Research Article Open Access
Effect of Xylitol and Fluoride Dentifrices on Human Enamel Surfaces Following Acid-Etching: Scanning Electron Microscopic Study
John Hicks1*, Jun Wu2and Catherine M Flaitz31Departments of Pathology and Immunology, and Pediatrics, Baylor College of Medicine and Texas Children’s Hospital, and Department of Pediatric Dentistry, University of Texas Health Science Center at Houston School of Dentistry, Houston, TX, USA
2 Pediatric Dentistry Private Practice, Bellaire Kids Dental, Houston, TX, USA
3Division of Pediatric Dentistry, College of Dentistry, The Ohio State University, Chief of Division of Dentistry, Nationwide Children’s Hospital, Columbus, OH, USA
- *Corresponding Author:
- Hicks J
Department of Pathology AB1195
Texas Children’s Hospital
6621 Fannin Street AB1195
Houston TX-77030, USA
Tel; 832-824-1869/832-824-2250
Fax: 832-825-1032
E-mail: hicks@bcm.edu
Received Date: November 20, 2016 Accepted Date: December 22, 2016 Published Date: December 30, 2016
Citation: Hicks J, Wu J, Flaitz CM (2016) Effect of Xylitol and Fluoride Dentifrices on Human Enamel Surfaces Following Acid-Etching: Scanning Electron Microscopic Study. Pediatr Dent Care 1: 127.
Copyright: © 2016 Hicks J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Neonatal and Pediatric Medicine
Abstract
Purpose: The purpose of this in vitro pilot study was to evaluate the effects of xylitol and sodium fluoride containing dentifrices on the surface topography of sound human enamel following acid-etching, using scanning electron microscopic (SEM) techniques. Methods: Enamel surfaces from caries-free human molar teeth (n=10) underwent debridement, and dental prophylaxis. Each tooth was sectioned into 5 portions, with each tooth portion assigned to a specific treatment group: 1) acid-etching (35% phosphoric acid gel, 60 seconds); 2) acid-etching followed by synthetic saliva rinsing; 3) acid-etching followed by treatment with Fluoride Free Xylitol Toothpaste (25% xylitol) and synthetic saliva rinsing; 4) acid-etching followed by treatment with Fluoride and Xylitol Toothpaste (31% xylitol, 0.243% sodium fluoride) and synthetic saliva rinsing; and 5) acid-etching followed by treatment with Sodium Fluoride Toothpaste with No Xylitol (0.243% sodium fluoride) and synthetic saliva rinsing. Enamel surfaces from an additional 10 caries-free human teeth served as untreated control teeth that only underwent soft tissue debridement and prophylaxis. Dentifrice treatment was performed for 120 seconds twice daily with synthetic saliva rinsing between dentifrice applications followed by synthetic saliva rinsing at 37°C for 7 days with fresh synthetic saliva replenished on a daily basis. Following the experimental period, the tooth portions were prepared for scanning electron microscopy using standardized techniques. Results: Acid-etching alone created type 1 etching patterns with a finely porous enamel surface. With acidetching followed by synthetic saliva rinsing, the effects of acid-etching were obscured by a finely granular surface coating with fine porosities. With acid-etching followed by xylitol only dentifrice treatment and synthetic saliva rinsing, enamel surfaces showed fine granular coatings with no obvious porosities. Enamel surfaces that were acid-etched then treated with the xylitol with fluoride dentifrice and rinsed with synthetic saliva possessed homogenous surface coatings with areas with finely granular to globular deposits. Acid-etched enamel surfaces that were treated with the fluoride dentifrice without xylitol and rinsed with synthetic saliva had surface coatings with numerous relatively large globoid deposits, morphologically resembling calcium fluoride deposits. Clinical significance: The clinical application of the results from the current in vitro pilot study relate to the procedure of acid-etching teeth for adhesive dental materials. With acid-etching, there is a residual exposed etched enamel surface following sealant, restoration or orthodontic bracket placement. Application of xylitol containing fluoridated dentifrice may facilitate fluoride uptake from the dentifrice and calcium and phosphate uptake from saliva, aid in the elimination of the surface effects of acid-etching, and allow for remineralization of the acid-damaged enamel surface that was not protected by adhesive resin placement.
