversed all these manifestations even in the absence of caloric restriction.
Conclusion: moderate exercise training seems to be an effective strategy to reverse almost all risk factors of cardiovascular diseases and NAFLD associated with metabolic syndrome.
Keywords: Obesity; Oxidative stress; Non-alcoholic fatty liver disease; Hypertension
Introduction
Obesity is one of the serious public health problems in the world. It is strongly associated with many chronic diseases such as dyslipidemia, hypertension, diabetes, coronary atherosclerotic heart disease, cancer and nonalcoholic fatty liver disease (NAFLD) [1,2].
The major effects of obesity on cardiovascular (CV) health are mediated through the risk of metabolic syndrome (insulin-resistance, dyslipidemia, and hypertension), such that an absence of these risk factors in obese individuals may not be associated with increased mortality risk [3].
The liver is a major regulator of metabolite flow in the body. Hepatocytes remove many materials from the circulation and release them or their products at a moderated rate. This capacity for uptake, especially of lipids, may be of key importance to initiate steatosis [4] which, in turn, increases the rate of mitochondrial beta-oxidation of fatty acids and ketogenesis that can promote lipid peroxidation and accumulation of reactive oxygen species (ROS) in the hepatocytes with subsequent inflammatory response, which initiate nonalcoholic steatohepatitis (NASH)/fibrosis [5].
Moreover, Adipose tissue is not only the primary site for storage of excess energy but it also serves as an endocrine organ capable of synthesizing a number of biologically active compounds (adipocytokines) that regulate the metabolic homeostasis [6]. Dysfunction of these adipocytokine pathways has been recognized as a key etiological factor of obesity-induced disorders [7].
Another major determinant for many obesity-induced sequelae is the low-grade inflammation of the enlarged adipose tissue and the persistent release of inflammatory adipocytokines such as tumor necrosis factor-α (TNF) and interleukin-6 (IL-6), which can adversely affect various non-adipose target tissues [8].
Both high-fat diet and lack of or declines in daily physical activity are the most important factors for obesity development [9]. Increasing physical activity has become an important part of a nonpharmacological strategy to control obesity, reverse existing cardiovascular diseases and associated risks factors linked to obesity [3] and prevent or attenuate hepatic steatosis [10]. However, these data do not identify the physiological or cellular mecanisms that elicit this improvement [11]. In most clinical investigations, however, training rehabilitation of obese patients with cardiovascular risks factors is often associated with lifestyle changes (modified diet, smoking cessation, etc.) and/or medications [12,13], and it is difficult to discern the direct therapeutic contribution of exercise alone.
Noteworthy, human and rodent exercise studies have indicated that exercise training can alter circulating adipokine concentration as well as adipokine expression in adipose tissue. Thus, the profound changes to white adipose tissue in response to exercise training may be part of the mechanisms by which exercise improves whole-body metabolic health [14]. However, the effect of exercise on adipokine levels depends on the type and duration of exercise; hence, it is difficult to compare and standardize the results reported by various studies [15].
The present study was designed to examine the effects of moderate intensity swimming exercise training on cardiovascular, metabolic and hepatic changes produced by HFD induced obesity and to demonstrate some underlying mechanisms.
Material And Methods
This study was conducted on 30 healthy adult male albino rats of local strain weighing 151-190 g, were obtained from the animal house of Faculty of Veterinary Medicine- Zagazig University. Rats were kept in steel wire cages (40 x 30 x 18 cm-5/cage) in the physiology animal house in Faculty of Medicine, Zagazig University, under hygienic conditions. They were fed the commercial rodent chow with free access to water, kept at room temperature and were maintained on a natural light/dark cycle. Rats were adapted to the new environment for one week before the experiment going on. All investigations were conducted in accordance with the guiding principles for the care and use of research animals and were approved by the Institutional Research Board.
Rats were randomly divided into three equal groups: group (I): normal diet fed (control) group; rats were fed on normal chow diet consisted of 5% of energy derived from fat, 18% from proteins and 77% from carbohydrates; 3.3 kcal/g for 12 weeks [16], group (II): a high fat diet induced obesity group; rats were fed on high fat diet consisted of 58% of energy derived from fat, 18% from protein and 24% from carbohydrates; 5.6 kcal/g for 12 weeks [16] (Diets were obtained from Faculty of Agriculture, Zagazig University), and group (III): a high fat diet induced obesity followed by exercise training group; rats were fed a high fat diet chow for 12 weeks then subjected to a moderate intensity swimming exercise training protocol for 8 weeks.
The rats in the training group were subjected to a swimming exercise performed one hour per day, six day per week for eight weeks in a cylindrical tank of 80 cm high, 120 cm diameter and filled with heated water 50 cm deep at (30-32°C). The pre-training period lasted for three-weeks (the first week lasted only 15 min, the second week lasted only 30 min, and the third week lasted only 45 min), and duration was gradually increased such that the rats were able to perform exercise for one hour per day. At the completion of exercise, rats were towel-dried and returned to their respective cages. The animal groups that were not trained were confined to stand in groups of three or four in a plastic tank (120 cm diameter filled with water to a height of 5 cm at 30-32°C) [17,18]. No deaths occurred during or after exercise in any groups.
Anthropometric measures
Measurement of body weight: By using a digital balance (Germany) at the start and the end of experiment.
Measurement of rat length: Taken as the distance from the nose tip to the anus at the start and the end of experiment [19].
Calculation of Body Mass Index [BMI]: BMI=body weight (gm)/ length2 (cm2). The cutoff value of obesity is BMI more than 0.68 gm/cm2 [19].
Measurement of abdominal circumference (AC): A plastic tape was used to measure the abdominal circumference at the largest zone of the rat’s abdomen [20].
Measurement of systolic, diastolic & mean arterial blood pressure (MABP) and heart rate (HR)
Systolic & diastolic blood pressures are measured in millimeters of mercury (mm Hg) and HR measured as beat /min by non-invasive blood pressure monitor (NIBP; 250 system, BioPAC system, INC) [21].
MABP=diastolic + (systolic-diastolic)/3
Sample collection
Blood samples were collected from retro-orbital venous plexus 48 h after the last training to avoid immediate effects of exercise and food was removed from the animal cages the night before [22]. Serum was separated by centrifugation of blood at 3000 rpm for 20 minutes and kept deep frozen at (-20°C) until used to measure the serum levels of glucose, insulin & HOMA-IR, serum total cholesterol (TC), triglyceride (TG), very low-density lipoprotein (VLDL), low-density lipoprotein (LDL), high-density lipoprotein (HDL) & atherogenic index, serum adiponectin, leptin, irisin, , tumor necrosis factor alpha (TNF- α), interleukin-6 (IL-6), malondiadehyde (MDA), superoxide dismutase (SOD), alanine aminotransferase (ALT), aspartate aminotransferase (AST), Alkaline Phosphatase (ALP), lactate dehydrogenase (LDH) and albumin. Livers were also excised and processed for histopathological studies.
Biochemical analysis
Measurement of serum glucose and insulin: Serum glucose was estimated as described by [23] using specific glucose kit (Bioscience, Egypt) and analyzed by spectrophotometers device (URIT-810, China). Insulin was measured by enzyme amplified sensitivity immunoassay (EASIA) as described by [24] using specific insulin kit (BioSource Belgium) and analyzed by spectrophotometers device.
Calculation of Insulin resistance (HOMA-IR): Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated according to the following formula [HOMA-IR = insulin (μU/mL) x glucose (mg/dl)/405] [25].
Measurement of serum lipids profile: Total cholesterol (TC) and triglycerides (TG) were measured by enzymatic colorimetric method described by using specific cholesterol and triglycerides kits (Spinreact Spain) and analyzed by spectrophotometers device. High density lipoproteins (HDLc) was measured by precipitating reagent method described by [23] using HDLc precipitating reagent kit (Spinreact, Spain) and analyzed by spectrophotometers device. Low density lipoproteins (LDLc) and very low density lipoproteins (VLDLc) were estimated by using [26] formula.
LDLc=TC-HDLC -(TG/5) VLDLC =TG/5
Calculation of atherogenic index (AI): The atherogenic index was calculated from the following formula: AI=Log (triglycerides/HDLc) [27].
