Research Article Open Access
Effect of Preparation Method on Antioxidant Activity of Ayurvedic Formulation Kumaryasava
| Rahul Manmode1*, Jagdish Manwar2, Mustafa Vohra3, Satish Padgilwar2 and Nitin Bhajipale2 | |
| 1Department of Chemistry, University of Massachusetts, Lowell-01854, USA | |
| 2Department of Quality Assurance, Institute of Pharmacy, Akola-444 004, India | |
| 3Department of Alcohol Technology, Vasantdada Sugar Institute, Pune-412 307, India | |
| Corresponding Author : | Dr. Rahul Manmode Department of Chemistry, 1st University Avenue University of Massachusetts Lowell, Lowell, USA Tel: +001-978-934-3732 E-mail: rahulmanmode@gmail.com |
| Received September 28, 2012; Accepted October 26, 2012; Published October 29, 2012 | |
| Citation: Manmode R, Manwar J, Vohra M, Padgilwar S, Bhajipale N (2012) Effect of Preparation Method on Antioxidant Activity of Ayurvedic Formulation Kumaryasava. J Homeop Ayurv Med 1:114. doi: 10.4172/2167-1206.1000114 | |
| Copyright: © 2012 Manmode R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Traditional Medicine & Clinical Naturopathy
Abstract
Kumaryasava is an alcoholic Ayurvedic formulation prepared by the fermentation of Aloe vera. Flowers of Woodfordia fruticosa are added as inoculums for fermentation process. The aim of the present investigation to find the effect of its preparation method on antioxidant activity of Kumaryasava. Three different formulations of Kumaryasava were prepared by fermentation using three different inoculums viz. Woodfordia fruticosa flowers, Madhuca indica flowers and yeast Saccharomyces cerevisiae SC1011. During fermentation process, relationship between alcohol generation and sugar utilization in each formulation was studied. In vitro antioxidant activity of all formulations was evaluated by 1,1-diphenyl-2-picryl hydrazyl (DPPH) scavenging, hydrogen peroxide scavenging and total reducing power. The results were compared to standard antioxidant ascorbic acid. All the tested formulations showed marked in vitro antioxidant activity in which WFKA (W. fruticosa flowers based Kumaryasava) showed prominent activity. Obtained IC50 values of WFKA in DPPH scavenging assay, hydrogen peroxide scavenging assay and total reducing power assay were 481.78, 50.13 and 49.60, respectively. From the results it is concluded that formulation (WFKA) prepared by traditional method showed higher in vitro antioxidant activity relative to others.
| Keywords |
| Aloe Vera; Woodfordia fruticosa; Madhuca indica; Alcohol; Antioxidant activity |
| Introduction |
| The use of Ayurvedic formulations has led to the sudden increase in the number of Ayurvedic drug manufactures due to the toxicity and side effects of allopathic medicines. Kumaryasava is an Ayurvedic formulation containing Aloe Vera (leaves) is the major crude drug along with 17 other minor ingredients [1]. A. vera (Synonym: Aloe barbadensis, family Liliaceae), also known as Kumaari, is a shrubby plant with succulent and elongated leaves. The plant is widely cultivated throughout India, especially in the coastal region of Maharashtra, Gujarat and South India [2]. Leaves of the plant were reported to exhibit various effects like wound healing, anti-inflammatory and immunomodulatory activity [3-6]. Topical polyherbal formulation containing pulp of A. vera leaves as a main ingredient is used for the treatment of acne [7]. Ayurvedic Pharmacopoeia of India recommends Kumaryasava for the treatment of abdominal lump, epilepsy, digestive impairment and menopause [1]. |