Keywords
Enamel; Scanning electron microscopy; Xylitol, Fluoride; Acid-etching; Synthetic saliva
Introduction
Dental caries is the most prevalent infectious disease in US children [1]. Over 40% of children under the age of 5 years have dental caries in their primary dentition [2,3]. Dental caries can cause significant pain, lead to odontogenic infections, affect growth and development, and diminish overall quality of life [4-6]. This infectious disease continues to develop in coronal enamel and root surfaces throughout adolescence and into adulthood.
Dental caries is a complex dynamic disease process, involving periods of demineralization and remineralization. Multiple factors contribute to this process, including acidogenic organics S. mutans (Streptococcus mutans, Streptococcus sobrinus, Lactobacillus spp), salivary factors (flow rate, buffering capacity, antimicrobial activity, immunologic factors), and tooth mineral composition (fluoride, hydroxyapatite, fluoridated hydroxyapatite, variety of calciumphosphate mineral phases) [7-12]. To prevent dental caries, the dental profession reinforces effective oral hygiene measures, reducing cariogenic refined carbohydrate ingestion, and home use of fluoridated toothpastes, gels and rinses. In recent years, xylitol containing products, including toothpastes, chewing gums S. mutans and mouthrinses, have been widely promoted for use in caries prevention [13-16].
Xylitol is a non-fermentative sugar alcohol (polyol), which inhibits oral bacterial enzymes involved in glycolysis [17]. Xylitol competes with sucrose for cell wall transporters and other glycolytic metabolic processes. Some strains of take up xylitol and convert it into xylitol-5-phosphate, which accumulates within the bacteria, leading to intracellular vacuolization affecting bacterial viability [18,19]. Xylitol has the ability to inhibit the growth and metabolism of S. mutans, decrease acid and glucan production, and reduce S. mutans and cariogenic bacteria levels in plaque and saliva [20-22]. Additionally, xylitol contains the tridentate ligand (H-C-OH) that can interact with polyvalent cations, such as calcium ions. Xylitol has been reported to be a “calcium ion carrier” that may enhance remineralization by facilitating calcium ion movement into the deeper layers of demineralized enamel [23]. Furthermore, some in vitro studies have reported that xylitol reduces demineralization, enamel erosion and abrasion [24].
Fluoride is well known to be effective in preventing caries and enhancing remineralization of enamel and root surface caries [7- 13,25-31]. Fluoride inhibits bacterial metabolism by rapidly diffusing into bacteria as hydrogen fluoride during periods when plaque pH decreases due to bacterial production of organic acids. Hydrogen fluoride dissociates within the bacterial cell, acidifies the cellular environment, and releases fluoride ions, which interfere with bacterial metabolic enzymes, such as enolase [7]. Enolase is a critical enzyme for bacteria in metabolizing carbohydrates. This inhibition of carbohydrate metabolism results in decreased acid production by the bacteria. Fluoride also inhibits demineralization and facilitates remineralization [12,25-28]. During demineralization, the carbonate-rich and calciumdeficient regions of hydroxyapatite crystals are very susceptible to acid attack. Low levels of fluoride in plaque and saliva act as a “catalyst”, facilitating mineral deposition within carious lesions [25,26]. Fluoride in solution surrounding carbonate-rich hydroxyapatite can be incorporated into HAP crystals to form fluoride-containing HAP (FHAP) and other fluoride containing calcium-phosphate mineral phases, which have substantial resistance to an acidic challenge [12].