Measurement of serum Leptin: was measured according to the method described by [28], using commercial ELISA kit, (Catalog Number RAB0005, provided by Sigma-Aldrich Co).
Measurement of serum TNF-α level: was measured according to the method described by [29], using commercial ELISA kit, (Catalog Number RAB0480, provided by Sigma-Aldrich Co).
Measurement of serum IL-6 level: was measured according to the method described by [30], using IL-6 ELISA Kit (Catalog Number RAB0306 provided by Sigma-Aldrich Co).
Measurement of serum adiponectin level: was measured according to the method described by [31] using commercial ELISA kit, (Catalog Number RAB0005, provided by Sigma-Aldrich Co).
Measurement of serum irisin level: was measured according to the method described by [32] using irisin ELISA kit (EK-067–16; Phoenix Pharmaceuticals, Burlingame, CA).
Measurement of serum MDA level: was measured according to the method described by [33], using Biodiagnostic kit (Biodiagnostic company, Dokki, Giza, Egypt).
Measurement of serum SOD activity: was measured according to the method described by [34], using kit provided by (Biodiagnostic company, Dokki, Giza, Egypt).
Measurement of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels: were measured according to the method described by [35], using rat ALT & AST enzyme-linked immunosorbent assay kit, (Catalog Number: 2011-11-0595, Shanghai Sunred biological technology, China).
Measurement of serum alkaline phosphatase level: was measured by colorimetric method according to the method described by [36].
Measurement of serum lactate dehydrogenase (LDH): was measured according to the method described by [37], using commercial kit (Catalog Number 279 001, provided by Egyptian Company for Biotechnology).
Measurement of serum alkaline phosphatase level: was measured according to the method described by [36] using commercial kit (Catalog Number 15-1711 provided by Sigma-Aldrich Co).
Measurement of serum albumin: was measured by using the bromocresol green method according to the method described by Stoskopf [38].
Tissue sampling and histopathological examination
Immediately after collecting blood samples, rats were killed by decapitation after light ether anesthesia. The abdominal cavities of the rats were opened to remove the livers. All removed livers were fixed in 10% buffered formalin solution for duration of 48-60 hour. After this, tissue samples were processed through ethyle alchol and xylene series, and embedded in paraffine blocks. Liver specimens were sectioned (5μm thick), then stained with hematoxylin and eosin [39]. The slides were examined under a light microscope by an expert pathologist in a blinded fashion.
Statistical analysis
Results were presented as mean x ± SD for and analyzed using version 18 SPSS program (SPSS Inc. Chicago, IL, USA). One way Analysis of variance (ANOVA) was used followed by student- least significant differences (LSD) test to compare statistical differences between groups. Pearsons test was done to detect correlations between parameters. P value less than 0.05 was considered to be significant (Figures 1-4).
Results
The present study showed that HFD (group II) significantly increased body weight, BMI, AC, serum glucose, insulin & HOMA-IR, serum total cholesterol, triglyceride, LDL, VLDL & atherogenic index, serum leptin, TNF-α, IL-6, MDA, ALT, AST, ALP, LDH. It also significantly increased Systolic, diastolic & mean arterial blood pressures and heart rate (p<0.001) with significant positive correlations versus BMI., but, it significantly decreased serum albumin, HDL, adiponectin, SOD and irisin levels (p<0.001) with significant negative correlations versus BMI when compared to control group ( group I) (Tables 1-4).
| Parameters | Group | Group I | Group II | Group III |
|---|---|---|---|---|
| Initial BW (gm) | x ± Δ | 172.4 ± 8.54 | 166.2 ±7.31 | 169 ± 4.94 |
| P value of LSD | -- | NSa | NSb | |
| Final BW (gm) | x ± Δ | 265 ± 8.273 | 418.5 ± 12.695 | 342.1 ± 12.749 |
| P value of LSD | -- | P<0.001a | P<0.001a,b | |
| Final BMI (gm/cm2) | x ± Δ | 0.54 ± 0.72 | 0.77 ± 0.06 | 0.69 ± 0.08 |
| P value of LSD | -- | P<0.001a | P<0.001a, P<0.05 b | |
| AC (cm) | x ± Δ | 15.1 ± 1.19 | 21.9 ± 2.07 | 18.2 ± 1.03 |
| P value of LSD | -- | P<0.001a | P<0.001a,b | |
| r with BMI | -- | r=0.934, p<0.001 | -- |
Table 1: Body weights, Final BMI & AC of all studied groups. a = versus group-1, b = versus group-2 and NS = non-significant (P>0.05).
| Groups | Parameters | Group I | Group III |
|---|---|---|---|
| Serum Glucose (mg/dl) | x ± ΣΔ | 84.5 ± 10.31 | 10.63 ± 10.63 |
| P value of LSD | -- | P<0.001a,b | |
| r with BMI | -- | -- | |
| Serum Insulin (μIU/ml) | x ± ΣΔ | 20.499 ± 3.38 | 33.949 ± 5.45 |
| P value of LSD | -- | P<0.001a , P<0.01b | |
| r with BMI | -- | -- | |
| HOMA-IR | x ± ΣΔ | 4.285 ± 0.96 | 10.983 ± 2.51 |
| P value of LSD | -- | P<0.001a ,b | |
| r with BMI | -- | -- | |
| Serum Cholesterol (mg/dl) | x ± ΣΔ | 71.78 ± 11.53 | 98.08 ± 6.78 |
| P value of LSD | -- | P<0.001a,b | |
| r with BMI | -- | ||
| Serum Triglyceride (mg/dl) | x ± ΣΔ | 70.703 ± 14.32 | 101.045 ± 8.61 |
| P value of LSD | -- | P<0.001a,b | |
| r with BMI | -- | ||
| Serum HDL (mg/dl) | x ± ΣΔ | 41.8 ± 7.15 | 35.35 ± 5.77 |
| P value of LSD | -- | P<0.05a, P<0.01b | |
| r with BMI | -- | -- | |
| Serum LDL (mg/dl) | x ± ΣΔ | 15.84 ± 3.13 | 42.51 ± 6.003 |
| P value of LSD | -- | P<0.001a,b | |
| r with BMI | -- | -- | |
| Serum VLDL (mg/dl) | x ± ΣΔ | 14.14 ± 2.865 | 20.2 ± 1.722 |
| P value of LSD | -- | P<0.01a, P<0.001b | |
| r with BMI | -- | -- | |
| Atherogenic index | x ± ΣΔ | 0.226 ± 0.044 | 0.46 ± 0.087 |
| P value of LSD | -- | P<0.001a,b | |
| r with BMI | -- | -- |
Table 2: Serum Glucose & Insulin, HOMA-IR, serum Cholesterol, Triglyceride, HDL, LDL, VLDL and atherogenic index of all studied groups.
| Groups | Parameters | Group I | Group II | Group III |
|---|---|---|---|---|
| Serum leptin (ng/ml) | x ± ΣΔ | 3.15 ± 0.53 | 9.86 ± 0.70 | 6.6 ± 1.4 |
| P value of LSD | -- | P<0.001a | P<0.001a,b | |
| r with BMI | -- | r=0.963, p<0.001 | -- | |
| Serum adiponectin (ng/dl) | x ± ΣΔ | 7.32 ± 1.11 | 3.511 ± 0.74 | 5.51 ± 0.61 |
| P value of LSD | -- | P<0.001a | P<0.001a,b | |
| r with BMI | -- | r= -0.928, p<0.001 | -- | |
| Serum irisin (ng/ml) | x ± ΣΔ | 17.28 ± 1.83 | 8.19 ± 0.84 | 12.17 ± 1.15 |
| P value of LSD | -- | P<0.001a | P<0.001a, P<0.01b | |
| r with BMI | -- | r = -0.961, p<0.001 | -- | |
| Serum TNF-α (pg/ml) | x ± ΣΔ | 47.17 ± 3.77 | 63.16 ± 1.94 | 54.23 ± 2.06 |
| P value of LSD | -- | P<0.001a | P<0.001a,b | |
| r with BMI | -- | r=0.905, p<0.001 | -- | |
| Serum IL-6 (pg/ml) | x ± ΣΔ | 9.57 ± 1.636 | 23.66 ± 3.691 | 15.5 ± 2.427 |
| P value of LSD | -- | P<0.001a | P<0.001a,b | |
| r with BMI | -- | r=0.9, p<0.001 | -- | |
| Serum MDA (nmol/ml) | x ± ΣΔ | 40.2 ± 5.28 | 66.01 ± 8.43 | 52.18 ± 5.15 |
| P value of LSD | -- | P<0.001a | P<0.001a,b | |
| r with BMI | -- | r=0.931, p<0.001 | -- | |
| Serum SOD (U/L) | x ± ΣΔ | 52.99 ± 4.34 | 33.95 ± 5.45 | 44.09 ± 4.45 |
| P value of LSD | -- | P<0.001a | P<0.001a,b | |
| r with BMI | -- | r=-0.906, p<0.001 | -- |
Table 3: Serum leptin, adiponectin, irisin, TNF-α, IL-6, MDA and SOD of all studied groups.
