Research Article Open Access
Effect of Temperature and Salts on Phenol Bio-Availability in Polluted-Sandy- Soils: A Practical Biotechnological Approach before Microbial Bioremediation
| Gularte HF1*, Diaz ME2, Rendueles M1, Gutierrez A1, Jaili E3 and Diaz M1 | |
| 1Chemical Engineering and Environmental Technology, University of Oviedo, Spain | |
| 2Physical and Analytical Chemistry Departments, Faculty of Chemistry, University of Oviedo, Spain | |
| 3Work and Sustainable Development, Soil and Mineral Research Office, Spain | |
| Corresponding Author : | Gularte HF Chemical Engineering and Environmental Technology University of Oviedo, Spain Tel: +447806620217 Fax: +34985103434 E-mail: fgularte@gmail.com |
| Received March 04, 2014; Accepted August 28, 2014; Published August 30, 2014 | |
| Citation: Gularte HF, Diaz ME, Rendueles M, Gutierrez A, Jaili E, et al. (2014) Effect of Temperature and Salts on Phenol Bio-Availability in Polluted-Sandy- Soils: A Practical Biotechnological Approach before Microbial Bioremediation. J Bioremed Biodeg 5:240. doi:10.4172/2155-6199.1000240 | |
| Copyright: © 2014 Gularte HF, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Despite the huge amount of accidental spills of phenols and other similar compounds, it is still unclear for real cases how to work and clean huge amounts of polluted sandy soil with high phenol concentration, especially with factors related to the adsorbed fraction and its bio-availability for a bio-treatment process. This new approach is about how by the control of clean operation conditions, like natural soil characteristics, pH, salts (type and concentration), solid-liquid relation, stirring speed and temperature, it is possible to decrease or increase the adsorbed fraction and bio-availability of phenol in ex-situ reactors. The results of this work allowed enhancing phenol bio-availability and reducing its absorption in studied polluted soils. In addition, this showed how soil complexity works and interacts with analytes in media and opens the door for a real world process to bio-remediate similar soils in all kinds of environmental conditions.
| Keywords |
| Soil; Biotechnology; Bioremediation; Bio-availability; Sandy-soils; Adsorption; Microbial-nutrition-optimization |
| Introduction |
| In accidental spills of phenolic compounds on soils it is crucial to make an immediate isolation to prevent these pollutants to reach the water-table and contaminate human water resources. Several methods have been developed to clean phenol polluted soils in low concentration cases, especially at lab scale under ideal conditions. However, high phenol-concentration pollution cases in real-world soils have been poorly evaluated due to their complexity. Among the available methods to clean large amounts of soils under extreme and heterogenic conditions, ex-situ bioremediation is a feasible process [1-4]. |
| The phenol soil ex-situ bioremediation is a method where a soil as soon as possible is taken out from polluted area and is put into a reactor, where pollutants are degraded into less toxic substances by microorganisms [5-7]. However, it is still unclear in real cases how to work and clean huge amounts of polluted soils with high pollutant concentrations, especially considering factors related to the adsorbed fraction and its bio-availability for a bio-treatment process. |
| In this sense, a new practical approach of high phenol polluted soil cases is shown in this work, about how by the analysis and control of clean operation conditions, like soil properties, pH, salts (kind and concentration), solid/liquid relation, stirring speed and temperature, it is possible to change the adsorbed fraction and bio-availability of this compound in ex situ reactors for sandy soil bioremediations. All sets of tests and data were carried out before microbial activity in this process. |
| Many works have been published with respect to phenol soil bioremediation, however, not all have taken into account the adsorption factor during the cleaning process for similar experimental conditions. That means that these results cannot be assumed without doubts [5,7-10]. There is still a lot of work to be done in relation with soil properties, isothermal equations and how the operation conditions influence the adsorption equilibrium during a phenol soil bioremediation process. |
| The methodology for this research (see section 4) is presented in four subsections: the first one shows how the samples were taken; the second one shows how the samples were analyzed; the third one shows how the modelling and mathematical calculation of isotherms, bioavailability and distribution of phenol and turbulence of reactors were carried out; the fourth one shows how the different tests were carried out. |
| The results of this research on bioremediation of phenolic sandy soils (see section 5) are presented in four subsections: the first one shows how natural characteristics of these soils are and how these factors can enhance the microbial activity in this process; the second one shows how pH, salts (by type and concentration), solid-liquid relation and stirring speed can be used to have reproducible and comparative results during bioremediation processes in these soils independent of their nature; the third one shows how to choose isotherm equations in heterogeneous systems and how soil characteristics can influence the adsorption equilibrium during a real process of this kind; the fourth one shows how temperature and salts (depending on type and concentration) can increase or decrease the adsorption and bio-availability of phenol in this process. |
| At the end, the results of this work allowed enhancing phenol bioavailability, reducing its absorption and set the operation condition needed to bio-remediate the studied polluted sandy soils. Also, this showed how soil complexity works and interacts with analytes in media. However, more work is required to be done in order to understand completely how pollutants interact in natural heterogeneous soil systems in real high-concentration cases. The top of this is to have a universal method to face all possible cases of this and similar kind and predict their dynamics and interactions. The work presented here is to set the first steps to solve and meet this ambitious and leading goal. |
| Materials and Methods |
| Sampling methods |
| In this section the main aspects on sampling during the whole process of this research in solid, liquid and solid-liquid phases are described. |
| Soils |
| • Field scale |
| Seven random sandy soils were used to carry out different tests in this work. These were taken from different areas in central Asturias of Spain at random spots as follows (GPS coordinates, latitudes and longitudes respectively): a. 43° 35' 12.1812", -5° 56' 35.0412"; b. 43° 21' 18.396", -5° 52' 18.5586"; c. 43° 21' 14.832", -5° 52' 18.5298"; d. 43° 33' 4.572", -5° 37' 19.8402"; e. 43° 32' 55.8666", -5° 37' 25.6836"; f. 43° 21' 6.2166", -5° 52' 30.561"; g. 43° 21' 10.476", -5° 52' 26.0142". For the collection of each sample herbs and soil top layers were removed with a hand fork. Each sample was taken from field surface with a maximum depth of 20 cm with a hand trowel. The samples were collected in plastic bags and transported immediately to the laboratory at room temperature without preservatives. At laboratory, samples were immediately sieved (2 mm) and then dried until constant weight in an oven at 105°C. Thereafter, these were stored in sealed hermetic plastic bags at room temperature. |
| • Laboratory scale |
| Before each test at laboratory, random samples of each soil were taken [11]. Laboratory representative soil test samples were obtained from a soil batch of 500 g, according to the quartering scheme by dividing it into four even parts, being two opposite quarters discarded. The retained quarters were then combined and the operation was repeated until the required size sample was obtained. |
| Liquids: In all cases not more than 5% of total volume in each reactor was taken in each test. It meant that total volume and number of samples in each analytical method were managed in this regard. |
| Analytic methods |
| In this section the main aspects used to analyze samples during the whole process of this research in solid and liquid phases are described. |
| Soil phase |
| • pH |
| The pH was determined by the method of suspension soil/water: 1/2 by adding 20 mL of water to 10 g air-dry soil sample [12]. |
| • Cation exchange capacity |
| Cation exchange capacity (CEC) was determined by the Mehlich method using barium chloride buffered with triethanolamine to pH 8.2 [13]. |
| • Organic matter |
| Organic matter (OM) was determined by the oxidation method of Walkley-Black based on the soil wet digestion with potassium dichromate [11]. |
| • Electrical conductivity |
| The electrical conductivity of soil samples was determined in a 1/2 soil/water suspension by weighing 10 g dry soil into a flask [14]. |
| • Volumetric density |
| Volumetric density, or bulk density, was determined by ovendrying the sample soil at 105ºC until constant weight [12]. |
| • Particle size |
| The particle size was determined by the sieve method. Particle size distribution was estimated by passing 10 kg of each soil through a series of sieves with mesh sizes in three ranges, from 2 mm to 0.02 mm, from 0.02 mm to 0.002 mm and particles smaller than 0.002 mm. |
| • Morphology |
| The soil morphologies were analyzed by a Scanning Electron Microscopy (SEM). Dry soil samples were dispersed on a graphite adhesive tap place on an aluminium stub, coated with a thin layer of gold and examined with a Jeol-6100 Meb SEM [15]. This method was used to confirm the particle size and their results are not shown. |
| Liquid phase |
| • pH |
| The pH was determined with a glass electrode [14]. |
| • Phenol |
| Phenol concentration was determined from liquid fraction, filtered by 0.45 μm filter, by the method of the amino-anti pyrine at 550 nm [16]. |