The use of professionally applied fluoride treatments (1.23% acidulated phosphate fluoride) or professionally prescribed fluoride pastes (5,000 ppm fluoride) create enamel with increased fluoride content. When high fluoride concentrations in enamel are present during acid dissolution of HAP, calcium fluoride is formed [27,28]. When calcium fluoride is exposed to acid phosphate or phosphate ions during the demineralization process, calcium fluoride and bioavailable phosphates may be hydrolyzed to form FHAP. Calcium fluoride may also act as a reservoir for both calcium and fluoride ions on the tooth surface and within the tooth surface and dental plaque, and release these ions during acidogenic challenges, which further inhibits HAP dissolution and encourages FHAP formation during periods of demineralization. Studies examining the effects of xylitol in combination with fluoride on enamel remineralization have reported inconsistent findings [24,29-36]. Some studies report that: 1) xylitol decreased demineralization (29) and reduced enamel erosion [30]; 2) xylitol enhanced the effect of fluoridated toothpaste on enamel remineralization and decreased enamel erosion and abrasion [24]; and 3) xylitol with sodium fluoride in combination led to significantly and increased remineralization compared with fluoride exposure alone [31,32]. In contrast, other studies report that xylitol alone showed no significant remineralizing effect, and xylitol and fluoride in combination did not produce greater remineralization compared with fluoride treatment alone [32-35]. These conflicting results may be attributed to substantial variations in experimental design (in vitro vs. in vivo), human versus bovine tooth type, xylitol concentration (10% to 70%), fluoride concentration (2 to 1100 ppm), and techniques for assessing demineralization and remineralization (polarized light microscopy, microradiography, quantitative light-induced fluorescence, scanning electron microscopy [SEM]). Well-designed investigative studies are necessary to determine whether xylitol alone or the synergistic effects of xylitol and fluoride treatment affect enamel remineralization and to identify mechanisms of action.
The purpose of this in vitro study was to evaluate the effects of xylitol and sodium fluoride containing dentifrices on the surface topography of sound human enamel following acid-etching, using scanning electron microscopic (SEM) techniques.
Materials and Methods
Many xylitol containing dentifrices are available, but their formulations vary in xylitol concentration, presence or absence of fluoride, and other ingredients. Epic brand dentifrices were selected for this in vitro study (Fluoride Free Xylitol Toothpaste, and Fluoride and Xylitol Toothpaste, Epic Dental, Provo UT 84603), because these dentifrices have well-defined components and xylitol concentrations, with and without the addition of sodium fluoride.
Ten human third molar teeth with macroscopically sound enamel as determined by stereo zoom microscopy, were removed for oral surgical reasons, underwent soft tissue debridement, and dental prophylaxis. Each tooth was sectioned into 5 portions to decrease the effects of tooth to tooth variation with the experimental treatments. Each tooth portion from a single tooth was assigned to a specific treatment group:
1) Acid-etching (35% phosphoric acid gel for 60 seconds (Scotchbond Etchant, 3M ESPE Dental Products, St. Paul MN 55144) followed by air-water spray for 30 seconds to inactivate acid-etching followed by distilled water rinsing for 60 seconds;
2) Acid-etching, as described above, followed by synthetic saliva (20mM NaHCO3, 3mM NaH2 PO4 , 1 mM CaCl2-2H2O) rinsing at 37oC for 7 days with fresh synthetic saliva replenished on a daily basis [37];
3) Acid-etching, as described above, followed by xylitol only dentifrice containing 25% xylitol (Fluoride Free Xylitol Toothpaste, Epic Dental, Provo UT 84603) for 120 seconds twice daily with synthetic saliva rinsing between dentifrice applications, followed by synthetic saliva rinsing at 37oC for 7 days with fresh synthetic saliva replenished on a daily basis;
4) Acid-etching, as described above, followed by xylitol with fluoride dentifrice containing 31% xylitol and 0.243% sodium fluoride (Fluoride and Xylitol Toothpaste, Epic Dental, Provos UT 84603) for 120 seconds twice daily with synthetic saliva rinsing between dentifrice applications, followed by synthetic saliva rinsing at 37°C for 7 days with fresh synthetic saliva replenished on a daily basis; and
5) Acid-etching, as described above, followed by fluoride dentifrice containing 0.243% sodium fluoride and containing no xylitol (Kid’s Crest Cavity Protection Sparkle Fun Toothpaste, Procter and Gamble, Cincinnati OH, 45202) for 120 seconds twice daily with synthetic saliva rinsing between dentifrice application, followed by synthetic saliva rinsing at 37°C for 7 days with fresh synthetic saliva replenished on a daily basis.
The purpose of acid-etching sound enamel was to create a more reactive enamel surface, and also to simulate the clinical situation when acid-etching is performed for placement of an adhesive restoration, sealant or orthodontic bracket in the dental operatory [27,38,39].