| Groups | Parameters | Group I | Group II | Group III |
|---|---|---|---|---|
| Systolic blood pressure (mmHg) | x ± ΣΕ | 127.1 ± 1.792 | 169 ± 4.163 | 144.1 ± 3.479 |
| P value of LSD | -- | P<0.001a | P<0.001a,b | |
| r with BMI | -- | r= 0.904, p<0.001 | -- | |
| diastolic blood pressure (mmHg) | x ± ΣΕ | 88.2 ± 3.824 | 125.42 ± 6.893 | 103.8 ± 4.91 |
| P value of LSD | -- | P<0.001a | P<0.001a,b | |
| r with BMI | -- | r= 0.933, p<0.001 | -- | |
| MABP (mmHg) | x ± ΣΕ | 101.233 ± 2.39 | 139.95 ± 5.82 | 117.233 ± 3.55 |
| P value of LSD | -- | P<0.001a | P<0.001a,b | |
| r with BMI | -- | r= 0.955, p<0.001 | -- | |
| heart rate (beat/min) | x ± ΣΕ | 321.5 ± 12.38 | 532.5 ± 14.13 | 402.8 ± 12.06 |
| P value of LSD | -- | P < 0.001a | P < 0.001a,b | |
| r with BMI | -- | r=0.946, p<0.001 | -- | |
| Serum ALT (mg /dl) | x ± ΣΕ | 44.93 ± 8.25 | 137.2 ± 9.33 | 89.9 ± 7.12 |
| P value of LSD | -- | P < 0.001a | P<0.001a,b | |
| r with BMI | -- | r=0.991, p<0.001 | -- | |
| Serum AST (mg /dl) | x ± ΣΕ | 133.9±12.56 | 190.8 ± 13.91 | 160.7 ± 17.83 |
| P value of LSD | -- | P<0.001a | P<0.001a,b | |
| r with BMI | -- | r=0.835, p<0.05 | -- | |
| Serum ALP (U/L) | x ± ΣΕ | 67.07 ± 4.015 | 101.1 ± 8.283 | 83.6 ± 3.930 |
| P value of LSD | -- | P<0.001a | P<0.001a,b | |
| r with BMI | -- | r=0.933, p<0.001 | -- | |
| Serum LDH (U/L) | x ± ΣΕ | 258.22 ± 4.976 | 571.4 ± 7.09 | 440.84 ± 6.927 |
| P value of LSD | -- | P<0.001a | P<0.001a,b | |
| r with BMI | -- | r=0.9, p<0.001 | -- | |
| Serum albumin (g/dl) | x ± ΣΕ | 4.2 ± 0.25 | 1.852 ± 0.51 | 2.938 ± 0.53 |
| P value of LSD | -- | P<0.001a | P<0.001a,b | |
| r with BMI | -- | r=-0.928, p<0.001 | -- |
Table 4: Systolic, diastolic &mean arterial blood pressures, heart rate and serum ALT, AST, ALP, LDH & albumin of all studied groups.
Whereas, chronic moderate exercise training ( group III) significantly decreased body weight, BMI, AC, serum glucose (P<0.001), insulin(P<0.01) & HOMA-IR, serum total cholesterol, triglyceride, LDL, VLDL & atherogenic index, serum leptin, TNF-α, IL-6, MDA, ALT, AST, ALP, LDH (P<0.001) and it also significantly increased Systolic, diastolic & mean arterial blood pressures and heart rate (P<0.001) , it significantly decreased serum albumin, adiponectin, SOD (P<0.001), HDL and irisin levels (P<0.01) when compared to HFD- induced obesity group ( group II) (Tables 1-4).
Discussion
Obesity is a worldwide epidemic and is recognized as a risk factor for many disorders including type-2 diabetes, essential hypertension and nonalcoholic fatty liver disease (NAFLD). However, the mechanism that links obesity with high blood pressure and NAFLD has not been fully elucidated [40]. NAFLD constitutes spectra of liver diseases ranging from intrahepatic fat accumulation (steatosis) to various degrees of necrotic inflammation and fibrosis (nonalcoholic steatohepatitis; NASH) with or without hepatic fibrosis/cirrhosis [41]. NAFLD can be improved through weight reduction and exercise without medical therapy [42]; however, the mechanism of improvement remains unknown even though many studies have been conducted to address this issue [43].
The present study was designed to examine the effect of moderate intensity exercise on existing cardio metabolic and hepatic complications linked to obesity including hyperlipidemia, insulin resistance, hypertension and NAFLD.
In the present study, we observed a significant increase in final body weight and BMI in high fat diet fed group of rats, which indicates the occurrence of an overall obesity. In addition, these HFD fed animals showed a significant increase in abdominal circumference (AC) which indicate the occurrence of visceral (=abdominal or central) obesity. These results are in agreement with those of many investigators [40,44].
Our results are in agreement with those of [45-47] who reported that chronic consumption of HFD in rats induced metabolic syndrome (MS) as evidenced by visceral obesity, hyperglycemia, dyslipidemia, endothelial dysfunction and hypertension and found that exercise improved all of these features of metabolic disease without necessarily switching to a normal caloric diet.
Metabolic syndrome is strongly linked to the development of coronary heart disease and stroke mainly through an increased risk of insulin resistence and other abnormalities including dyslipidemia and high blood pressure [48]. Results of our research work showed that HFD induced obesity in rats resulted in a significant increase in systolic, diastolic and mean arterial blood pressures. These findings are in consistence with the study of [49] who reported that in rats fed a HFD, systolic BP and diastolic BP were significantly elevated compared to a standard diet-fed rats and suggested that a short period of high fat diet intake might increase Ca2+ channel numbers or alter channel regulation, leading to increased transmembrane Ca2+ influx that is associated with significantly elevated blood pressure. Furthermore, The changes in hemodynamic parameters (BP↑ & HR ↑) observed in the present study in HFD-induced obesity group may be partially attributed to activation of sympathetic -renin-angiotensin system, dyslipidemia, increased oxidative stress and pro-inflammatory cytokines such as (IL-6 &TNF-α) and diminished endogenous NO production [17,40].