| Traditionally, Kumaryasava is prepared by the fermentation of leaves of A. vera. During the preparation, flowers of Woodfordia fruticosa (Family-Lythraceae) are added as inoculums for alcoholic fermentation. This fermentation process uses the natural yeasts present on the flowers of W. fruticosa [8,9]. The yeast present on the flowers may vary depending on the source location and hence about one month period is recommended to complete the fermentation process. Alcohol generated during the fermentation process causes the extraction of water insoluble active principle components from the crude drugs. According to Ayurvedic Pharmacopoeia of India, Kumaryasava should contain not less than 5% v/v and not more than 10% v/v self-generated alcohol (40-80 g/l). W. fruticosa flowers, based formulations are useful in various conditions like leucorrhoea, dysfunctional uterine bleeding, burning sensation in stomach, weakness and rheumatoid diseases [10-12]. |
| Madhuca indica (synonym: Madhuca latifolia) belonging to family Sapotaceae is a large forest tree found largely in the central and north Indian plains and forests [13]. Flowers of the tree contain 28.1-36.3% w/w of fermentable sugars [14]. Presence of yeast Saccharomyces cerevisiae, well known for alcohol production, was documented in the literature [15]. Therefore, these flowers are used to produce fermented alcoholic drink mahuwa (country liquor). Tribals of Bastar in Chattisgarh, Orissa and Jharkhand consider the mahuwa drink as part of their cultural heritage. The flowers are also used in making of some Ayurvedic formulations like Abhayarista, Kutajarista, Partadyarista etc. [1]. |
| Inspite of availability of numerous sources of inoculums like flowers of M. indica or yeast strain S. cerevisiae, even today Kumaryasava is being prepared by traditional method using W. fruticosa flowers [16,17]. This process takes upto 1-month period to complete the fermentation process. Therefore, there is urgent need to reduce this long time to fewer days by trying the other inoculums those contain fermentation yeast. Literature survey indicated that no report on kinetics of alcohol generation and sugar utilization during making of Kumaryasava by traditional method. We are the first to report the kinetics of fermentation process in traditional method of preparation of same formulation. This data will be helpful to decide when to stop fermentation process. |
| Antioxidant molecules are able to reduce oxidative stress the by preventing the oxidation of other molecules. This stress is caused by free radicals which initiate chain reaction and seek stability through electron pairing with biomolecules like lipids, proteins, DNA in the cells and causes lipid peroxidation along with protein and DNA damage [18]. Antioxidant activity of extract of A. vera leaves is well documented [19-21]. During the making of Kumaryasava, minor ingredients like Nordostachys jatamansi, Piper cubeba, Syzygium aromaticum etc., also contribute to the antioxidant activity. Therefore, all minor ingredients were omitted to avoid their interference in antioxidant activities [22-24]. In vitro antioxidant activity of formulations was evaluated by 1,1-diphenyl-2-picryl hydrazyl (DPPH) scavenging, hydrogen peroxide scavenging and total reducing power. The data of antioxidant study will explain at least in part how preparation method affects the biological activity. Jaggery was used as source of sugar to produce alcohol. |
| Materials and Methods |
| Plant material and chemicals |
| Leaves of A. vera were collected from nearest farm (Figure 1). Flowers of W. fruticosa were collected from the Solapur district and flowers of M. indica were collected from Washim district of Maharashtra, India. All the samples were then immediately transferred into the sterilized plastic bag. The plant materials were authenticated from Agharkar Research Institute, Pune, India. Commercial grade of jaggery was purchased from local market. The chemicals and solvents used in this experimental work were of the highest quality available. |