| Modelling and mathematical equations |
| In this section a description is made of the main aspects used to modelling the isotherms, bio-availability and distribution of phenol and turbulence conditions of reactors during tests. This information allows replication of all developed calculations. |
| Isotherms |
| The correlation orders were determined by a linear regression of equivalent equation as follows [17-20]. |
| • Freundlich |
| Freundlich equation was used to test and show the tendency of phenol adsorption equilibrium in soils. This is represented by the following equation: |
| q = kC1/n |
| Equation 3-1 |
| Where q is adsorbed phenol at equilibrium (mg/g), k and n are empirical constants for each soil sample (mg(1-1/n)·L(1/n)/g and dimensionless quantity respectively) and C is the liquid phenol concentration at adsorption equilibrium (mg/L). |
| In order to obtain the constant values, it is required to: 1º Get natural logarithm on both sides of equation 3 – 1 |
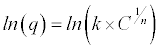 |
| Equation 3 - 2 |
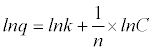 |
| Equation 3 - 3 |
| 2º Get the equivalent linear equation from equation 3 - 3: |
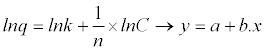 |
| Equation 3 - 4. |
where 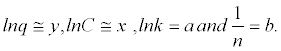 |
| 3º Do the graph from equation 3 - 4 and get the constant values of k and n by intersection values of y versus x for this equation, of the inverse of logarithm and fraction. |
| • Constant adsorption |
| Constant adsorption equation was used to test and show the tendency of phenol adsorption equilibrium in soils. This is represented by the following equation: |
| q=kC |
| Equation 3 - 5 |
| where q is adsorbed phenol at equilibrium (mg/g), k is a constant for each soil sample (L/g) and C is the liquid phenol concentration at adsorption equilibrium (mg/L). |
| If we have that q = y , C = x and k = a in equation 3 - 5, we have : |
| y = ax |
| Equation 3 - 6 |
| In order to obtain the constant value of k it is needed to find the slope of equation 3 - 5 or 3 - 6 as follows: |
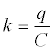 |
| Equation 3 - 7. |
| The easiest way to get this value is to find the slope in a graphical representation of equation 3 - 7 in Cartesian diagram. |
| • Constant separation coefficient |
| Constant separation coefficient equation was used to test and show the tendency of phenol adsorption equilibrium in soils. This is represented by the following equation: |
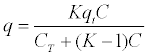 |
| Equation 3 - 8 |
| where q is adsorbed phenol at equilibrium (mg/g), K is a constant of separation coefficient (dimensionless numbers), C is the liquid phenol concentration at adsorption equilibrium (mg/L) and CT is the maximum phenol concentration at equilibrium where there is the maximum adsorption capacity of the soil (mg/L). |
| In order to find the constant values of K and qt, it is needed to: |
| 1º Find experimentally the value of CT. |
| 2º Find the inverse of both sides of equation 3 - 8: |
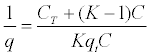 |
| Equation 3 - 9. |
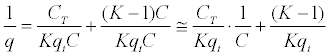 |
| Equation 3-10 |
| 3º Transform equation 3 - 10 to a linear expression: |
If we have that 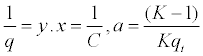 and and 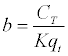 in equation 3 - 9 we have: in equation 3 - 9 we have: |
| y = a + bx |
| Equation 3-11 |
| 4º Do the graph from equation 3 - 11 and get the constant values of K and qt by intersection values. |
| Relative bio-availability and adsorption equilibrium constant |
| • Relative bio-availability |
| In this work the term "bio-availability of phenol" was considered as equal to available phenol at liquid phase in milligrams. The relative bioavailability was determined by the percentage of increment of phenol at liquid phase with respect to an empirical reference point as follows: |
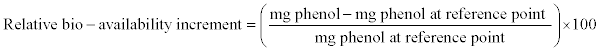 |
| Equation 3 - 12 |
| The term is used from a concept that microorganism consume first the dissolved substrates. This empirical consideration was developed in this work to show the differences between similar tests at different room temperatures. |
| • Adsorption equilibrium constant |
| In this work was selected a k value at equilibrium to express the relation of adsorbed phenol and phenol at liquid phase as follows for temperature tests: |
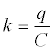 |
| Equation 3 - 13. |
| where q is adsorbed phenol at equilibrium (mg/g) and C is the liquid concentration of phenol at adsorption equilibrium (mg/L). |
| Reynolds number: Reynolds number (NRe) was used to describe the turbulent conditions of reactors during tests. The NRe is expressed according the equation: |
| Equation 3 - 14. |
| in which D is impeller diameter or equivalent (m), N is rotation speed (rad/s), ρ is liquid density (kg/m3) and μ is liquid viscosity (kg/ (m·s)). |
| For calculations, the diameter of the tank, D, was 0.03 m, N was measured in revolutions per minute (rpm) and transformed into radian per second (rad/sec), and ρ and μ were assumed to be 1000 kg/m3 (water density) and 0.00201 kg/(m•s) (water viscosity) at 20ºC, respectively. Also throughout the tank an NRe>10000 was considered as fully turbulent conditions and a value between 10<NRe<10000 was considered as a transition, where there was turbulence between laminar and turbulent [21]. |
| Experimental tests |
| Experimental system was designed in different tests in order to determine the effect of temperature, pH, stirring speed and salts on bio-availability, environmental quality for microbial growth or phenol adsorption. These tests were carried out in slurry reactors with polluted sandy soils. Each test was conducted in triplicate and its results were represented in mean values. |
| Soil samples were placed in 250 mL Erlenmeyer flasks (reactors), in different solid/liquid (w/w) ratios, depending on the test. Initial phenol concentrations were prepared from appropriate dilution of a phenol standard solution (2000 mg/L). The same procedure was followed when nutrient salt solutions were considered. |
| Reactors were placed in an orbital incubator, New Brunswick G.25 model, protected from sunlight. To avoid interferences due to microbial degradation processes, sodium azide 0.02% (w/w) was used as antimicrobial agent. The tests were as follows: |
| pH and salts: Different salt solutions were used to control systems’ pH, their compositions are shown in Table 1. Each solution is described by letter A, B, C, D, E or F. Even though all soils were used for this test, focus was on samples with a pH approximately lower or equal to seven (pH ≤ 7), in this case soils T4, T5 and T6 as it is shown in Table 2. Other experimental conditions were kept constant: temperature equal to 28°C, S/L equal to 0.2 and stirring speed equal to 150 rpm. |
| Slurry reactor |
| • Soil concentration |
| The effect of soil concentration on bio-availability and adsorption of phenol was tested at S/L interval from 0.01 to 0.82 (weigh/weigh relation). During this test other experimental conditions were kept constant: pH equal to 7 ± 0.5, temperature equal to 28ºC and solution E (Table 1). The soil sample for this test was T1. |
| Stirring speed: The effect of stirring speed on bio-availability and adsorption of phenol was tested at the interval 150 ± 50 rpm (the circular motion had as a radius of 3 cm). During this test other experimental conditions were kept constant: pH equal to 7 ± 0.5, S/L equal to 0.2, temperature equal to 28ºC and solution E (Table 1). Soil samples were T1, T2 and T3. |
| Isotherms: The same experimental conditions were used to find the different isotherm equations for soils T1, T2, T3, T4, T5, T6 and T7. Solution F, described in Table 1, was used to carry out this test with initial phenol concentrations between 30 to 300 mg/L. Other experimental conditions were kept constant: temperature equal to 28ºC, stirring speed of 150 rpm and S/L relation equal to 0.2. |
| Temperature: System temperature was evaluated at two different intervals. The first interval corresponded to slight variations, it was around 28 ± 2°C for soils T1, T2, T3, T4, T5, T6 and T7 (Table 2). Its specific experimental conditions were S/L equal to 0.2 and solution F (Table 1); the second one was conducted with greater variations, it was around 27.5 ± 22.5°C for soil sample T1. Its specific experimental conditions were S/L equal to 0.1, solution F (Table 1) and different phenol initial concentrations in an interval from 27 to 134 mg/L. Other experimental conditions were kept constant in both tests: pH equal to 7 ± 0.5 and stirring speed equal to 150 rpm. |
| Salts: The effect of salts (taking into account their nature and concentrations) on the phenol bio-availability and soil adsorption capacity was conducted by study of adsorption equilibrium on soil sample T1. Salts, such as sodium chloride (NaCl), magnesium sulphate (MgSO4), ferric chloride (FeCl3), potassium hydrogen phosphate (K2HPO4), sodium carbonate (NaHCO3), chloride ammonium (NH4Cl), calcium chloride (CaCl3) and potassium sulphate (K2SO4), were used to test these properties [5]. Salt concentrations were 0, 12, 60 and 353 mg/L, and with initial phenol concentrations of between 30 and 300 mg/L. Other experimental conditions were kept constant during this process: temperature equal to 28°C, stirring speed equal to 150 rpm, S/L equal to 0.2, pH equal to 7 ± 0.5 and solution F (Table 1). |
| Results and Discussion |
| In this section a summary is presented of the major results related to the study of bioremediation cases in polluted phenolic soils before microbial activity. It is structured in four main sections: In the first one, the main aspects related to soil characteristics and what these mean in a soil environment are described; in the second one, a description is made of which variables need to be controlled to have equivalent conditions necessary for making a comparative analysis in a bioremediation process; in the third one, a description is made of which isotherm equations and what soil properties could be used to describe soil adsorption equilibrium and; in the fourth one, it is described how the control of experimental variables can increase or decrease phenol affinity for soil adsorption. |