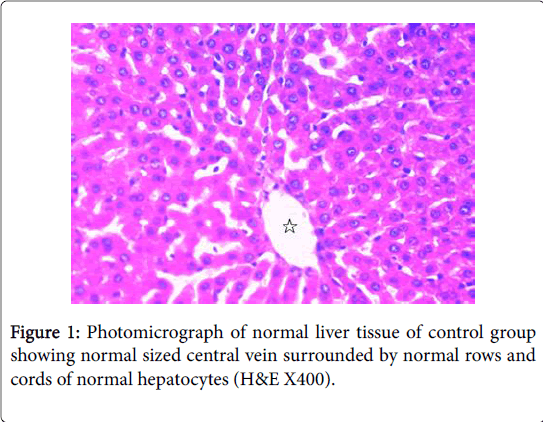
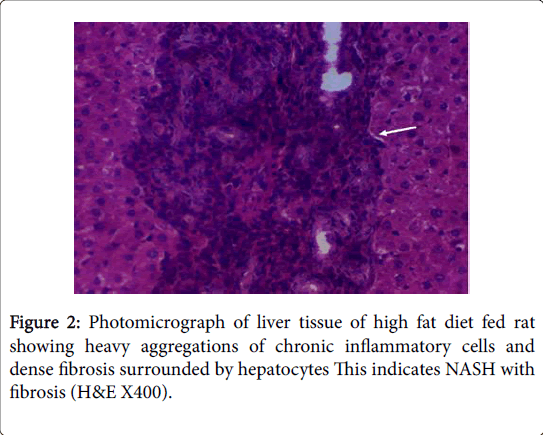
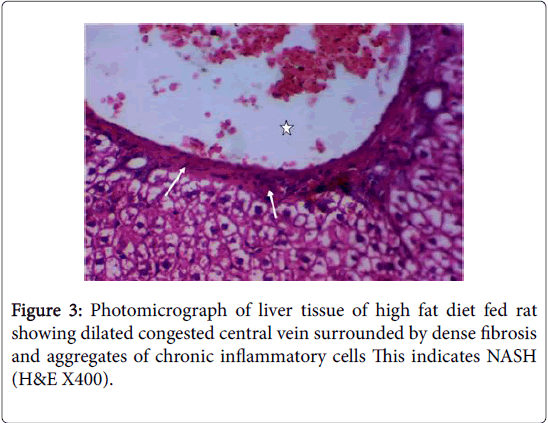
Moreover, Liver injury is evidenced in HFD fed rats in this study by the histopathological changes in hepatic tissue as indicated by fatty infiltration with the presence of foci of mixed inflammatory cell infiltration and fibrosis that may indicate the development of NASH and cirrhosis. These histopathological changes were associated with significant increases in serum levels of ALT, AST, ALP and LDH with significant positive correlations versus BMI, and with significant increases in serum levels of albumin with significant negative correlations versus BMI. These findings are in accordance with the findings of [50,51]. Both ALT and AST are leakage enzymes, and their elevation in the circulation indicates significant hepatocellular damage [52]. The most common cause of NAFLD in developed countries can be attributed to increased caloric intake that exceeding the rates of caloric expenditure [53]. Regional mobilization of circulating triglycerides and fatty acid transport is altered in obese patients with NAFLD. Lipoprotein lipase (LpL) hydrolyzes circulating triacylglycerol followed by tissue uptake through fatty acid transport proteins (FATPs) together with fatty acid translocase (FAT/CD36) [54]. LPL activity in adipose tissue in response to insulin seems to be blunted in obese patients, while associated with increased hepatic LPL and FATP expression [55].
Insulin resistance (IR) as evidenced by the significantly increased serum glucose and insulin levels in addition to increased HOMA-IR in the present study, in HFD fed group, may contribute to the development of hypertension and NAFLD.
Insulin can be considered as both inflammatory and antiinflammatory, in physiological condition insulin stimulates endothelial NO production to exert a vasorelaxant and anti-inflammatory effect. Whereas, in the state of insulin resistance, the insulin-stimulated NO pathway is selectively impaired and the compensatory hyperinsulinemia may activate mitogen-activated protein kinase (MAPK) pathway, resulting in enhancement of vasoconstriction, proinflammation, sodium & water retention and elevation of blood pressure [56] .
IR and hyperinsulinemia also play a key role in the pathogenesis of NALFD. IR in NAFLD is predominantly peripheral and occurs in the skeletal muscle and adipose tissue. Peripheral IR in the skeletal muscle causes reduced glucose uptake, which leads to hyperglycemia. In adipose tissue, IR impairs the anti-lipolytic action of insulin, which leads to an increased release of FFA. Elevated plasma concentrations of insulin, glucose, and fatty acids then impair the β-oxidation of fatty acids by negative feedback and promote the uptake of hepatic fatty acids and triglycerides & de novo lipid synthesis through the expression of sterol-regulatory element-binding protein (SREBP-1) and C/enhancer-binding protein (CCAAT/EBP) [57].
In addition, we found that HFD for a period of 12 weeks also produced a significant increase in the serum TC, TG, LDL-C and VLDL-C levels together with a significant decrease in serum HDL-C levels in HFD-induced obesity in rats. These findings are supported by the work of [40,47] which indicated that obesity adversely affects plasma lipids by increasing TC, TG, LDL-c and decreasing the levels of HDL-C. A significant increase in atherogenic index (AI) has also been demonstrated in our study in HFD fed group.
To compensate for the increased hepatic triglycerides, the liver forms an atherogenic lipid profile that is strongly associated with adverse cardiovascular outcomes [58]. SREBP-2 and low-density lipoprotein (LDL) receptor are down regulated in NAFLD patients, leading to inhibition of cholesterol uptake and very low-density lipoprotein (VLDL) synthesis in liver cells, resulting in an increase in hepatic triglycerides (TG) [59]. Increased TG levels can further disturb the atherogenic lipid profile by lowering high-density lipoprotein cholesterol (HDL-C) (an anti-AS factor) and increasing small dense LDL particles and oxidized LDL (ox-LDL) as a key molecular connection between NAFLD and atherosclerosis (AS) [60]. Steatosisstimulated fatty-acid oxidation in the liver, systemic release of proatherogenic molecules like tumor necrosis factor-α, interleukin-6 and oxidized LDL cholesterol, increased IR and macrophage activation have been also suggested as possible explanations for accelerated atherosclerosis and increased CVD burden in NAFLD patients [61,62]. The atherogenic role of hepatic inflammation is also supported by the fact that patients with NASH have increased atherosclerosis when compared with patients with simple steatosis [63]. Therefore, NAFLD seems to be an early risk factor for atherosclerosis [64].
After the initial development of steatosis, the liver becomes extremely vulnerable. Multipe series of injurious factors, including oxidative damage, dysregulation of multiple adipokines, apoptpsis and activation of hepatic stellate cell, may lead to hepatocyte injury and finally to the progression from simple steatosis to NASH and fibrosis [65].
An important detectable parameter in our model is the presence of oxidative stress, which was indicated by the significant increase in serum level of MDA indicating lipid peroxidation associated with the significant decrease in serum SOD level in HFD-fed rats. Oxidative stress and free radicals are known to be involved in a variety of human pathologies including atherosclerosis, obesity and hypertension [66].
High serum FFAs levels activate mitochondrial, peroxisomal and microsomal fatty acid (FA) oxidation promoting the release of reactive oxygen species (ROS) which contribute to apoptosis and nuclear & mitochondrial DNA damage in NASH [67]. Moreover, ROS and products of lipid peroxidation can lead to fibrosis by activating hepatic stellate cells, which synthesize collagen and perpetuate the inflammatory response, causing fibrogenic response [68].
Human studies also support the role of oxidative stress (OS) in the development of hypertension, especially in obesity. An imbalance in superoxide and NO production may account for reduced vasodilation together with sympathetic nervous system excitation by OS in the brain could play an important role in the pathogenesis of obesityassociated hypertension [69].
Our results also revealed that HFD induced obesity resulted in significant changes in various adipocytokines. There were significant increases in serum TNFα, IL-6 and leptin levels with significant positive correlations versus BMI but there were significant decreases in serum adiponectin and irisin levels with significant negative correlations versus BMI in the HFD fed group. This is in agreement with previous studies showing that the concentration of adiponectin decreases in obesity and increases after weight loss [70]. Moreno- Navarrete et al. [71] also reported that circulating irisin decreased in obesity and negatively associated with BMI. However, at variance of our results, Stengel et al. [72] reported that obese patients have higher circulating irisin levels compared with normal weight controls and positively correlated with body weight and BMI. On the other hand, [73] didn’t find a positive or negative correlation between circulating irisin levels and BMI.
Obesity is considered a state of chronic inflammation. It leads to increased production of monocyte chemotactic protein (MCP-1) by the adipocytes, which attracts more macrophages to the adipose tissue itself. Once the macrophages in the adipose tissue are activated, a selfperpetuating inflammatory cascade is triggered by secretion of proinflammatory cytokines like TNF-α and IL-6 [74]. Moreover, lipid accumulation in the liver induces Bax (pro-apoptotic Bcl-2 family member) translocation to lysosomes causing their destabilization and release of lysosomal cysteine protease cathepsin B leading to activation of inhibitor of nuclear factor kappa-B kinase (IKK-β) in hepatocytes that activates nuclear factor-kappaB (NFκB), and enhances gene expression of proinflammatory cytokines including TNF-α and IL-6 [75].