| Collection of S. cerevisiae SC1011 |
| Standard culture of yeast S. cerevisiae SC1011 was collected from Vasantdada Sugar Institute, Pune, India. The strain was maintained on MGYP agar slants (3 g malt extract, 10 g glucose, 3 g yeast extract, 5 g peptone and 20 g agar per liter of water, pH 4.5) and preserved at 4°C for routine use. |
| Preparation of Jaggery Media (12°brix) |
| About 6-7 g of jaggery was dissolved in 350 ml of distilled water. This media was then transfered to measuring cylinder (250 ml capacity). Brix hydrometer of range 10-20 was dipped into the media and brix value was observed. The brix value was adjusted to 12 by addition of water or jaggery and pH was adjusted to 4.5. To the media, 0.01% w/v of diamonophosphate and 0.01% w/v of urea was added as a source of nitrogen, autoclaved at 15 lbs at 121°C for 15 min, cooled and used in further experimental work. |
| Preparation of inoculums of S. cerevisiae SC1011 |
| The inoculums of S. cerevisiae SC1011 was prepared by transferring one loopful of yeast culture into 100 ml of sterile 12°brix jaggery medium and incubated at 32.5°C for 24 h in shaker incubator at 180 rpm. After incubation, cell count of yeasts were measured using neubauer counting chamber (methylene blue indicator was used to stain viable cells). |
| Preparation of formulations |
| Leaves of A. vera were cut into bits and the skin was removed to obtain a pulp. In three separate 1 L conical flasks, 250 g jaggery was dissolved in 300 ml of distilled water by boiling. The flasks were cooled and final volumes of media were made up to 500 ml. To each flask, 100 g of pulp was added and they were autoclaved at 121°C for 20 min. After sterilization, flasks were cooled and flasks were inoculated with W. fruticosa flowers (16 g), M. indica flowers (10 g) and inoculums of S. cerevisiae SC1011 (10 ml, cell count- 3 × 107 cells/ml), separately. Formulation inoculated with W. fruticosa flowers, M. indica flowers and yeast S. cerevisiae SC1011 were named as WFKA, MIKA and SCKA, respectively. All the flasks were incubated in BOD incubator at 32.5°C for 30 days. At the intervals of 3 days, 15 ml of fermentation broth was withdrawn from each formulation aseptically, centrifuged at 6000 rpm, and supernatant layers were used for the analysis of alcohol generated (g/l) and residual sugar (% w/v). |
| Analysis of broth |
| Kinetics of fermentation was studied by determining the relationship between alcohol generation and sugar utilization. Amount of alcohol generated was determined by dichromate oxidation method and that of residual sugar was determined by titration method [25,26]. |
| Evaluation of in-vitro antioxidant activity |
| After completion of fermentation process, all the formulations were filtered through three layers of muslin cloth over other. Filtered formulations were centrifuged at 3000 rpm for 20 min and were used for evaluation of in vitro antioxidant activity by various assay methods. Ascorbic acid (100 μg/ml) was used as standard antioxidant for comparing results of sample solution. |
| DPPH scavenging assay |
| Ability of formulation to scavenge DPPH free radical was carried out by slightly modifying the procedure given in the literature [27]. Solution of DPPH was prepared by dissolving 33 mg of compound in 1 L of analytical grade methanol and was then store in dark amber colored bottle. 1 ml of various concentration (100, 200, 400, 800 and 1000 μl/ml) of formulation in water were taken in different test tubes. To each tube, 5 ml of DPPH solution was added, shaken and tubes were immediately kept in dark at 27°C for 20 min. After incubation, the tubes were centrifuged at 3000 rpm for 5 min and absorbance of supernatant layer was measured at 517 nm using UV-Vis Spectrophotometer (Shimadzu, model 160). Control solution was also prepared and zero was set using solvent methanol. Percentage DPPH scavenging effect was calculated from the following formula: |