| Soil characteristics |
| Soil properties determine how effective bioremediation processes can be, otherwise without their consideration the processes can be misinterpreted. |
| In this section characteristics of soil samples are described. This is structured in six subsections. In these subsections are described all aspects related to pH, cation exchange capacity, organic matter, electrical conductivity, volumetric density, morphology and particle size and what these mean in a soil environment. |
| Natural soils are heterogeneous systems where bioremediation processes are difficult to understand and optimize in case of accidental leak of phenols. The interrelation of soil properties can enhance or reduce the natural bioremediation of these compounds. A basic soil characterization is required to know why and how to optimize the cleaning process with the lowest price despite the heterogeneity of natural soils [22-25]. However, there is lack of information in this sense when it comes to real contaminated sandy soils and their environments for ex-situ phenol bioremediation processes. |
| The object of this test was to find out the basic soil properties of different samples in order to determine how a bioremediation process could be carried out under natural conditions. Also it was analyzed which factors could be optimized to have a favourable environment for microbial activity in an ex-situ bioremediation process. |
| This test was carried out taking samples of each soil. All samples were homogenized before each analysis. The details are described in section 3.2.1. |
| In Table 2 are listed the main characteristics of pH, cation ion exchange, organic matter, electrical conductivity, volumetric density and particle size of soil samples. |
| pH: pH range of soil samples was between 9.2 and 4.8 approximately as it is shown in Table 2. Under these conditions soils, from number T1 to T7, were classified as strongly alkaline, moderately alkaline, neutral, neutral, moderately acid, very strongly acid and moderately alkaline respectively [6,25,26]. |
| Previous works have shown that soils with a pH between 6 and 8, like soils T3 and T4, have better readily available macronutrients and microbial activity [27,28]. On the one hand, it has been reported that soils with pH values below 6, like soils T5 and T6, have generally low availability of calcium, magnesium, phosphorus, nitrates, molybdemum if they are present in soils. Also it has been reported that at this pH the solubility of metallic ions like aluminum, iron, zinc, copper, manganese and boron is high. It is important to highlight that during this test the natural soil conditions did not have very low pH values, they were never less than pH 3. This is important because at this pH there is a high mobility of very toxic heavy metals like lead, chromium and cadmium if they are present in the system according to previous works [29,30]; on the other hand, it has been reported that soils with pH over 8, like soils T1, T2 and T7, have high availability of calcium, magnesium and phosphorus ions if they are present in soil sample. Soils with these pH values have poor availability of aluminum, iron, zinc, copper, manganese, boron and other heavy metals [31,32]. |
| Additionally about nutrient availability, according to literature soil pH determines micro-organism population. Soils with pH outside of the range 6 to 8, like soil samples T1, T2, T5, T6 and T7, have low favourable conditions for microbial activities because this factor contributes to the availability of macronutrients like nitrogen, phosphorus and sulphur in soil samples [31,32]. |
| Cation exchange capacity: Cation exchange capacity (CEC) values between 5.7 and 28.9 mEq/g approximately of soil samples, as it is shown in Table 2, meant that these soils had the ability to retain cations, some of which are plant and microbial nutrients according to the literature. However, in the same way, soils with very low CEC values, like soils T2, T3 and T5, may need an artificial increment of anions to support their living micro and macro-organisms if it is required. |
| Also, soils with high CEC values, like samples T1, T4, T6 and T7, have more stability against external changes as it has been reported [23,24]. This capacity is related to the potential to retain cation ions and positive charged compounds, which reduces the risk that pollutants in soil move to groundwater. This means that CEC values showed indirectly the order of soil surface electronegativity and it could be related with clay and organic compound concentrations. However, during experimental tests this last consideration was partially true, soils with the highest CEC values, soils T1, T4, T6 and T7, did not have the highest values of clay and organic matter porcentage. The reason for this was, perhaps, the presence of different kinds of these in soil samples [12,33,34]. Moreover, it is not completely clear based on Table 2 data if there was a significative relation of these variables in soil properties. |
| The relation of CEC and pH of soils was complex due to the heterogeneity of soil samples. From a theoretical point of view, soils with the lowest CEC values should have the lowest pH values and visceverse [35]. This was true for soils T3 and T5, these samples with the lowest CEC values of 11.88 and 5.63 mEq/g had the lowest pH values of 6.66 and 5.65 respectively as it is shown in Table 2. This criterion was valid too for soils T1, T4 and T7, where a high CEC value meant a high pH value. Nevertheless there was a soil sample which did not follow this tendency. Soil T6 with the highest CEC value had the lowest pH value of all samples. Also, as in the case of clay and organic matter, it was not entirely clear on the basis of Table 2 data how the correlation of these variables was and their interdependence. Furthermore, it is important to highlight that this is not necessarily explained by these experimental data. |
| Organic matter: The experimental tests showed that most of soil samples had low concentration of organic matter (OM) as it is shown in Table 2, it was less than 0.7% in most of them. Only two soils, T1 and T7, had organic matter above this value, around 3.78 to 2.98% respectively. |
| These results showed that soils T2, T3, T4, T5 and T6 had very poor conditions for plant and microorganism growth according to the literature [36,37]. Also, in the same way, these samples with less than 0.7% of OM did not have very good encouraged granulation, tilth, porosity, bulk density, water infiltration and availability, plasticity, cohesion, adsorption capacity and nutrient souce [38,39]. |
| It has been reported that similar soils, like soils T1 and T7, had relative buffering capacity around its pH value for its OM concentration [36]. These were around the value of strongly alkaline, 9.16, to moderately alkaline, 8.01, respectively for these samples. |
| Additionally, it was not clear that OM was the main factor to determine the CEC value of all soil samples. Only for soils with high OM concentration, soils T1 and T7, this variable had a linear increment, where a high CEC value meant a high OM value. In other cases, specially with soils T2, T4 and T6, with intermedia CEC values, OM concentration did not have a clear relation with it. This was, probably, due to its characteristics, nature or other soil properties. Moreover, the effect of OM concentration over other soil variables in soil samples was also not evident at naked eye to its complex nature [38]. |
| Electrical conductivity: Soil samples had different electrical conductities (EC) in this order: T4>T7>T3>T6>T2>T1>T5 as it is shown in Table 2. This was related to soil disolved components because they carried electrical current in liquid phase under experimental conditions. |
| The relation of EC and pH of soil samples was not clear because soils with high conductivity had neutral alkaline pH values, like soils T4 and T7. Also samples with low EC had high and low pH values, like soils T1 and T5. These factors, maybe, were more related to soil nature and its composition [40]. |
| Soils with high EC had high CEC values like soils T2, T3, T4, T6 and T7. It is important to highlight that it has been reported that soils with high EC have high bioavailability of their nutrients, cations and anions, as disolved salts [41-45]. Nevertheless, in this sense, experimental EC values below 200 μS/cm were a clear indication that there were not enough available nutrients in some soil samples and it could show low organism activity, especially for soils with low EC and low OM at the same time, like soils T2, T3, T4, T5 and T6. |
| Other important variable related with EC was the clay concentration. Soils with the highest EC had the highest clay%, like soils T3, T4 and T7. This is so because the clays were the support of cations that later were mesured in disolution with other anions by EC [46,47]. |
| Volumetric density: The volumetric densities of soil samples were around 0.7 to 1.30 g/cm3 as it is shown in Table 2. The interelation of this variable with pH and CEC was not evident for all soil samples [42]. This factor was more related with sand and silt proportions and their paking arragement [48]. Soil samples with the highest sand and silt porcentage had approximately the highest volumetric density, that was the case of soils T5, T2 and T1. However, this was not a linear correlation in all soil samples due to soil morphology, especially for soils T6, T7, T4 and T3. |