TNF-α is a key link in obesity-induced IR and stimulates the hormone sensitive lipase resulting in increased serum FFA and their influx in the liver [76]. Increased IL-6 may also promote partial liver injury and atherosclerosis [77]. IL-6 can activate macrophages to secrete matrix metalloproteinase-1, induce mononuclear cells to participate in the development of vessel plaque, promote synthesis of LDL receptor and influx of LDL into macrophages, enhance lipid deposition and stimulate vascular smooth muscle cell proliferation [78].
Leptin also plays a crucial role in aggravation of NASH in obese individuals [79]. Leptin levels are enhanced by pro-inflammatory cytokines such as IL-1 and TNF-α and help to perpetuate the loop of chronic inflammation in obesity and down regulates the transcription of the preproinsulin gene and insulin excretion which could be connected with high leptin levels in IR [80,81]. Hyperleptinemia also elevates the sympathetic drive via the corticotropin-releasing factor [82], elevates renal sympathetic nerve activity resulting in sodium retention, volume expansion and blood pressure elevation [83] and promotes the release of vasoconstrictive substances such as angiotensin II and endothlin-1 thereby increasing the blood pressure [84]. Although leptin resistance refers to the condition of diminished cellular and metabolic responsiveness to leptin, a condition of partial or selective leptin resistance seems to exist whereas the sympathoexcitatory effects are maintained leading to hypertension and tachycardia [85].
On the other hand, plasma concentrations of adiponectin were found to be significantly lower in obese subjects [86]. Adiponectin may be a promising drug candidate in the treatment of liver diseases through its insulin-sensitizing and anti-inflammatory effects [87]. Moreover, adiponectin can also stimulate vascular endothelial nitric oxide synthase (eNOS) mRNA expression, and progressively reduce atherosclerotic lesions by inhibiting VSMC proliferation & migration to suppress plaque disruption and inhibiting the endothelial cell (EC) inflammatory reaction, another key molecular pathway involved in AS [88].
Likewise, plasma irisin level is also reduced in obese patients with NAFLD and could behave as a protective factor against liver steatosis [89]. Irisin may modulate the peroxisome proliferator-activated receptor alpha (PPARα) signaling pathway, a key regulator of lipid metabolism, leading to an improvement in hepatic steatosis and insulin sensitivity [90]. Moreover, Lu et al. [91] found that irisin treatment reduced the atherosclerotic plaque area, inflammatory cell infiltration and inflammatory factor expression on the vascular wall. It can also inhibit high glucose-induced endothelial cell apoptosis, promote endothelial cell proliferation, and alleviate endothelial cell dysfunction [92].
Noteworthy, our experimental findings showed that exercise even in the absence of reduced caloric intake, is associated with reduction in HFD-induced visceral obesity. This is in agreement with those from clinical studies [93].
Exercise also significantly reduced the dyslipidemia observed in the HFD fed group and improved the atherogenic index in our study. Previous studies have shown also that physical training and caloric restriction are effective at improving lipid profiles in both humans [94] and animals [45]. Exercise training could contribute to attenuating hepatic TG accumulation via suppressed de novo lipogenesis and/or TG synthesis through suppression of hepatic SREBP-1c. It also increased the expression and phosphorylation of hepatic 5' AMPactivated protein kinase (AMPK) in rats and thereby the traininginduced β-oxidation of fatty acids and attenuation of lipogenesis to be an effective and non-pharmacological means to combat HFD-induced fatty liver and its metabolic complications [43,95].
In the present study and in other studies [47], exercise training and/or switching from a HFD to a control diet improved lipid profiles and decreased the atherogenic index (AI). Touati et al. also observed that the AI was less in the exercise-trained rats than in the rats with modified diet.
Concerning plasma triglycerides (TG), our experiments showed that HFD induced obesity was associated with a significant increase in serum levels of TG that were improved by exercise training. This is in contrast to the results of Touati et al. who found that metabolic syndrome (MS) induced by HFD consumption did not induce change in the plasma triglyceride levels, despite hyperinsulinemia and hyperglycemia. Gami et al. has shown that plasma hypertriglyceridemia is strongly correlated with the prevalence and incidence of metabolic syndrome (MS) and cardiovascular disease.
Our study also revealed a significant improvement of insulin resistance indicated by the significant decrease in serum glucose and insulin levels in addition to decreased HOMA-IR in HFD-fed group subjected to exercise training. Regular physical activity mends insulin function and glucose tolerance in patients with obesity & insulin resistance [96]. It also augments the oxidative capacity of skeletal muscles due to an increase in fatty acid transport proteins, which improved the rate of whole body fat oxidation [97].
Moreover, the present study demonstrated that obese rats subjected to moderate intensity exercise training was associated with a significant decrease in SBP, DBP and MABP. These results are in agreement with those of many investigators who showed that diet modification and/or regular exercise training resulted in a significant reduction of ABP and even prevention of hypertension in obese rats [47,98]. Exercise and diet modification both ameliorate endothelial dysfunction by increasing expression and activity of e NOS, generating NO and modulating ROS production leading to reversal of hypertention and risk factors of the MS [47,99].
Touati et al. have noted that a decrease in blood pressure was more effective in trained obese rats than in sendentary obese rats with modified diet. They showed that exercise with or without diet modification not only restored but also increased the endothelium and No-dependent aortic relaxant’s response to acetylcholine whereas switching from a HFD to a control diet in sedentary rats improved but did not completely normalize it. Similarly, they observed that plasma Thiobarbituric acid reactive substances levels were more diminished in training rats independently of diet used than in sendentary rats with modified diet. Taken together, these findings indicate that exercise was more effective in reversing endothelial dysfunction and oxidative stress than converting to normal caloric diet.
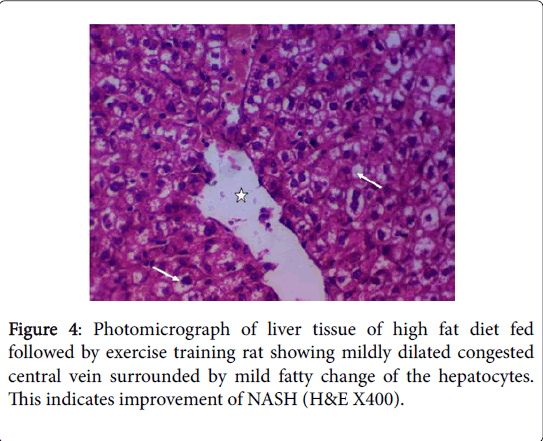
The beneficial effects of exercise training on the reversal of HFDinduced fatty liver were also demonstrated in our study by the significantly decreased serum ALT, AST, ALP and LDH and the improvement in liver histopathology. Many studies have also reported reductions in hepatic lipid content in NAFLD patients after exercise training intervention programs that did not induce weight loss which suggest that exercise per se can reverse hepatic steatosis [100]. Nevertheless, these data do not identify the physiological or cellular adaptations that elicit this improvement [11].
Exercise training also significantly decreased the serum levels of TNFα, IL-6 and leptin but significantly increased the serum levels of adiponectin and irisin in the HFD fed group subjected to exercise training. These results are supported by those of [101], who revealed that aerobic exercise training resulted in a significant decrease in serum leptin level that may be added to the beneficial effects of exercise. Exercise training especially that which is associated with reduced fat mass corrects the dysfunction in adipokine and cytokine expression and the magnitude of benefits may vary with the type and amount of exercise [102].
Exercise stimulates the expression of fibronectin type-III domain containing protein 5 (Fndc5), a membrane protein that is cleaved and secreted as irisin [103]. Irisin increases energy expenditure and it could be responsible for a better control of certain diseases related to insulin resistance such as fatty liver disease [104]. Moreover, Mazur-Bialy et al. [105] reported that irisin alleviates the inflammatory activation of macrophages by suppressing the phosphorylation of MAPK and consequently a lower NF-κB activation leading to reduction in both the expression and release of pro-inflammatory cytokines such as IL-1β, TNFα, IL-6 and MCP-1.