Ten additional human extracted 3rd molar teeth with macroscopically sound enamel surfaces, as determined by stereo zoom microscopy, underwent soft tissue debridement and dental prophylaxis to serve as untreated control surfaces. These control surfaces were not acid-etched, not exposed to dentifrices, and not exposed to synthetic saliva.
All tooth portions from the control and experimental groups were critical-point dried, and coated with platinum-palladium for SEM examination (JEOL 6100 SEM, JEOL USA Inc, Peabody, MA 01960, 20 kv, 2000X original magnification). The enamel surface topographic findings among the experimental and the untreated control l groups were compared descriptively.
Results
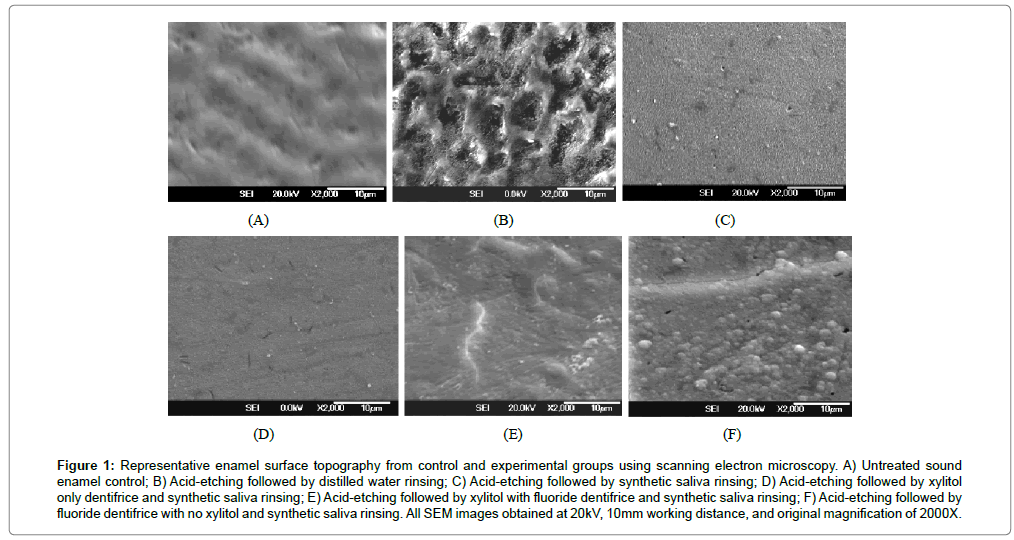
The enamel surfaces from the untreated control group (Figure 1A) demonstrated intact surfaces with typical termination of enamel prisms on the enamel surface with central prism cores appearing as slight depressions and the prism peripheries being slightly elevated. The enamel surfaces from the control group were considered to be intact and without surface coatings or surface debris. Enamel surfaces from the acid-etching only treatment group (Figure 1B) showed loss of the typical intact enamel surface demonstrated with the untreated control group (Figure 1A). Acid-etching alone (Figure 1B) resulted in a type 1 etching pattern with partial loss of the central prism core and retention of the prism periphery. This resulted in accentuated exposed prism architecture and a markedly porous surface. In contrast, enamel surfaces from the group that had undergone acid-etching followed by synthetic saliva rinsing and no exposure to dentifrice (Figure 1C) demonstrated fine granular surface coatings with very fine porosities that obscured the effects of the acid-etching procedure. The porosities with this treatment group were very fine and did not resemble the porosities seen with the enamel surfaces that had undergone acidetching only (Figure 1B). There was no exposure of etched prism cores with this treatment group. Enamel surfaces from the group that had undergone acid-etching followed by the xylitol only dentifrice and synthetic saliva rinsing (Figure 1D) demonstrated surface coatings with a fine granular texture and no obvious porosities. The effects of acid-etching were obscured. Enamel surfaces from the group that had undergone acid-etching followed by xylitol with fluoride dentifrice and synthetic saliva rinsing (Figure 1E) demonstrated relatively homogenous dense surface coatings with focal areas that were partially obscured by granular to somewhat globular fine deposits. The surface coatings obscured the effects of acid-etching. Enamel surfaces from the group that had undergone acid-etching followed by fluoride only dentifrice and synthetic saliva rinsing (Figure 1F) possessed surface coatings with readily identified relatively large globoid deposits of less than 1 micrometer in diameter. There were numerous, somewhat confluent globoid deposits on the surface of the coatings. The morphologic features of these globoid deposits are similar to those described for calcium fluoride deposits. The effects of acid-etching were obscured by these surface coatings (Figures 1B and 1F).