| It has been reported that high soil volumentric densities are related indirectly with poor nutrient and environmental conditions to support micro and macro-organismos in high concentrations [49]. This could be the case of soils T1, T2, T3 and T5 because they had the highest volumetric density values. |
| The analysis of soil samples with these highest volumetric density values and other soil properties can show how the environmental conditions were for these soils for living organisms, and it can be explained as follows: a high volumetric density and a low anion concentration (high CEC and low EC) in soil T1 meant low mineral nutrient availability; a high volumetric density and a low OM concentration in soils T2 and T3 meant low organic nutrient availability; a high volumetric density and low cation and OM concentrations in soil T5 meant low mineral and organic nutrient availability . |
| In the case of soils T1, small black coal particles, 100% organic matter, were observed in soil grains. Without this huge visible amount of organic matter the volumetric density of this soil should have been bigger [38,48]. |
| Additionally, in case of macro-organism growth conditions, it was observed that none of soil samples exceeded the volumetric density for roots and plant growth of 1.40 g/cm3 [50]. |
| Particle size: All soil samples were texture clasified as sandy soils due to their size distribution of particles as it is shown in Table 2. In all cases the percentage of sand was over 71%, silt was under 16.80% and clay was under 18.86%. This means that all soils were part of the same hydraulic group and had a high hydraulic conductivity (HC). The HC for soil samples was determined by the texture triangle and its value was around 10 to 100 μm/s, it meant a low water retention time in these samples [51,52]. |
| • Sand |
| The high proportion of sand, over 90%, in soils T1, T2 and T5, was a direct indicator that these soils had the lowest water retention time and the highest rate of nutrient release of soil samples [52,53]. Also other previous studies have mentioned that these kind of soils, with a high sand%, have a high aeration rate in natural conditions that increase the microbial activity [54]. |
| • Silt and clay |
| The soils with the highest silt and clay proportion were soils T3, T4, T6 and T7 of all soil samples as it is shown in Table 2. According to the literature, these soils had the highest water retention time, stability about release of nutrient elements and retention of soil moisture [52]. Even the activity of silt and clay of these samples was related to kind and amount of these components, the soils with more silt and specially more clay had more surface areas per gram than other soils with more sand%. In the same way, based on literature, these soils were more chemical and physical active in direct relation to its clay% [42,55]. |
| The analysis of soil sample properties showed that these factors had a heterogeneous nature in all cases. Without an artificial experimental homegenization of all soil natural conditions a phenol bioremediation process in all of these samples could have unpredictive results, away from optimal microbial conditions to achieve remediation goals. |
| Previous considerations of experimental conditions |
| Real ex-situ soil bioremediation environments are complex systems that are not easy to understand, for that reason well designed and reproducible tests are required. This, as well as the support of mathematical models, can be the key to understand, predict and try to control as much as possible the reality in these microbial processes. |
| In this section the main previous considerations to have stable and reproducible conditions during the experimental tests are mentioned. This is structured in two main subsections: (1) pH and salts and (2) soil concentration and stirring speed. As it can be deduced by its names in these subsections are described the effect of pH, salts, soil-liquid relation and stirring speed on phenol soil adsorption equilibrium. |
| Given the situation of complex natural soil samples, as it has been shown in previous section, it was requiered to have a set of experimental conditions where a comparative and reproducible analysis could be done with independence of soil sample. |
| The object of this was to find out which pH, salts (type and concentration), solid-liquid relation and stirring speed had to be used to have reproducible and comparative results during the experimental tests. |
| The results presented in this section were obtained from tests carried out in slurry reactors, Erlenmeyer flasks of 250 mL, under different experimental conditions. The details are described in section 3.4. |
| pH and salts: The chemical nature of phenol as a weak acid allows researchers to know the ionic charge of this compound as a function of media pH (RO-, H + and R-OH). The pH of the system is known to have great influence on phenol solubility, availability and adsorption in solid particles for industrial and water processes. This especially has been developed for systems with low phenol concentrations, high organic matter concentrations and homogeneous conditions. However, this ideal behaviour changes when there are complex systems involved like in the case of real soil bioremediation environments. It is still required to know how phenol adsorption works in natural enviroments and set methods to reproduce, control and predict its behaviour [5]. |
| Given the heterogeneous nature of the soil samples, it was necessary to develop a method to adjust pH and salt concentrations of the medium to carry out a comparative analysis under the same experimental conditions. |
| In soil bioremediation processes there is a trend to use pH-ranges and nutrient salts that provide the maximum metabolic activity of the microorganisms in system. Nevertheless, there is a lack of information about its reproducibility under real operation conditions due to the heterogeneity of natural soils. |
| In case of phenols, there are some works, of many, that have proposed as the best pH to soil biodegrade this compound the range between 4 and 10 [5,7,56-58]. However, in these works their experimental conditions are different and heterogeneous between samples, even for the same set of tests. For that reason this kind of studies are hardly comparable and its technique are difficult to applicate for real ex-situ bioremediation processes without doubts. |
| The aim of this test was to develop a method that provides a stable and reproducible environment of pH and salt concentrations to enable carry out a comparison under the same experimental conditions of phenol adsorption in all studied soil samples. |
| Bicarbonate salt has been used to carry out the test of pH control based on the results of Rancaño (2000) for similar conditions. This author, when he studied the bioremediation of complex wastewater systems with phenolic pollutants and suspended particles, noted that it was possible to adjust system pH between 6 and 9 with sodium bicarbonate as a buffer and obtain high microbial activity. |
| Different solutions were prepared and used to regulate the pH, its compositions are shown in Table 1. Even all soils have been used to this test, however this has been focused on samples with pH ≤ 7.5 or more unstable caracteristics about this factor as it is shown in Table 2 and Figure 1. Furthermore, other experimental conditions were kept constant. More information about how this test was carried out can be consulted in section 3.4.1. |
| • Soil pH |
| Naturally, the system pH tended to stabilize at around natural pH of studied soil sample. A summary of this information is represented together in Figure 1 for all soil samples. |
| A comparative analyisis of pH results showed small differences between media pH (Figure 1, for solution A, Table 1) and natural pH (Table 2) for each soil. These were due to small differences in the analytical techniques and experimental conditions. During the evaluation of this test the soil samples were suspended in more water than in the case of the determination of soil pH as it is shown in the methodology section. However, these differences in absolute values were less than 0.7 pH units in the worst case, for soil T6, and less than 0.02 pH units in the best case, for soil T7. This meant that it was possible to consider the final operation pH as approximately equal to the natural soil pH in each case. The final pH value in the system under this operation condition for each sample were: T1 equal to 8.69, T2 equal to 8.38, T3 equal to 7.23, T4 equal to 7.25, T5 equal to 5.49, T6 equal to 4.1 and T7 equal to 8.01 approximately. |
| Also, during this test it was observed that soil medias with more unstable pH conditions were soils T4 and T6. However, these differences were not significative under tested conditions after 75 hours. |
| • Change of pH by salt addition |
| To have different pH values in soil samples meant that phenol adsorption reached its adsorption equilibrium in each case under different environmental conditions. To reduce the uncertainty and doubts about how comparable the results of this work were, all pH values of systems were corrected by the addition of different salts. In this section is described how this process was carried out. |
| In Figure 2, letter A, it is represented together media pH of soils T4, T5 and T6 under natural conditions as it is shown in Table 1, solution A. In mean terms the pH value of these samples was 5.61 ± 1.59, 1.89 pH units less, in absolute value, from the ideal 7.5 pH required as a mean value developed by Rancaño [59]. |
| The fist step was to increase the bicarbonate salt concentration to 500 mg/L in systems as it is described in table 1, solution B. This increment in all cases allowed increasing the pH and stabilized its value in the required pH interval of 6 to 9 after only 12 hours. Nevertheless, for the media of soil T6, the pH stabilization was not reached even after 72 hours under this condition. Despite this result, the mean pH value in all studied systems after this test was 7.9 ± 1.56. In Figure 2, letters A and B, it can be seen how the pH of media under natural conditions (represented by letter A) changed to the new pH values due to the addition of bicarbonate salt. (represented by letter B). |