Exercise also controls the release and activity of at least two cytokines, TNF-α & IL-6 that could contribute to the natural protective effects of physical activity. Physical exercise upregulates IL-6, improving insulin sensitivity by increasing skeletal muscle glucose uptake and promoting fatty acid oxidation [106]. Muscle-produced IL-6 also exerts anti-inflammatory effects through its inhibitory effects on TNF-α, IL-1β, and activation of interleukin-1 receptor antagonist (IL-1ra) and IL-10 [107]. Nevertheless, IL-6 overexpressed in adipocytes and has been reported to be increased in obese patients [108]. Moreover, IL-6 can induce insulin resistance in hepatocytes, adipocytes and skeletal muscle [109]. These data suggest that a strict balance is required to keep metabolism stable [110].
Many studies also reported the antihypertensive effect of exercise. It is able to reduce heart rate, improve the sensitivity of aortic baroreceptors, which contributes to a more efficient regulation of blood pressure [111]. Decreased activity of both the sympathetic nervous system and renin-angiotensin system were also documented with regular exercise training leading to lowering of HR and BP [22].
In summary, the data from this study demonstrated that high fat diet-induced obesity is associated with insulin resistance, dyslipidemia, altered adipocytokines production, decreased antioxidant levels, hemodynamic changes, fatty liver disease (steatohepatitis) and hepatic dysfunction. These criteria of metabolic syndrome can be corrected by moderate exercise training. In addition, moderate exercise training seems to be an effective strategy to reverse almost all factors of cardiovascular diseases and NAFLD risks associated with metabolic syndrome. Thus, exercise prescription might be recommended either alone or as adjuvant of drug therapy for treatment/attenuation of the serious complications of HFD-induced obesity.
Further studies will be necessary for a better understanding of the precise effect of exercise training in ameliorating the cellular and molecular mechanisms by which HFD contribute to cardiovascular diseases and NAFLD development and progression. Moreover, further research is needed, namely in humans, in order to establish the preferred type, duration and intensity of training that should be practiced in order to maximize the benefits of exercise.
Acknowledgment
To Prof. Kamal EL-Kashishy, Pathology Department, Faculty of Medicine, Zagazig University, for performing the histopatholgical study.
References
- Rosa E, Zanella M, Ribeiro A, Kohlmann Junior O (2005) Visceral obesity, hypertension and cardio-renal risk: a review. Arquivos Brasileiros de Endocrinologia and Metabologia 49: 196-204.
- Tock L, Prado W, Caranti D, Cristofalo D, Lederman H, et al. (2006) Nonalcoholic fatty liver disease decrease in obese adolescents after multidisciplinary therapy. Eur J Gastroenterol Hepatol 18: 1241-1245.
- Kachur S, Lavie C, Schutter A, Milani R, Ventura H (2017) Obesity and cardiovascular diseases. Minerva Medica 108: 212-228.
- Bradbury M (2006) Lipid metabolism and liver inflammation. I. Hepatic fatty acid uptake: possible role in steatosis. Am J Physiol Gastrointest Liver Physiol 290: 194-198. â€
- Marra F, Gastaldelli A, Baroni G, Tell G, Tiribelli C (2008) Molecular basis and mechanisms of progression of non-alcoholic steatohepatitis. Trends Mol Med 14: 72-81.
- Saely C, Geiger K, Drexel H (2012) Brown versus white adipose tissue: A mini-review. Gerontology 58: 15-23.
- Cao H (2014) Adipocytokines in Obesity and Metabolic Disease. J Endocrinol 220: 47-59.
- Gregor M, Hotamisligil G (2011) Inflammatory mechanisms in obesity. Annu Rev Immunol 29: 415-445. â€
- Westerterp K (2006) Perception, passive overfeeding and energy metabolism. Physiology and behavior 89: 62-65.
- Schultz A, Mendonca L, Aguila M, Mandarim-de-Lacerda C (2012) Swimming training beneficial effects in a mice model of nonalcoholic fatty liver disease. Exp Toxicol Pathol 64: 273-282.
- Haus J, Solomon T, Kelly K, Fealy C, Kullman E, et al. (2013) Improved hepatic lipid composition following short-term exercise in nonalcoholic fatty liver disease. J Clin Endocrinol Metab 98: 1181-1188.
- Roberts C, Ng C, Hama S, Eliseo A, Barnard R (2006) Effect of a short-term diet and exercise intervention on inflammatory/anti-inflammatory properties of HDL in overweight/obese men with cardiovascular risk factors. J Appl Physiol 101: 1727-1732.
- Tjønna A, Lee S, Rognmo Ø, Stølen, T, Bye A, et al. (2008) Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome. Circulation 118: 346-354. â€
- Stanford K, Middelbeek R, Goodyear L (2015) Exercise Effects on White Adipose Tissue: Beiging and Metabolic Adaptations. Diabetes 64: 2361-2368.
- Albu A, Lupu D (2015) Adipokines, systemic inflammation and exercise. Palestrica Third Millennium-Civiliz Sport 16: 257-261.
- Svegliati-Baroni G, Candelaresi C, Saccomanno S, Ferretti G, Bachetti T, et al. (2006) A model of insulin resistance and nonalcoholic steatohepatitis in rats. Ame J Pathol 169: 846-860.
- Souza HC, Penteado DM, Martin-Pinge M, Barbosa N, Teixeira Vde P et al. (2007) Nitric oxide synthesis blockade increase hypertrophy and cardiac fibrosis in rats submitted to aerobic training. Arq Bras Cardiol 89: 99-104.
- Huang C, Wang T, Tung Yand LinW (2016) Effect of exercise training on skeletal muscle SIRT1 and PGC-1α expression levels in rats of different age. Int J Med Sci 13: 260-270.
- Novellie Y, Galhardi C, Ebaid G, Rodrigues H, Mani F (2007) Anthropometrical parameters and markers of obesity in rats. Lab Anim 41: 111-119.
- Gerbaix M, Metz L, Ringot E, Courteix D (2010) Visceral fat mass determination in rodent: Validation of dual-energy x-ray absorptiometry and anthropometric techniques in fat and lean rats. Lipids Health Dis 140: 1-9.
- Borow K, Newburger J (1982) Noninvasive estimation of central aortic pressure using the oscillometric method for analyzing systemic artery pulsatile blood flow: comparative study of indirect systolic, diastolic, and mean brachial artery pressure with simultaneous direct ascending aortic pressure measurements. Am. Heart J 103: 879-886.
- Lemos ET, Nunes S, Teixeira F, Reis F (2011): Regular physical exercise training assists in preventing type 2 diabetes development: focus on its antioxidant and anti-inflammatory properties. Cardiovasc Diabetol 10: 12.
- Tietz N (1995) Clinical guide to laboratory tests, (3rd edn) WB Saunders Co Philadelphia PA.
- Temple R, Clark P, Hales C (1992) Measurement of insulin secretion in type 2 diabetes: problems and pitfalls. Diabetic Medicine 9: 503-512.
- Matthews D, Hosker J, Rudenski A, Naylor B, Treacher D, et al. (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412-419.
- Friedewald W, Levy R, Fredrickson D (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18: 499-502.
- Jurgonski A, Juskiewiez J, Zdunezyk Z, Bogusaw K (2012) Caffeoylquinic acid-rich extract from chicory seeds improves glycemiaatherogenic index and antioxidant status in rat. Nutrition 28: 300-306.
- Considine RV, Considine EL, Williams CJ, Hyde TM, Caro JF (1996) The hypothalamic leptinreceptors in humans: The hypothalamic leptin receptors in human: Identification of incidental sequence polymorphism and absence of the db/db mouse and fa/fa rat mutation. Diabetes 45: 992-994.
- Fernando B, Marley R, Holt S, Anand R, Harry D, et al. (1998) N-acetylcysteine prevents development of the hyperdynamicciculation in the portal hypertensive rat. Hepatology 28: 689-694.
- Engvall E, Perlmann P (1971) Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry 8: 871-874.
- Kishore U, Reid K (2000) C1q: structure, function, and receptors. Immunopharmacology 49:159-170.
- Yang X, Yuan H, Li J, J Fan J, Jia S, et al. (2016) Swimming intervention mitigates HFD-induced obesity of rats through PGC-1α-irisin pathway. Eur Rev Med Pharmacol Sci 20: 2123-2130.
- Satoh K (1978) Serum lipid peroxide in cerebrovascular disorders determined by a new colorimeteric method. Clin Chim Acta 90: 37-43.