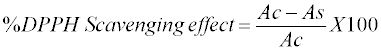 |
| Where, Ac is the absorbance of control solution, and As is the absorbance of sample solution. |
| Hydrogen peroxide scavenging assay |
| Ability of formulation to scavenge hydrogen peroxide (H2O2) was carried out by method reported in literature [28]. H2O2 solution (100 mM) was prepared in phosphate buffer (pH 7.4). About 0.5 ml of solution of each formulation of various concentration (10, 20, 40, 60 and 100 μl/ml) of formulations in water was added to test tube separately containing 4.5 ml of H2O2 solution. Tubes were incubated at room temperature for 10 min and absorbance of solution was measured at 230 nm against a solution in phosphate buffer without H2O2. Percentage of H2O2 scavenging activity was calculated from the following formula: |
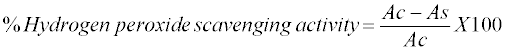 |
| Where, Ac is the absorbance of control solution, and As is the absorbance of sample solution. |
| Reducing power assay |
| Reducing ability of formulations was measured according to procedure given in literature [29]. The different concentrations of formulation (10, 20, 40, 60 and 100 μl/ml) were mixed with phosphate buffer (2.5 ml, 0.2 M, pH 6.6) and potassium ferricyanide (2.5 ml, 1%). To this, about 2.5 ml of trichloroacetic acid (10%v/v) was added, and mixture was incubated at 50°C for 20 min. After incubation, mixture was centrifuged at 3000 rpm for 20 min. 2.5 ml of supernatant layer was mixed with 2.5 ml of water and 0.5 ml of FeCl3 (0.1%), and absorbance was measured at 700 nm wavelength. |
| Statistical analysis |
| The data of antioxidant testing are expressed as mean ± standard error of mean (SEM). Statistical analysis was performed using a oneway analysis of variance (ANOVA) and differences were considered to be significant at p<0.05. |
| Results |
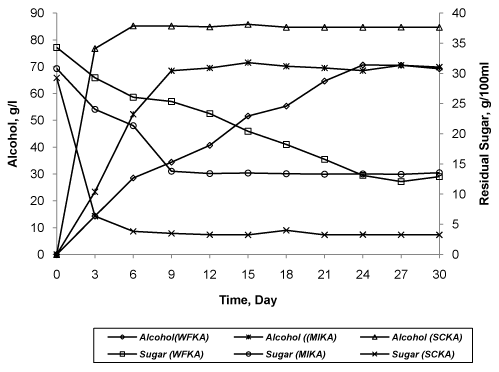
| Alcohol generation in formulation SCKA, MIKA and WFKA was stopped after 6 days, 15 days and 21 days, respectively. In SCKA, fermention process was stopped after 6 days with the alcohol generation of 85.21 g/l. this amount of alcohol was remained constant till 30 days. Initial amount of residual sugar was 29.27 g/100 ml. Within a period of first 6 days, 86.91% of initial sugar was utilized for this alcohol production and then no further utilization was observed. In finished SCKA formulation, amount of alcohol and residual sugar was 84.70 g/l and 3.26 g/100 ml, respectively. In MIKA, flowers of M. indica have produce 71.53 g/l of alcohol after 15 days using 56.05% of initial amount of sugar. Initial amount of residual sugar was 30.81 g/100 ml. Over the period of next 15 days, negligible (0.167% w/v) reduction in amount of alcohol was noted. Nevertheless, amount was sugar was constant in this period. In finished formulation, amount of alcohol and residual sugar was 69.86 g/l and 13.54 g/100 ml, respectively. In WFKA, which was prepared traditionally using flowers of W. fruticosa, fermentation process was completed after 24 days. Initial amount of residual sugar was 34.3 g/100 ml. In this period, amount of alcohol produced was 70.65 g/l at a rate of production was 2.94 g/l per day. Over the remaining period, amount of alcohol and sugar was nearly constant. Till last day, 62.36% of initial amount sugar was utilized for production of 69.21 g/l of alcohol. In finished formulation, 12.91 g/100 ml of sugar was present (Figure 2). |