| In order to have media pH of soil T6 around the required pH interval, it was required to increase the bicarbonate salt to 600 mg/L as it is shown in Table 1, solution C. As it can be seen in Figure 2, letter C, the increment bicarbonate salt to this concentration allowed reaching the required pH interval for all samples in less time, especially for soil T6. This pH value was around 8.36 ± 0.45. The pH equilibrium for T4 and T5 was reached after just two hours, ten hours less than the previous test. In case of media of soil T6, this pH equilibrium was reached after just 24 hours, 48 hours less than the previous test. An important factor to highlight in this point is that this salt concentration allowed sustaining the pH equilibrium without significant variation independent of soil nature. This last interesting conclusion was the reason for choosing 600 mg/L as an ideal bicarbonate concentration for subsequent tests. |
| When the effect of other salts in the system was evaluated (solution D, Table 1), like FeCl3, K2HPO4, NaHCO3, NH4Cl, K2SO4 and CaCl3 [60-64], an imbalance in the pH values of the systems was observed again as it can be appreciated in Figure 2, letter D. This imbalance of pH was more significant for soil T6 media, with a difference in absolute value of 3.01 units of pH. The new mean pH value in all systems after this test was 7.02 ± 1.93. Even the difference in pH values of other systems was small with respect to previous test, in absolute values of pH units of 0.73 for T4 media and 0.23 for T5 media, it was, therefore, required to find a better method to control the pH. The reason for this was that the presence of new salts in the media changed its properties in an unpredictable way with respect to pH due to the heterogeneous nature of soil samples. Again, the setback was on how to compare different medias with different experimental conditions. |
| Given the difficulty in controlling the pH of the systems, and after a lot of tests, about how to have a stable pH in all cases under operation conditions, it was found that a stable condition could be reached by using HCl and NaOH as a pH regulator and salts as a buffer. The composition of this salt buffer solution is described in Table 1, solution E. As it can be seen in Figure 2, letter E, the pH was stable and similar in all soil media independently of their nature. The mean value of pH for all soil samples after this test was 7.19 ± 0.07. |
| Other works have reported the possibility of using HCl and NaOH, and buffer phosphate as solutions to control the pH but none of them has considered bicarbonate salts as a pH buffer in the presence of other nutrient salts [6,10,63,64]. |
| The new developed experimental condition, regarding the control pH of the media, with reference to solution E of Table 1, allowed achieving two important things: similar operating condition for pH and salt concentrations in all cases independently of their nature and the possibility to compare all the results. This with the idea to have robust conclusions in this work. |
| Soil concentration and stirring speed: Stirring factor determines the relation between soils, solvents, microorganisms, nutrients and pollutants in ex-situ soil bioremediation studies. The start point is to know how the operation conditions influence and change the soil adsorption equilibrium to avoid any misinterpretation of initial conditions in the global process [5]. |
| • Soil concentration |
| Different soil concentrations were tested to analyze the influence of this factor on final phenol soil adsorption equilibrium in sandy soil samples. |
| There is a trend in soil ex-situ bioremediation to use indistinctly soil concentrations without any consideration of this factor [9,14,65] and in many cases it is still unclear how the optimization of this variable can improve the process goals. Even when this factor is considered in these processes the effect of this variable on soil adsorption properties are poorly described in real cases for sandy soils [30,66,67]. |
| These tests were carried out by putting different solid/liquid (S/L, relation weight/weight) relations under the same experimental conditions to determine phenol adsorption kinetics and equilibrium point as it is shown in section 3.4.2.1. The results of these showed that it is possible to reach the adsorption equilibrium after 6 hours independently of the S/L relation in the system (the results are not shown). |
| Even when the equilibrium was reached almost at the same time in all tests, small differences were observed about phenol adsorption equilibrium for each S/L relation. Only for a small interval, from 0.01 to 0.11 S/L relations (0.01 ≤ S/L ≤ 0.11), was possible to have approximately ideal suspension conditions for particles, this being without significant interference between them for adsorption factors. Even for cases with S/L equal to 0.2 approximately (S/L ≈ 0.2) these differences were reasonably negligible for this heterogenic case. They were around 20 mg/L in mean terms. The same tendency has been reported by Fukui et al. [68] in tests with different S/L relations. This means that for any point under similar conditions, the equilibrium is constant independent of the S/L relation. |
| Also during these tests it was observed that soils formed conglomerate structures in the centre of flasks almost immediately after starting the tests (less than 1 hour). The high density of particles was the main reason, even when the stirring speed and turbulence of the media were high, where the Reynolds number was equal to 13860 (NRe=13860) [21]. This effect was more significant when the soilliquid relation was over 0.2 (S/L>0.2). Likewise, this soil configuration provided different experimental conditions during the adsorption process in the same reactor; there were particles in suspension, semisuspension or without-suspension at the same time. This structure can be described as a conglomerate in the bottom of flasks. This factor could be the reason why no proportional phenol soil adsorption equilibriums were reached under the same experimental conditions for bigger S/L. As it has been reported, this phenomenon is related with mass transfer rate and interfacial contact areas [69]. |
| After the analysis of these results, it was clear that the better S/L relation was around 0.01 to 0.2 for the purpose of this work (0.01 ≤ S/L ≤ 0.2). This allowed getting the most stable conditions and the highest soil concentration during phenol soil adsorption. Further, this analysis showed that the mechanism of adsorption equilibrium over 0.2 S/L relations (S/L>0.2) was unclear, because it is possible that the S/L factor can have other unconsidered and unexpected influences over other variables in the system for a bioremediation process. For that reason it is needed to improve the knowledge in this line with other works with specific tests [5,70-72]. |
| • Stirring speed |
| In this section the results of the effect of stirring speed on phenol adsorption equilibrium in different sandy soil samples are presented. |
| Previous works have shown that stirring speed in reactors can determine the behaviour of soil adsorption equilibrium in solid/ liquid suspensions for different organic compounds. In this sense, to maximize the diffusion and mass transport relation for complex soil bioremediation systems is determinant because this determines the interfacial area available for mass exchange between solute - solvent - adsorbent, which in this study are phenol - water - sandy soil respectively. In this relation, the result reproducibility was the key factor to find. Together with this, the other goal was to get the optimal operation conditions [5]. |
| This test was carried out with three different soil samples with the same S/L relation for stirring speeds of 100, 150 and 200 rpm. This and other considerations for this test are shown in section 3.4.2.2. The other experimental conditions were kept constant. |
| This test showed, for soils T1, T2 and T3, how the increment of stirring speed, from 100 to 200 rpm, did not alter significantly the phenol equilibrium point independently of soil sample (the results are not shown). This meant that the tested S/L relation of 0.2 had maximum soil concentration without significant effects on equilibrium as it was noted during soil concentration tests and described in the previous section. The empirical reason was that even there were settled particles in the vessel bottom, these particles still had free movement and better mass transfer rate around this reactor part without significant soil layers over them. For similar conditions, where all soil particles are in a suspension state, or equivalent, similar results have been found [70,73,74]. |
| However, when other stirring speeds for cases with S/L over 0.2 value (S/L>0.2) were evaluated, it was not possible to have reproducible results for all soil samples independently of their nature. The main reason was that the conglomerates in the vessel bottoms formed heterogenic structures difficult to replicate even for the same experimental conditions as it was mentioned in the previous section. Also other important factor for this was that the lab mixer had a circular movement and this did not allow splitting these soil conglomerates for high soil concentrations. |
| At this point, during experimental tests, it was clear that soils with density (ρ) between 1.10 and 1.30 g/cm3 (1.1 ≤ ρ ≤ 1.30), for soils T1, T2 and T3 respectively (Table 2), could be fully mixed in reactors at 150 rpm for S/L equal to or below 0.2 (S/L ≤ 0.2). Furthermore, it was clear that soils with similar or less density (ρ ≤ 1.30), with equal or less heavy particles, could be fully mixed at this stirring speed as well (soils T4, T5, T6 and T7. Table 2). For other cases, with S/L relations over 0.2 (S/L >0.2), more specific tests are needed with other alternative soilliquid mixer devices. The information available in the bibliography about solid mixing techniques [69,71] and devices [21] can be useful for this purpose. |