Discussion
Saliva plays a critical role in the prevention and remineralization of enamel and root surface caries [7,10-12]. Saliva contains calcium, phosphate, proteins, immunoglobulins, antibacterial substances, and buffers which can neutralize the acids produced by plaque bacteria, raise the pH, reverse the diffusion gradient of calcium and phosphate during demineralization, and enhance remineralization [7,10-12]. Calcium and phosphate ions can diffuse into subsurface carious lesions, resulting in precipitation of mineral phases on partially demineralized HAP crystals in enamel and dentin leading to increased crystal size, create de novo mineral crystals in demineralized enamel and dentin with HAP crystal loss, and enhance resistance to demineralization [7,10-12]. With the current study, dentifrice application followed by synthetic saliva mimics, to a certain extent, the oral cavity situation in which dentifrices are rapidly intermixed with saliva creating a saliva-dentifrice slurry, which is important in prolonging exposure of dental plaque and tooth surfaces to xylitol and fluoride provided by the dentifrice, and calcium, phosphate and caries protective organic agents provided by saliva. The topographic findings with the treated enamel surfaces in the current study demonstrated that synthetic saliva rinsing after acid-etching (Figure 1C) effectively masked the effects of acid-etching (Figure 1B), and may initiate the “remineralizing” process for acid-damaged sound enamel surfaces. This is important because enamel surfaces overlying white spot lesions (enamel caries) remain intact for considerable periods of time prior to cavitation. White spot lesions typically have a finely porous, but intact enamel surface, and are amenable to remineralization by saliva, and various remineralizing agents in toothpastes, gels and rinses. Of particular interest is the comparison between the acid-etched enamel surfaces that were exposed to xylitol followed by synthetic saliva rinsing (Figure 1D) and those that were acid-etched only followed by synthetic saliva (Figure 1C). With the addition of xylitol dentifrice, there was only a slight different between these groups. The surface coating with xylitol produced a fine degree of granularity and without the fine porosities noted with surface coatings with acid-etching followed by synthetic saliva rinsing. Although acid-etched surfaces exposed to the xylitol with fluoride dentifrice followed by synthetic saliva had dense surface coatings with focal areas with partially obscured fine granular deposits (Figure 1E), the surface coatings lacked the numerous globoid and somewhat confluent deposits noted with the acid-etched surfaces treated with the fluoride dentifrice with no xylitol followed by synthetic saliva (Figure 1F). Only the fluoride dentifrice with no xylitol alone group showed these globoid deposits, that were suggestive of calcium fluoride deposition. This finding may indicate that the xylitol component within the dentifrice may have interfered with deposition of globoid precipitates on the etched enamel surfaces.
Figure 1: Representative enamel surface topography from control and experimental groups using scanning electron microscopy. A) Untreated sound enamel control; B) Acid-etching followed by distilled water rinsing; C) Acid-etching followed by synthetic saliva rinsing; D) Acid-etching followed by xylitol only dentifrice and synthetic saliva rinsing; E) Acid-etching followed by xylitol with fluoride dentifrice and synthetic saliva rinsing; F) Acid-etching followed by fluoride dentifrice with no xylitol and synthetic saliva rinsing. All SEM images obtained at 20kV, 10mm working distance, and original magnification of 2000X.
Calcium fluoride deposition on enamel surfaces serves as a reservoir for long-term release of fluoride to the tooth surface, dental pellicle and plaque [7,11,12,40]. During an acidogenic attack, calcium fluoride deposits on the enamel surface or within plaque release calcium and fluoride ions, which may inhibit HAP dissolution to a certain extent, and encourage FHAP and fluoridated calcium-phosphate mineral phase formation by reaction with acid phosphate or phosphate ions [7,11,12,40].