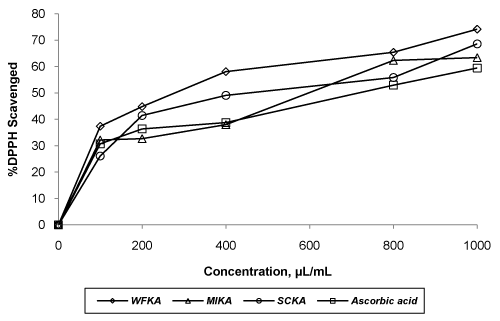
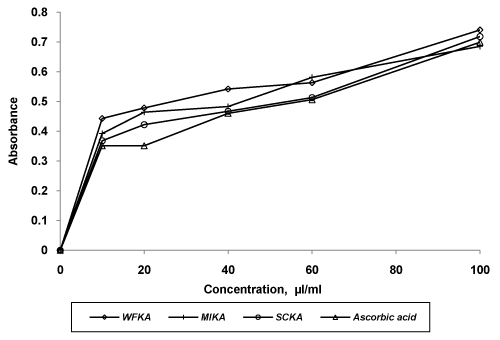
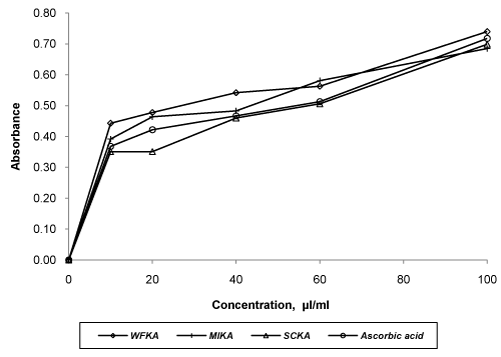
| Evaluation of in vitro antioxidant activity of all formulations was carried out using three model systems viz. DPPH scavenging assay, reducing power assay and hydrogen peroxide scavenging assay. All the formulations and ascorbic acid solution were found to decreases the concentration of DPPH significantly (p<0.05) with the concentration of 100 μl/ml to 1000 μl/ml. The order of DPPH scavenging activity was WFKA>MIKA>SCKA >ascorbic acid. IC50 values WFKA, SCKA, MIKA and ascorbic acid were 481.78, 607.16, 642.64 and 723.77, respectively (Figure 3). Hydrogen peroxide scavenging capacity of formulations was carried out using different concentration. SCKA indicated lesser activity than those of WFKA and MIKA. The order of activity shown by formulations was WFKA> MIKA >SCKA>ascorbic acid. IC50 values of WFKA, MIKA, SCKA and ascorbic acid were 50.13, 71.41, 86.18 and 92.91, respectively (Figure 4). In reducing power assay, the order of reducing power shown by formulations was WFKA>MIKA >SCKA>ascorbic acid. IC50 values of WFKA, MIKA, SCKA and ascorbic acid were 49.6, 53.4, 59.4 and 64, respectively (Figure 5). |
| Discussion |
| Three different formulations of Kumaryasava i.e. WFKA, MIKA and SCKA were prepared by fermentation using various sources of inoculums like W. fruticosa flowers, M. indica flowers and yeast S. cerevisiae SC1011, respectively. To avoid any interference with activity, all minor ingredients were omitted during preparation. For all, jaggery media (50% w/v) was as source of sugar for alcohol [30]. Both non-traditional inoculums (M. indica flowers, S. cerevisiae SC1011) have produced recommended amount of alcohol i.e. 40 to 80 g/l within a shorter period relative to traditional method. In SCKA, rate of alcohol generation and rate of sugar utilization was faster than other formulation. In this case, fermentation process was stopped after 6 days. Flowers of M. indica were found to intermediate type of inoculums. It minimizes the duration of fermentation time of 30 days to just 15 days. In WFKA, it takes about 24 days to complete the fermentation process. In this case, rate of alcohol generation and sugar utilization was much less than others. Slow alcohol generation may cause incomplete extraction of water insoluble active principles of crude drugs, and perhaps Ayurvedic Pharmacopoeia of India might have recommended enough time (one month) for complete extraction. In all formulations, good co-relationship was observed between the alcohol generation and sugar utilization. These data of fermentation may be used as a new method to classify the inoculums with respect to their maximum for alcohol generation capacity. |
| Medicinal plants contain phenolic compounds, which are responsible for different kinds of biological effects including antioxidant activity [31,32]. The free radical scavenging activities of all formulations were evaluated using metabolic solution of DPPH. DPPH is a stable nitrogen-centered free radical. DPPH has advantages of being unaffected by such a metal ion due certain side reactions, such as metal ion chelation and enzyme inhibition brought about by various additives. Antioxidant molecule quench DPPH radicals (by providing hydrogen atom or by electron transfer, conceivably via a free radical attack on the DPPH molecule) and convert them to a colorless/yellow product (2,2-diphenyl-1-hydrazine or a substituted analogs of hydrazine) resulting in a decreasing absorbance at a 517 nm [33]. Substances to