| Equilibrium |
| In ex-situ bioremediation processes, it is necessary to know how the pollutants are distributed around phases to find the best experimental conditions to achieve process goals. Without this consideration, it is not possible to predict the availability of pollutants in the liquid phase and know if the microorganisms can degrade them easily under a set of experimental conditions. |
| This section of equilibrium is structured in two subsections. They are related with results of isotherm equations and soil properties related with phenol adsorption equilibrium. |
| The key factor, before microbial activity in a bioremediation process, is to predict the adsorption behaviour and understand its properties, in the equilibrium solvent-solute-adsorbent, to implement all required actions to quickly recover the polluted soil. There are a lot of studies about how to predict adsorption behaviour with different isotherm equations and about which soil factors are more important in this process in ideal solid-liquid conditions. However, natural soil bioremediation cases with high pollutant concentrations are poorly evaluated. |
| The object of these tests was to find a universal isotherm equation to predict the adsorption behaviour of high phenol concentrations in natural sandy soils and find which soil properties can influence this adsorption equilibrium under real bioremediation conditions before microbial activity. |
| sotherms: Equations used to describe the adsorption isotherms in solid-liquid systems are derived from the models developed for solidgas systems [17]. Despite this, excellent results have been obtained to represent real cases of this kind in low concentration or in conditions where the nature of the adsorbent is homogeneous. The challenge here is to find equivalent equations for heterogeneous conditions in natural environments like those that are easily to find in soil bioremediation processes. |
| In this section, results of equation validations for phenol soil adsorption isotherms during experimental phase are shown. |
| Even adsorption isotherm equations that come from solid-gas systems, as it was mentioned above, there are many of them that are especially improved for ion exchange studies in solid-liquid systems [18-20]. More studies related to the adsorption equilibrium of contaminants in natural soil systems have been done for years. Most of them are specialized in adsorption equilibriums where the pollutants and soils are related in ideal conditions with low pollutant concentrations and high soil adsorption capacities, such as activated carbon, polymeric resins or pure minerals [20,75-80]. However, heterogeneous soil adsorption processes with high loadings of pollutants have been poorly evaluated due to its complexity [81-83]. |
| The object of these tests was to find an equation that it could be used to describe phenol soil adsorption isotherms independently of sandy soil nature in an ex-situ bioremediation process before microbial activity. |
| In order to carry out these tests, different soil samples were settled in different reactors under the same experimental conditions. This allowed us to do a real comparative analysis of the efficiency of tested equations. The specific details of this are described in section 3.4.3. of methodology section. In order to fit the equations to experimental data a graphical representation method was used as it is shown in section 3.3.1. |
| The correlation order for phenol adsorption isotherms was different for each soil sample and each tested equation. The order of accuracy between the experimental data and calculated data was bigger in the case of the Freundlich equation in relation to others. The fit of the experimental data to tested equations was as follows, in the order of accuracy from the highest to the lowest: Freundlich (r2=0.95)>constant separation factor (r2=0.91)>linear adsorption model (r2=0.21). |
| The small variation between Freundlich and separation constant isotherm, represented by correlation factor r2 of 0.95 and 0.91 respectively, showed that both equations can be used to represent the phenol adsorption isotherms on sandy soils. However, the order of accuracy and the less complexity of Freundlich model made this model more suitable for representing these experimental data. |
| It is interesting to note that equations whose theoretical considerations assume multilayer adsorption were those which obtained the highest correlation coefficients [17]. |
| These adsorption isotherms and experimental data are shown in Figure 3. |
| This figure shows how certain soils, such as soils T1, T3, T6 and T7, possessed a greater adsorption affinity for phenol molecules than others, such as soils T2, T4 and T5. Freundlich isotherms, represented by continuous lines, helped to distinguish this tendency due to the fact that they correctly fit to experimental data regardless of media heterogeneous nature. |
| Despite the good representation of the experimental data, Freundlich equation did not always show a correlation coefficient over 0.95 (r2>0.95), this was the case of soils T2 (r2=0.90), T4 (r2=0.95), T5 (r2=0.89) and T6 (r2=0.93). This meant that other soil factors also contributed to determine soil adsorption properties, and this phenomenon was not the only one influenced by the multilayer adsorption. |
| Other similar studies have shown, for systems such as those described here, that it is not possible in all cases to have greater grade of precision due to soil heterogeneous factors [84,85]. |
| After this test and result, it was clear that Freundlich equation could be used to represent all cases of phenol soil adsorption in studied sandy soils. Additionally, there were empirical evidences that other soil factors influenced phenol adsorption equilibrium for some soils. For that reason additional analyses were needed to clarify this issue. This analysis is presented in the following section. |
| Soil properties: Standard protocols for ex-situ soil bioremediation processes are needed in order to achieve the process goals independently of soil nature and environmental conditions. As it was mentioned, the soil properties and their control can be key factor to enhance availability of pollutants in medium if it is analyzed correctly in soil adsorption process. |
| In this section, a comparative analysis of soil properties and how these influenced the phenol soil adsorption process for studied soil samples is described. |
| In ex-situ soil bioremediation processes the pollutant distribution in the soil determines the guidelines to follow in the cleaning of these systems. Most studies which are related to this issue are focused on groundwater contamination, where contamination levels are generally low [10,80,82,83,86]. In these and other similar studies, the highest correlation coefficients (r2) of adsorption processes generally correspond to the tests conducted in systems with a high concentration of organic matter. Other factors prevailing in this connection is the percentage of clay and cation exchange capacity [25,75,87-90]. However, it is not completely clear how these factors influence adsorption conditions with heterogeneous natural soils in high phenol concentrations. |
| The object of this analysis was to find which soil properties could influence the adsorption equilibrium under real bioremediation conditions before microbial activity. |
| The experimental data used to carry out this analysis were taken from the test described in the previous section (5.3.1) All details of experimental conditions and related information are described there. |
| The experimental results showed that the assumption that soils with the highest proportion of organic matter (OM), clay% and cation exchange capacity (CEC) have the highest adsorption properties is not always true. |
| In case of soil OM, as it is shown in Table 2, from a theoretical point of view, soils T1, T4, T6 and T7 should have had the highest phenol adsorption properties because they had the highest OM concentrations, from 3.78 to 0.53%. Based on this criterion the order of preference of phenol for these soils should have been: soil T1>soil T7>soil T6>soil T4. However, as it can be seen in Figure 3, this was partially true because these soils were in the top 5 with the highest preference for phenol adsorption, but a soil with much less OM like soil T3, with just OM of 0.11%, had a bigger affinity for phenol than soils T4, T6 and T7. The reason of this could be the organic matter nature of each soil. In the case of soil T3, maybe its OM had more affinity for phenol molecules than the others. Also, the reason of this behaviour could be the links and high affinity between phenol molecules and other components in this soil [17]. |
| In the case of soil clay concentration, as it was shown in table 2, from a theoretical point of view, the soils that should have had a bigger affinity for phenol molecules should have been, in this order, soil T3>soil T4>soil T7>soil T1. The experimental results showed, as it can be seen in Figure 3, that the order of affinity for phenol molecules of these soils was: soil T1>soil T3>soil T7>soil T4. Even when it was clear in these soils that the highest clay concentration did not mean the highest phenol affinity, there was clear indication that the soil affinities for phenol molecules were related with the combination of different soil properties. However this was not evident at naked eye, especially for soils T2, T5 and T6. Only for soils with high clay concentration and at the same time high OM concentration, like soils T1, T3 and T7, this relation was evident. However, it was not clear how these factors were related. A possible explanation for this is that OM in clay% could be the main reason for this tendency but more tests are required to prove this theory [88]. |