Xylitol is a 5-carbon sugar alcohol (polyol) used in food, pharmaceutical, and oral health products in more than 35 countries [41]. It is found naturally in various trees, fruits, and vegetables. The Food and Drug Administration has approved xylitol as a dietary food additive since 1963 [41]. From a dental standpoint, xylitol is an interesting natural product that is not fermented by dental plaque bacteria. Xylitol primarily acts on cariogenic bacteria in the oral environment, and has certain protective benefits [31,38,40-44]. There are several clinical studies evaluating the effectiveness of xylitol on prevention of dental caries [31,38,41-49]. Children who consumed xylitol for 3 weeks or more had both short and long term reductions in salivary and plaque levels of S. mutans [44,45]. Clinical studies also have demonstrated decreases in caries prevalence and increment, and delayed caries onset among children who were exposed to daily xylitol use for 12 to 40 months [45,46]. There is also evidence that maternal consumption of xylitol can reduce acquisition of S. mutans and dental caries in young children [47,48]. It is well known that maternal transmission of cariogenic bacteria is associated with oral colonization and development of caries in young children. Avoiding colonization of the oral cavity by S. mutans and other cariogenic bacteria in early childhood has been shown to correlate with decreased caries incidence and decreased early childhood caries [41-49].
The addition of xylitol to fluoridated dentifrices may provide additional caries preventive benefits [13,24,31,32,40,50,51]. In the oral cavity, xylitol has an inhibiting effect on cariogenic bacteria in plaque and leads to a reduction in bacterial acid production. Studies have shown anticaries properties of fluoride dentifrices containing xylitol, as well as lower S. mutans levels in plaque and saliva after 6 months use of xylitol containing dentifrices [50]. With xylitol usage, the pH of cariogenic plaque has been shown to increase above the critical pH (pH 5.2) at which enamel dissolution occurs, even when plaque bacteria have access to fermentable carbohydrates [17,18,20,22]. Furthermore, xylitol can induce remineralization of the deeper layers of demineralized enamel by facilitating calcium ion movement and bioavailability [23].
A xylitol and fluoride containing dentifrice has shown lower glucose retention in the oral cavity compared to a non-xylitol containing dentifrice [51]. In a clinical study, a toothpaste containing 0.243% sodium fluoride and 10% xylitol was shown to significantly reduced caries during a 3-year period [13]. In a 30-month clinical study using a 0.836% (1,100 ppm) sodium monofluorophosphate and 10% xylitol dentifrice, caries was significantly reduced when compared to a similar fluoride dentifrice without xylitol [52]. In another clinical study, the combination of 500 ppm sodium fluoride and 5% xylitol in toothpaste was shown to significantly enhance remineralization [40].
The clinical application of the results from the current study relate to the procedure of acid-etching teeth prior to adhesive sealant, restoration or orthodontic bracket placement. With acid-etching, there is residual exposed etched enamel surfaces following sealant, restoration or orthodontic bracket placement. Application of a xylitol containing fluoridated dentifrice may facilitate fluoride uptake from the dentifrice, and calcium and phosphate ion uptake from saliva, aid in the elimination of the surface effects of acid-etching, and allow for remineralization of the acid-damaged enamel surface that was not protected by adhesive resin placement. The addition of xylitol to a fluoridated dentifrice would provide additional anticaries effects on acidogenic bacteria in the plaque, and potentially act as a calcium ion carrier to facilitate remineralization of white spot lesions or clinically undetectable lesions in the presence of fluoride released from the dentifrice. Unfortunately, this in vitro study could not determine the anticaries effect of xylitol on cariogenic bacteria. It would be expected in the oral cavity that the addition of xylitol to a fluoridated dentifrice would further enhance the caries protective effect of fluoride containing toothpaste alone by adversely affecting cariogenic bacteria.
Conclusions
Acid-etching of enamel surfaces created porous enamel surfaces that may provide a more reactive surface for interaction with caries preventive agents. From the results of this in vitro pilot study, the dentifrices containing xylitol alone, xylitol with fluoride, and fluoride alone with no xylitol created surface coatings following synthetic saliva rinsing that may protect acid-etched enamel surfaces from cariogenic challenges, and initiate the remineralization process for etched enamel surfaces that are left exposed after placement of adhesive resin sealants, restorations or orthodontic brackets. Dentifrice with fluoride alone with no xylitol appears to form surface coatings with globoid deposits resembling calcium fluoride, and may provide additional caries protective effects. Further studies, including an acidic challenge of enamel surfaces treated with xylitol containing dentifrices with and without fluoride using artificial caries techniques and analyses of plaque glycolysis need to be conducted to elucidate the role of dentifrices with and without xylitol on the dynamic demineralization and remineralization process in caries development and reversal.