- Nishikimi M, Roa N, Yogi K (1972) The occurance of superoxide anion in the reaction of reduced phenazinemethosulphate and molecular oxygen. Biochem Biophy Res Commun 46: 849-854.
- Belfield A, Goldberg D (1971) Hydrolysis of adenosine monophosphates by acid phosphatases as measured by a continuous spectrophotometric assay. Biochem Med 4: 135-148.
- Kachmar J, Moss D (1976) Enzymes. In Fundamentals of clinical chemistry. NW Tietz, editor, saunders, Philadelphia 652-6603.
- Altunkaynak Z (2005) Effect of high fat diet induced obesity on female rat livers (A histochemical study): Eur J Gen Med 2: 100-109.
- Bhandari U, Kumar V, Khanna N, Panda B (2011) The effect of high-fat diet-induced obesity on cardiovascular toxicity in Wistar albino rats. Hum Exp Toxicol 30: 1313-1321. â€
- Lima M, Leite L, Gioda C, Leme F, Couto C, et al. (2015) A novel Wistar rat model of obesity-related nonalcoholic fatty liver disease induced by sucrose-rich diet. J diabetes research.
- Huh J, Panagiotou G, Mougios V, Brinkoetter M, Vamvini M, et al. (2012) FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II.mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism 61: 1725-1738.
- Cho J, Lee I, Kim D, Koh Y, Kong J, et al. (2014) Effect of aerobic exercise training on non-alcoholic fatty liver disease induced by a high fat diet in C57BL/6 mice. J Exerc Nutrition Biochem 18: 339-346. â€
- Matsuo T, Takeuchi H, Suzuki H, Suzuki M (2002) Body fat accumulation is greater in rats fed a beef tallow diet than in rats fed a safflower or soybean oil diet. Asia Pac J Clin Nutr 11: 302-308. â€
- Roberts C, Vaziri N, Liang K and Barnard R (2001) Reversibility of chronic experimental syndrome X by diet modification. Hypertension 37: 1323-1328. â€
- Jun H, Hwang K, Kim Y, Park T (2008) Highâ€fat Diet Alters PP2A, TC10, and CIP4 Expression in Visceral Adipose Tissue of Rats. Obesity 16:1226-1231. â€
- Touati S, Meziri F, Devaux S, Berthelot A, Touyz R, Laurant P (2011) Exercise reverses metabolic syndrome in high-fat diet-induced obese rats. Med Sci Sports Exerc 43: 398-407.
- Gami A, Witt B, Howard D, Erwin P, Gami L, et al. (2007) Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol 49: 403-414. â€
- Aubin M, Lajoie C, Clément R, Gosselin H, Calderone A, et al. (2008) Female rats fed a high-fat diet were associated with vascular dysfunction and cardiac fibrosis in the absence of overt obesity and hyperlipidemia: therapeutic potential of resveratrol. J Pharmacol Exp Ther 325: 961-968. â€
- Xi Y, Wu M, Li H, Dong S, Luo E, et al. (2015) Baicalin attenuates high fat diet-induced obesity and liver dysfunction: dose-response and potential role of CaMKKβ/AMPK/ACC pathway. Cell Physiol Biochem 35: 2349-2359.
- Gutierrez RMP and Romero RV (2016) Effects of bixin in high-fat diet-fed-induced fatty liver in C57BL/6J mice. Asian Pacif J Tropical Biomed 6: 1015-1021.
- Huang T, Chang C, Kao E, Lin J (2015) Effect of Hibiscus sabdariffa extract on high fat diet–induced obesity and liver damage in hamsters. Food Nut Res 53: 222-230.                       Â
- Vernon G, Baranova A, Younossi ZM (2011) Systematic review: The epidemiology and natural history of nonâ€alcoholic fatty liver disease and nonâ€alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 34: 274-285.
- Struben VM, Hespenheide EE, Caldwell SH (2000) Nonalcoholic steatohepatitis and cryptogenic cirrhosis within kindreds. Am J Med 108: 9-13.
- Musso G, Gambino R, De Michieli F, Durazzo M, Pagano G, et al. (2008) Adiponectin gene polymorphisms modulate acute adiponectin response to dietary fat: Possible pathogenetic role in NASH. Hepatology 47: 1167-1177.
- Zhou M, Schulman I, Raij L (2010) Vascular inflammation, insulin resistance, and endothelial dysfunction in salt-sensitive hypertension: Role of nuclear factor kappa B activation. J hypertens 28: 527-535.â€
- Yki-Järvinen H (2010) Liver fat in the pathogenesis of insulin resistance and type 2 diabetes. Digest Diseas 28: 203-209.
- Alkhouri N, Kistangari G, Campbell C, Lopez R, Zein N, et al. (2012) Mean platelet volume as a marker of increased cardiovascular risk in patients with nonalcoholic steatohepatitis. Hepatology 55: 331-333.
- Nakamuta M, Fujino T, Yada R, Yada M, Yasutake K, et al. (2009) Impact of cholesterol metabolism and the LXRα-SREBP-1c pathway on nonalcoholic fatty liver disease. Intern J Molecular Medicine 23: 603-608.
- Czyżewska M, Wolska A, Ćwiklińska A, Kortas-Stempak B, Wróblewska M (2010) Disturbances of lipoproteins in the metabolic syndrome. Postepy Hig Med Dosw 64: 1-10.
- Gaggini M, Morelli M, Buzzigoli E, DeFronzo R, Bugianesi E, et al. (2013) Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients 5: 1544-1560.
- Bieghs V, Rensen P, Hofker M, Shiri-Sverdlov R (2012) NASH and atherosclerosis are two aspects of a shared disease: Central role for macrophages. Atherosclerosis 220: 287-293.
- Ekstedt M, Franzén L, Mathiesen U, Thorelius L, Holmqvist M, et al. (2006) Longâ€term followâ€up of patients with NAFLD and elevated liver enzymes. Hepatology 44: 865-873.
- Xu X, Lu L, Dong Q, Li X, Zhang N, et al. (2015) Research advances in the relationship between nonalcoholic fatty liver disease and atherosclerosis. Lipids Health Dis 14: 158.
- Harrison S, Kadakia S, Lang K, Schenker S (2002) Nonalcoholic steatohepatitis: What we know in the new millennium. Am J Gastroenterol 97: 2714-2724.
- Marseglia L, Manti S, D’Angelo G, Nicotera A, Parisi E, et al. (2015) Oxidative Stress in Obesity: A Critical Component in Human Diseases. Int J Mol Sci 16: 378-400.
- Takaki A, Kawai D, Yamamoto K (2013) Multiple hits, including oxidative stress, as pathogenesis and treatment target in non-alcoholic steatohepatitis (NASH). Int J Mol Sci 14: 20704-20728.â€
- Rolo A, Teodoro J, Palmeira C (2012) Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic Biol Med 52: 59-69.â€
- Nagae A, Fujita M, Kawarazaki H, Matsui H, Ando K, et al. (2009): Sympathoexcitation by oxidative stress in the brain mediates arterial pressure elevation in obesity-induced hypertension. Circulation 119: 978-986.â€
- Meyer L, Ciaraldi T, Henry R, Wittgrove A, Phillips S (2013) Adipose tissue depot and cell size dependency of adiponectin synthesis and secretion in human obesity. Adipocyte 2: 217-226.â€
- Moreno-Navarrete J, Ortega F, Serrano M, Guerra E, Pardo G, et al. (2013) Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J Clin Endocrinol Metab 98: 769-778.
- Stengel A, Hofmann T, Goebel-Stengel M, Elbelt U, Kobelt P, et al. (2013) Circulating levels of irisin in patients with anorexia nervosa and different stages of obesity-Correlation with body mass index. Peptides 39:125-130.
- Sanchis-Gomar F, Alis R, Pareja-Galeano H, Sola E, Victor V, et al. (2014) Circulating irisin levels are not correlated with BMI, age and other biological parameters in obese and diabetic patients. Endocrine 46: 674-677.
- Tilg H, Moschen A (2006) Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 6: 772-783.
- Basaranoglu M, Basaranoglu G, Sabuncu T, Sentürk H (2013) Fructose as a key player in the development of fatty liver disease. World J Gastroenterol 19: 1166-1172.