| In the case of soil CEC, as it was shown in Table 2, like in previous two analysis of OM% and Clay%, soils with the highest CEC, like soils T6, T4, T7 and T1, in this order, did not follow the theoretical criterion, they did not have the highest phenol affinity. The affinity order of these soils for phenol molecules was: soil T1>soil T7>soil T6>soil T4. The reason for this could be that the interrelation of soil properties, like OM%, clay% and CEC, determined the soil affinities for phenol molecules. However, this hypothesis could not allow finding in what proportion these variables were related. This was more evident for soil T4, with one of the highest CEC values. This soil did not have special preference for phenol molecules even when it has had a relative high OM% and clay%. |
| The tendency of experimental data relating to favourable properties that determined soil adsorption properties was tested by mathematical models to describe this process (Freundlich and linear equations). These correlations, which are not shown in this work, about OM%, clay% and CEC did not achieve greater precision settings. This meant that not only these soil properties on their own determined the observed tendencies in soil phenol adsorption isotherms. |
| When it was analyzed other soils properties in this sense, like pH, electrical conductivity, volumetric density and other particle sizes, that are not shown in this work, it was not possible to find out evident and direct relation between soil properties and adsorption capacities for studied soils. More specific analysis and tests are needed to find out how these variables are related [47,91-93]. |
| Different authors have reported that phenomenon like one observed in this work tends to occur in soil heterogeneous systems where the concentration of organic matter is less than 5% [22,40,81,83,86,90,94]. |
| Several authors, such as Schaffer et al. [93], Hu et al. [94], Allen et al. [95], Deitsch et al. [96], Ho and Mckay [97], agree that it is not possible to presuppose a high adsorption of a pollutant without knowledge of the major components of a soil, or direct adsorption test as like it has been done in this work. This means that the low concentration of soil organic matter, coupled with the high concentration of phenol and other soil properties were the reasons why a clear deviation was observed from the theoretical considerations in phenol soil adsorptions for studied soil samples. More specific tests have to be carried out in this sense if a method to predict phenol soil adsorption in sandy soils is required without a direct adsorption test. |
| Variable control and phenol bio-availability |
| In ex-situ soil bioremediation processes a common practice is to change environmental temperature and nutrient salt concentrations to increase the microorganism activity. However most of the time the effect of these variables is underestimated where it comes to soil adsorption equilibrium. The optimization of these factors can improve significantly the bio-availability of pollutants for microbial remediation. |
| This section is structured in two subsections. These are related with temperature and salt concentrations and their effect on phenol soil adsorption equilibrium in a bioremediation process before microbial activity. |
| Temperature: The effect of temperature on soil adsorption capacities of pure compounds with high content of organic matter has been widely described in literature. Presently, due to the great amount of polluted soils around the world, it is the time to know its effects on real polluted soil environments. |
| In this section the results of the effect of temperature on adsorption and bio-availability of phenol in sandy soil samples are presented. |
| In many phenol bioremediation processes it is common to see changes in temperature that enhance microbial activity. However, the effect of this variable on soil adsorption properties is unconsidered during this process in many cases [98,99]. Little is known about the effect of temperature on the adsorption equilibrium of phenol in natural sandy soils under high phenol concentration cases. |
| The object of these tests was to find how temperature could influence the adsorption and bio-availability of phenol during a process of ex-situ soil bioremediation in sandy soils before microbial activity. |
| Two different intervals of temperature were tested in this sense, one around 28 ± 2ºC and another one around 27.5 ± 22.5ºC. The other experimental conditions were kept constant and are described in section 4.4.4. The theoretical isotherms were represented by Freundlich equation. |
| The experimental data in the first set of tests showed, represented in Figure 4A, that small changes in system temperature, around 28 ± 2°C, caused slight differences in soil adsorption capacity and bio-availability of phenol for each soil sample. In the case of soil adsorption capacities (columns), these data showed that for four soil samples, T1, T2, T3 and T5, an increment in system temperature meant an increment in phenol adsorption with respect to the equilibrium. However, the opposite behaviour was observed for soils T6 and T7, where an increment system temperature meant a decrement of phenol adsorption with respect to the equilibrium. For soil sample T4, the change of system temperature in this interval did not affect significantly its adsorption capacity, its variation has been less than 5%; in case of phenol relative bio-availability with respect to the phenol equilibrium concentration (lines), it was noted that small differences between equilibrium conditions in that range of temperature were around ± 2.5% for each case in mean terms, as it can be seen in this figure. It meant that almost all equilibrium conditions were the same in that interval of temperature. The average standard deviation (SD) of concentration for each soil in this range of temperature was: T1SD= ±4.19; T2SD= ±2.45; T3SD= ±2.49; T4SD= ±0.94; T5SD= ±2.05; T6SD= ±1.64; T7SD= ±5.35. And for all soil samples together under these conditions it was ± 2.72 mg/L at equilibrium concentration. These small differences did not allow developing the real nature of this phenomenon. For this reason it was necessary to carry out more specific tests for this work. |
| The second set of tests about the effect of temperature on phenol adsorption capacity in the interval of 27.5 ± 22.5ºC showed, as it can be seen in Figure 4B, that an increment system temperature meant a decrement of phenol adsorption with respect to the equilibrium (columns). Its consequence was that there were more phenol molecules available in liquid phase for soil sample T1 (line). Between temperatures 5 and 1ºC it was noted that an increment in the system temperature meant an increment in soil phenol adsorption with respect to the equilibrium concentration. The reason of this behaviour, that it was the opposite of previous one, was probably due to the interrelation of phenol with some soil components whose concentration in liquid phase it had reduced [100,101]. Additionally, it was observed that reproducibility of results in this interval has had the highest standard deviation and this could be the reason of this behaviour even when triplicate analyses were done. For this reason an extra test on phenol adsorption was carried out. |
| When other experimental conditions to get the phenol adsorption isotherms for this soil in the same interval of temperatures were tested, more evident results were obtained, and they are represented in Figure 5. When the system temperature decreased, from 50 to 15°C, phenol soil adsorption equilibrium was displaced. This change in the system temperature increased the retention of phenolic molecules in the soil. The reason of this could be that at high temperature there was an increment of transport potential and at low temperature there was a decrement of this factor. However, it was not sufficiently clear which were the main interrelation forces between particle active zone, phenol molecules and water with tested experimental conditions [102-104]. |
| Also these data and their analysis, as it is shown in Figure 5, allowed finding out the complex behaviour of phenol soil adsorption at 5ºC. The reason for this could be, as it was mentioned before, the reaction of phenol with some salts in the soil structure. Nevertheless, its nature and composition was undetermined. Other more specific tests have to be done in order to understand this phenomenon. |
| The results of these tests showed that system temperature could enhance the availability of phenol in the liquid phase and reduce the phenol concentration in soil if there is an increment in temperature. Further, these results showed that the characteristics of this equilibrium are determined by the interrelation of phenol-water-soil. Without this information, it is impossible to find out this behaviour without empirical tests in each case. Other additional tests are required to find out what happens with soil adsorption properties in systems with high charge of phenol and heterogeneous soils at low temperatures. |
| Salts: Nutrient salt applications are common practice in soil bioremediation processes because these can enhance the microbial activity and reduce the recovery time. However, in all cases it is not completely clear if these substances can increase the pollutant soil adsorption and reduce its bio-availability. It is still required to know if "the remedy is worse than the disease" in this sense. |
| In this section the results of salts effect on phenol soil adsorption equilibrium in an ex-situ bioremediation process before microbial activity are presented. |