References
- US Dept of Health and Human Services (2000) Oral health in America: A report of the Surgeon General. Rockville, MD: US Dept of Health and Human Services, National Institute of Dental and Craniofacial Research, National Institutes of Health.
- Pierce KM, Rozier RG, Vann WF Jr (2002) Accuracy of pediatric primary care providers’ screening and referral for early childhood caries. Pediatrics 109: 82.
- Dye BA, Tan S, Smith V (2007) Trends in oral health status: United States, 1988-1994 and 1999-2004. National Center for Health Statistics. Vital Health Stat 11: 248.
- Low W, Tan S, Schwartz S (1999) The effect of severe caries on the quality of life in young children. Pediatr Dent 21: 325-326.
- Cunnion DT, Spiro A III, Jones JA (2010) Pediatric oral health-related quality of life improvement after treatment of early childhood caries: A prospective multi-site study. J Dent Child 77: 4-11.
- Sheller B, Churchill SS, Williams BJ, Davidson B (2009) Body mass index of children with severe early childhood caries. Pediatr Dent 31: 216-221.
- 7.Featherstone JDB (2000) The science and practice of caries prevention. J Am Dent Assoc 131-887-899.
- Featherstone JDB (2000) The science and practice of caries prevention. J Am Dent Assoc 131-887-899.
- Featherstone JDB (1999) Prevention and reversal of dental caries: role of low level fluoride. Community Dent Oral Epidemiol 27: 31-40.
- Featherstone JDB (2003) The caries balance: contributing factors and early detection. J Calif Dent Assoc 31: 129-133.
- Hicks J, Garcia-Godoy, Flaitz C (2003) Biological factors in dental caries: role of saliva and dental plaque in the dynamic process of demineralization and remineralization (part 1). J Clin Pediatr Dent 28: 47-52.
- Hicks J, Garcia-Godoy, Flaitz C (2004) Biological factors in dental caries: role of enamel structure and the caries process in the dynamic process of demineralization and remineralization (part 2). J Clin Pediatr Dent 28: 119-124.
- Hicks J, Garcia-Godoy, Flaitz C (2004) Biological factors in dental caries: role of remineralization and fluoride in the dynamic process of demineralization and remineralization (part 3). J Clin Pediatr Dent:28: 203-214.
- Sintes JL, Escalante C, Stewart B, McCool JJ, Garcia L, Volpe AR, Triol C (1995) Enhanced anticaries efficacy of a 0.243% sodium fluoride/10% xylitol/silica dentifrice: 3-year clinical results. Am J Dent 8: 231-235.
- Tanzer JM (1995) Xylitol chewing gum and dental caries. Int Dent J 45: 65-76.
- Suda R, Suzuki T, Takiguchi R, Egawa K, Sano T, Hasegawa K (2006) The effect of adding calcium lactate to xylitol chewing gum on remineralization of enamel lesions. Caries Res 40: 43-6.
- Lingstrom P, Lundgren F, Birkhed D, Takazoe I, Frostell G (1997) Effects of frequent mouthrinses with palatinose and xylitol on dental plaque. Eur J Oral Sci 105: 162-169.
- Takahashi N, Washio J (2011) Metabolomic effect of xylitol and fluoride on plaque biofilm in vivo. J Dent Res 90:1463-1468.
- Assev S, Waler SM, Rolla G (1996) Xylitol fermentation by human dental plaque. Eur J Oral Sci 104:359-362.
- Ravald N, Hamp SE (1981) Prediction of root surface caries in patients treated for advanced periodontal disease. J Clin Periodontol 8: 400-414.
- Assev S, Rolla G (1984) Evidence for presence of a xylitol phosphotransferase system in Streptococcus mutans OMZ 176. Acta Pathol Microbiol Immunol Scand B 92: 89-92.