- Plomgaard P, Bouzakri K, Krogh-Madsen R, Mittendorfer B, Zierath J, et al. (2005) Tumor necrosis factor-α induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes 54: 2939-2945.
- Kaneto H, Katakami N, Matsuhisa M, Matsuoka T (2010) Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis. Mediators Inflamm 2010: 453892.
- Schuett H, Luchtefeld M, Grothusen C, Grote K, Schieffer B (2009) How much is too much? Interleukin-6 and its signalling in atherosclerosis. Thromb Haemost 102: 215-222.â€
- Kwon D, Kang W, Nam Y, Lee M, Lee I, et al. (2012) Dietary protein restriction induces steatohepatitis and alters leptin/signal transducers and activators of transcription 3 signaling in lactating rats. J Nutr Biochem 23: 791-799.
- Paz-Filho G, Mastronardi C, Franco C, Wang K, Wong M, et al. (2012) Leptin: molecular mechanisms, systemic pro-inflammatory effects and clinical implications. Arq Bras Endocrinol Metabol 56: 597-607.
- Zuo H, Shi Z, Yuan B, Dai Y, Wu G, et al. (2013) Association between serum leptin concentrations and insulin resistance: A population-based study from China. PLoS One, 8: e54615.
- Beltowski J (2006) Role of leptin in blood pressure regulation and arterial hypertension. J Hypertens 24: 789-801.â€
- Antic V, Dulloo A, Montani J (2003) Multiple mechanisms involved in obesity-induced hypertension. Heart Lung Circul 12: 84-93.â€
- Koh K, Park S, Quon M (2008) Leptin and cardiovascular disease. Circulation 117: 3238-3249.â€
- Myers M, Heymsfield S, Haft C, Kahn B, Laughlin M, et al. (2012) Challenges and opportunities of defining clinical leptin resistance. Cell Metab 15: 150-156.â€
- Ostrowska L, Fiedorczuk J, Adamska E (2013) Effect of diet and other factors on serum adiponectin concentrations in patients with type 2 diabetes. Rocz Panstw Zakl Hig 64: 61-66.
- Pallavi M, Suchitra M, Rao P (2015) Role of adipokines in the pathogenesis of non-alcoholic fatty liver disease. J Clin Sci Res 4: 31-39.â€
- Hansen T, Ahlström H, Söderberg S, Hulthe J, Wikström J, et al. (2009) Visceral adipose tissue, adiponectin levels and insulin resistance are related to atherosclerosis as assessed by whole-body magnetic resonance angiography in an elderly population. Atherosclerosis 205: 163-167.â€
- Zhang H, Zhang X, Ma Z, Pan L, Chen Z, et al. (2013) Irisin is inversely associated with intrahepatic triglyceride contents in obese adults. J Hepatol 59: 557-562.
- Hondares E, Rosell M, DÃaz-DelfÃn J, Olmos Y, Monsalve M, et al. (2011) Peroxisome proliferator-activated receptor α (PPARα) induces PPAR γ coactivator 1α (PGC-1α) gene expression and contributes to thermogenic activation of brown fat involvement of PRDM16. J Biol Chem 286: 43112-43122.
- Lu J, Xiang G, Liu M, Mei W, Xiang L, et al. (2015) Irisin protects against endothelial injury and ameliorates atherosclerosis in apolipoprotein E-Null diabetic mice. Atherosclerosis 243: 438-448.â€
- Zhu G, Wang J, Song M, Zhou F, Fu D, et al. (2016) Irisin increased the number and improved the function of endothelial progenitor cells in diabetes mellitus mice. J Cardiovasc Pharmacol 68: 67-73.â€
- Slentz C, Duscha B, Johnson J, Ketchum K, Aiken L, et al. (2004) Effects of the amount of exercise on body weight, body composition, and measures of central obesity: STRRIDE-A randomized controlled study. Arch Intern Med 164: 31-39.â€
- Savage PD, Brochu M, Poehlman ET, Ades PA (2003) Reduction in obesity and coronary risk factors after high caloric exercise training in overweight coronary patients. Am Heart J 146: 317-323.â€
- Hou X, Xu S, Toolan KM, Sato K, Jiang B, et al. (2008) SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem 283: 20015-20026.
- Goodpaster B, Katsiaras A, Kelley D (2003) Enhanced fat oxidation through physical activity is associated with improvements in insulin sensitivity in obesity. Diabetes 52: 2191-2197.
- Tunstall R, Mehan K, Wadley G, Collier G, Bonen A, et al. (2002) Exercise training increases lipid metabolism gene expression in human skeletal muscle. Am J Physiol Endocrinol Metab 283: 66-72.â€
- Pinheiro A, Cunha A, Aguila M, Lacerda CM (2007) Beneficial effects of physical exercise on hypertension and cardiovascular adverse remodeling of diet-induced obese rats. Nutr Metab Cardiovasc Dis 17: 365-375.â€
- Sciacqua A, Candigliota M, Ceravolo R, Scozzafava A, Sinopoli F, et al. (2003) Weight loss in combination with physical activity improves endothelial dysfunction in human obesity. Diabetes Care 26: 1673-1678.â€
- Sullivan S, Kirk E, Mittendorfer B, Patterson B, Klein S (2012) Randomized trial of exercise effect on intrahepatic triglyceride content and lipid kinetics in nonalcoholic fatty liver disease. Hepatology 55: 1738-1745.
- Azizi M (2011) The effect of 8-weeks aerobic exercise training on serum LEPTIN in un-trained females. Procedia-Social Behav Sci 15: 1630-1634.â€â€â€
- GarcÃa-Hermoso A, Ceballos-Ceballos R, Poblete-Aro C, Hackney A, Mota J, et al. (2017) Exercise, adipokines and pediatric obesity: a meta-analysis of randomized controlled trials. Int J Obes (Lond) 41: 475-482â€.
- Boström P, Wu J, Jedrychowski M, Korde A, Ye L, et al. (2012) A PGC1-[agr]-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481: 463-468.â€
- Villarroya F (2012) Irisin, turning up the heat. Cell Metab 15: 277-278.
- Mazur-Bialy A, Pocheć E, Zarawski M (2017) Anti-inflammatory properties of irisin, mediator of physical activity, are connected with TLR4/MyD88 signaling pathway activation. International journal of molecular sciences 18: E701.
- Hoene M, Weigert C (2008) The role of interleukinâ€6 in insulin resistance, body fat distribution and energy balance. Obes Rev 9: 20-29.â€
- Febbraio M, Pedersen B (2005) Contraction-induced myokine production and release: Is skeletal muscle an endocrine organ? Exerc Sport Sci Rev 33: 114-119.
- Piva S, Tatsch E, De Carvalho J, Bochi G, Kober H, et al. (2013) Assessment of inflammatory and oxidative biomarkers in obesity and their associations with body mass index. Inflammation 36: 226-231.â€
- Dietze D, Koenen M, Röhrig K, Horikoshi H, Hauner H, et al. (2002) Impairment of insulin signaling in human skeletal muscle cells by co-culture with human adipocytes. Diabetes 51: 2369-2376.â€
- Arias-Loste M, Ranchal I, Romero-Gómez M, Crespo J (2014) Irisin, a link among fatty liver disease, physical inactivity and insulin resistance. Int J Mol Sci 15: 23163-23178.
- Chrysohoou C, Pitsavos C, Panagiotakos D, Kokkinos P, Stefanadis C, et al. (2003) The association between physical activity and the development of acute coronary syndromes in treated and untreated hypertensive subjects. J Clin Hypertens (Greenwich) 5: 115-120.â€
Citation: Soliman NA, Asalah AK, Moursi SM, Gamal SM, Eldeen MA (2018) Effect of Exercise Training on Metabolic Homeostasis and Some Hemodynamics (Some Hepatic and Cardiovascular Functions) in Experimentally Induced Obesity. J Obes Weight Loss Ther 8: 368. DOI: 10.4172/2165-7904.1000368
Copyright: © 2018 Soliman NA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 7510
- [From(publication date): 0-2018 - Sep 23, 2025]
- Breakdown by view type
- HTML page views: 6501
- PDF downloads: 1009