| In ideal conditions, ex-situ soil bioremediation is where microorganisms consume the pollutants and convert them into nonorganic soluble or gaseous compounds inert to the environment and innocuous to human health. During this process, the microorganisms capable of consuming these contaminants quickly increase their number. Critical micro and macro nutrients, or bio-stimulants, are required in this process, like carbon, nitrogen, phosphorus, potassium, sodium. Without these nutrients, the growth and activity of these organisms are impeded, and in some cases pollutant concentrations in the soil system can remain nearly untouched for years. In the case of phenol soil bioremediation there is a lack of information about the effect of bio-stimulants on soil adsorption capacity and phenol bioavailability in ex-situ sandy soil bioremediation processes when the phenol is in high concentrations [10,61,105]. |
| The object of these tests was to find out which were the effects of nutrients, including their kind and amount, on soil adsorption capacity and bio-availability of phenol in polluted sandy soils. |
| The effect of kind and amount of salts on phenol soil adsorption was determined by mixing the soil T1 with FeCl3, K2HPO4, NaHCO3, NH4Cl, CaCl3 y K2SO4 in proportion of 0, 12, 60 and 353 mg/L with a basic solution described in the methodology section (4.4.5) Then, after reaching the phenol adsorption equilibrium, the isotherms were determined by Freundlich equation. |
| These tests and results showed that phenol soil adsorption process in real environments is complex and difficult to predict. The salts, by kind and amount, increased, increased-decreased or decreased the phenol soil adsorption capacity. Also, these results have shown a competitive multilayer adsorption tendency. These results, represented by its isotherms, are shown in Figure 6. |
| The results showed that an increment of K2HPO4 salt around 12 mg/L did not alter significantly the phenol adsorption equilibrium. However, concentrations of this salt, above this value (>12 mg/L), increased considerably the phenol retention in soil matrix and reduced phenol availability in liquid phase as it is shown in Figure 6A; a similar behaviour was observed when NaHCO3 salt was tested in the system. When the concentration of this salt was over 12 mg/L there was an increment in phenol retention in soil matrix and a reduction of its availability in liquid phase as it is shown in Figure 6E, The interesting point to highlight here is that even when the concentration of this salt was around 60 mg/L the phenol retention in soil did not change significantly; in the case of these both salts, K2HPO4 and NaHCO3, even these are different, these prompted approximately the same behaviour in phenol soil retention. The reason was, perhaps, that soil structure changed its surface structure in the same manner and this enhanced the activated centres in the same proportion for phenol retention, and during certain period of time the system acted as a "salt buffer system", without change in phenol availability. |
| Results of the tests with FeCl3 salt in the system showed, represented in Figure 6B, an interesting behaviour. Concentrations of this salt between 60 and 353 mg/L decreased and increased respectively the phenol retention in soil matrix. The reason of this opposite behaviour with the same salt was, probably, the capacity of the soil to resist certain changes in the media without significant change, like a "buffer" of salts. This meant that for certain concentration of this salt, in this case of over 12 mg/L and under 60 mg/L, the phenol retention oscillated around the same value, maybe because this salt built a new unfinished soil surface structure. However, when this salt concentration was exceeded, concentrations of over 60 mg/L, the real effect of this new soil structure came out. In this case, as it can be seen in Figure 6B, for 353 mg/L, the FeCl3 salt increased much more the phenol retention in soil; a similar behaviour was observed when the tests with NH4Cl salt were carried out. In that case the oscillation in the equilibrium and phenol soil retention described before in this paragraph was more evident. These results showed, represent in Figure 6F, that at the first time, when the salt concentration was low, the phenol retention increased, but over a certain concentration, in this case around 60 mg/L, the system condition changed completely and showed a very different result where phenol was attracted strongly to soil matrix with salt concentration of 353 mg/L. |
| In the case of CaCl3 salt, an increment of its concentration meant, represented in Figure 6C, a reduction in phenol soil retention. This was, probably, because salt ions occupied with more affinity the active adsorption sites of soil; a similar behaviour with less intensity was observed with K2SO4 salt. An increment of this salt in the system increased slightly the phenol retention in soil matrix. However in this last case, the oscillation in phenol adsorption equilibrium conditions was minimal. This meant that this salt under operation condition could be considered as an inert for the purposes of this research. |
| In other studies similar trends have been found in relation of soil adsorption properties, some of them have shown increment [101, 106] or decrement [93,107] in soil adsorption capacities related with similar environmental conditions. The increment of salt concentrations change the soil retention capacity, however this relationship depends on characteristics of each system. However its characteristics are not very clear defined for natural and complex soils [93,108]. |
| The phenol adsorption isotherms for different salts under same experimental conditions showed how the soil adsorption properties could be changed for this factor and increase or decrease the phenol concentration in liquid phase and therefore its bioavailability. The effect on phenol adsorption equilibrium was in this order, from the highest to the lowest effect: FeCl3>K2HPO4>NaHCO3>NH4Cl>CaCl3>K2SO4. Also, in general terms, these were classified in those which increased (K2HPO4 and NaHCO3, Figure 6A and E), decreased (CaCl3 and K2SO4, Figure 6C and D) or increased-decreased (FeCl3 and NH4Cl, Figure 6B and F) the adsorption of phenol in soil matrix. This was a result of the interrelation of soil - salts - solvent - phenol molecules and it was based on which action and ionic or molecule structure take the least energy. However due to the complexity of the system, all details of its nature is undefined, maybe related with the electrostatic interrelations, ionic strength and chemical reactions [8,91,93]. Other and more specific tests, with pure soil structures, have to be done in order to find out which energetic properties control this phenomenon. |
| Conclusion |
| The results of this work showed that adsorption equilibrium in natural sandy soils is heterogeneous and complex. For this reason, an artificial homogenization is required in order to optimize and achieve the goals of phenol bioremediation of this kind of soil under whatever experimental conditions. |
| The experimental tests allowed developing a new artificial media, with NaHCO3, NH4Cl, K2HPO4*3H2O, FeCl3, MgSO4, CaCl2 and HCl/ NaOH as components, to achieve similar operation conditions to have a comparative analysis independently of soil nature in phenol bioremediation in sandy soils. |
| The ideal solid liquid relation (S/L) to carry out the soil bioremediation described in this work is less or equal to 0.2 units (S/L ≤ 0.2) for sandy soils with a density less or equal to 1.30 g/cm3 (ρ ≤ 1.30 g/cm3). Tests over these intervals can show undesired results in the optimizations and process goals in bioremediation treatments under these operation conditions. |
| The empirical tests have shown that Freundlich equation can describe all studied cases of phenol sandy soil isotherms with the least grade of uncertainty independently of sample nature. Further, these empirical tests have shown that strong deviation can be obtained in the ideal phenol soil adsorption isotherms if there are high phenol concentrations attached to low organic matter and clay concentrations and heterogeneous sample nature. |
| Likewise, the results of these tests showed that the system temperature can enhance the availability of phenol in the liquid phase and reduce the phenol concentration in soil if there is an increment of temperature and this is related with the nature of soil sample. The empirical tests have shown that the presence of nutrient salts in the medium during the phenol biodegradation process increases or decreases the phenol concentration in liquid phase and, therefore, its bioavailability due to adsorption factors. This is directly related to kind and amount of salts, and soil and system properties. |
| Acknowledgements |
| Gularte F. thanks the economic support of all his family, especially to his grandparents Guadalupe and Francisco, Lorena (mother), brother (Hector), Ronald, and the Spanish Agency of International Cooperation (AECI). Also, this author thanks all his supervisors, colleagues and support staff for their support in this amazing learning experience at the University of Oviedo. Gularte F gives special thanks to Mr Fernando Gonzalez for the web links that he provided for this work. Gularte also thanks all people that he found in his way during this work because in certain ways they indirectly contributed to this work. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
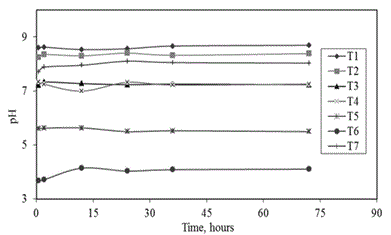 |
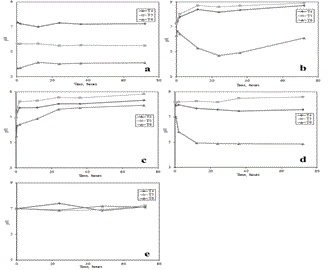 |
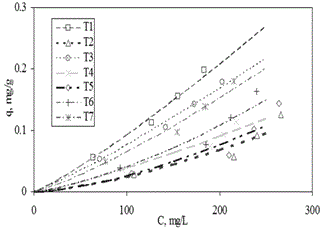 |
| Figure 1 | Figure 2 | Figure 3 |
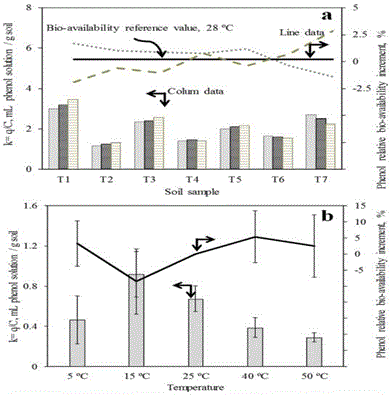 |
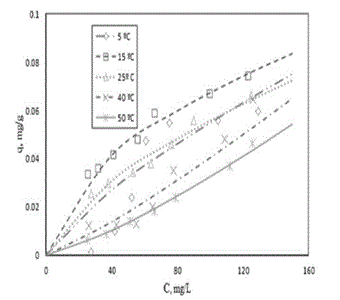 |
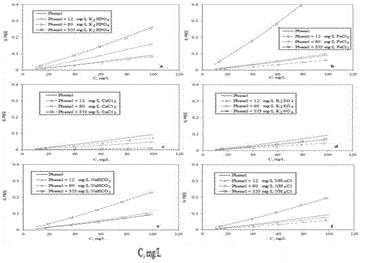 |
| Figure 4 | Figure 5 | Figure 6 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 16688
- [From(publication date):
September-2014 - Aug 29, 2025] - Breakdown by view type
- HTML page views : 12026
- PDF downloads : 4662