perform this above reaction can be considered as antioxidants [34]. Lower absorbance of the reaction mixture indicated higher DPPH scavenging activity. In biochemical systems, superoxide radicals are converted to hydrogen peroxide (H2O2) by superoxide dismutase. H2O2 has ability of penetrating biological membranes. It is very reactive but in presence of certain transition metal ions such as iron and copper, it can subsequently produce extremely reactive hydroxyl radicals [35]. Results show all formulations possess significant H2O2 scavenging activity. It has reported that the reducing power of plant is related to the antioxidant potential of active principles present [36]. Color change from yellow to different shades of green and blue depends upon the reducing power of each formulation. The presence of antioxidants in the Ayurvedic formulation causes the reduction of Fe3+ (Ferric cyanide complex) to Fe2+ (ferrous form). This Fe++ complex can be monitored by measuring the formation of Perl’s Prussian blue at 700 nm [37]. In this assay, absorbance of sample solution was increased with increase in concentration of formulations. Hence, the increase in absorbance indicates increasing trend of reducing power. Higher absorbance of the reaction mixture indicated greater reducing power. The reducing power of formulations was increased with increasing concentration. |
| Though the alcohol is earlier in formulation using non-traditional inoculums (M. indica flowers, S. cerevisiae SC1011), but they did not showed activity equal to formulation WFKA prepared by traditional method. All the formulations were found to exhibit higher antioxidant activity than that of ascorbic acid. This increase in activity may be due to presence of potent antioxidant principles in the leaves of A. vera and or may be due to the presence of jaggery in higher concentration. Jaggery also reported as good antioxidant agent [38]. Beside its use as a sugar, it may act as good antioxidant agent in the formulation. Among all, W. fruticosa flowers based Kumaryasava WFKA showed highest antioxidant activity by all models. This increased of in activity might be due to the presence of phenolic compounds in the flowers of W. fruticosa that possesses potent antioxidant activity [39,40]. |
| Conclusion |
| Even though the fermentation process is much slower using inoculums of W. fruticosa flowers relative to other inoculums, experimentally it is proved that their presence in the Kumaryasava highly contributes to the antioxidant activity. The phenolic compounds of the flowers might be responsible for this activity. Therefore, it has been concluded that the formulation prepared by traditional method reported in Ayurvedic Pharmacopoeia is the best formulation in respect of antioxidant activity. |
References
|
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
Relevant Topics
- Acupuncture Therapy
- Advances in Naturopathic Treatment
- African Traditional Medicine
- Australian Traditional Medicine
- Chinese Acupuncture
- Chinese Medicine
- Clinical Naturopathic Medicine
- Clinical Naturopathy
- Herbal Medicines
- Holistic Cancer Treatment
- Holistic health
- Holistic Nutrition
- Homeopathic Medicine
- Homeopathic Remedies
- Japanese Traditional Medicine
- Korean Traditional Medicine
- Natural Remedies
- Naturopathic Medicine
- Naturopathic Practioner Communications
- Naturopathy
- Naturopathy Clinic Management
- Traditional Asian Medicine
- Traditional medicine
- Traditional Plant Medicine
- UK naturopathy
Recommended Journals
Article Tools
Article Usage
- Total views: 8601
- [From(publication date):
December-2012 - Aug 29, 2025] - Breakdown by view type
- HTML page views : 3882
- PDF downloads : 4719