- Trahan L, Neron S, Bareil M (1991) Intracellular xylitol-phosphate hydrolysis and efflux of xylitol in Streptococcus sobrinus. Oral Microbiol Immunol 6: 41-50.
- Trahan L (1995) Xylitol: A review of its action on mutans streptococci and dental plaque - Its clinical significance. Int Dent J 45: 77-92.
- Miake Y, Saeki Y, Takahashi M, Yanagisawa T (2003) Remineralization effects of xylitol on demineralized enamel. J Electron Microsc (Tokyo) 52: 471-476.
- Rochel ID, Souza JG, Silva TC, Pereira AF, Rios D, Buzalaf MA, Magalhães AC (2011) Effect of experimental xylitol and fluoride-containing dentifrices on enamel erosion with or without abrasion in vitro. J Oral Sci 53: 163-168.
- Featherston JD, Nelson DG, McLean JD (1981) An electron microscope study of modifications to defect regions in dental enamel and synthetic apatites. Caries Res 15: 278-288.
- Featherstone JD, Goodman , Mclean JD (1979) Electron microscope study of defect zones in dental enamel. J Ultrastruc Res 67: 117-123.
- Flaitz CM, Hicks MJ (1996) Remineralization of caries-like lesions of enamel with acidulated calcifying fluids: a polarized light microscopic study. Pediatr Dent 18: 205-209.
- Comar LP, Wiegand A, Moron BM, Rios D, Buzalaf MA, Buchalla W, Magalhães AC (2012) In situ effect of sodium fluoride or titanium tetrafluoride varnish and solution on carious demineralization of enamel. Eur J Oral Sci 120: 342-348.
- Flaitz CM, Hicks MJ (1994) Role of the acid-etch technique in remineralization of caries-like lesions of enamel: a polarized light and scanning electron microscopic study. J Dent Child 61: 21-28.
- Comar LP, Wiegand A, Moron BM, Rios D, Buzalaf MA, Buchalla W, Magalhães AC (2012) In situ effect of sodium fluoride or titanium tetrafluoride varnish and solution on carious demineralization of enamel. Eur J Oral Sci 120: 342-348.
- Ekambaram M, Itthagarun A, King NM (2011) Comparison of the remineralizing potential of child formula dentifrices. Int J Paediatr Dent 21: 132-140.
- Garcia-Godoy F, Kao LM, Flaitz CM, Hicks J (2013) Fluoride dentifrice containing xylitol: in vitro root caries formation. Am J Dent 26: 56-60.
- Gaffar A, Blake-Haskins JC, Sullivan R, Simone A, Schmidt R, Saunders F (1998) Cariostatic effects of a xylitol/NaF dentifrice in vivo. Int Dent J 48: 32-39.
- Amaechi BT, Higham SM, Edgar WM (1999) Caries inhibiting and remineralizing effect of xylitol in vitro. J Oral Sci 41: 71-76.
- Hegde MN, Moany A (2012) Remineralization of enamel subsurface lesions with casein phosphopeptide-amorphous calcium phosphate: A quantitative energy dispersive X-ray analysis using scanning electron microscopy: An in vitro study. J Conserv Dent 15: 61-67.
- Zhou SL, Zhou J, Watanabe S, Watanabe K, Wen LY, Xuan K (2012) In vitro study of the effects of fluoride-releasing dental materials on remineralization in an enamel erosion model. J Dent 40: 255-263.
- Vashisht R, Kumar A, Indira R, Srinivasan MR, Ramachandran S (2010) Remineralization of early enamel lesions using casein phosphopeptide amorphous calcium phosphate: an ex-vivo study. Contemp Clin Dent 1: 210-213.
- Wefel JS, Harless JD (1981) The effects of topical fluoride agents on fluoride uptake and surface morphology. J Dent Res 60: 1842-8.
- Hicks J, Flaitz C, Ellis R, Westerman G, Powell L (2003) Primary tooth enamel surface topography with in vitro argon laser irradiation alone and combined fluoride and argon laser treatment: scanning electron microscopic study. Pediatr Dent 25: 491-496.
- Hicks J, Silverstone L (1983) The effect of acid-etching on caries-like lesions treated with stannous fluoride. J Dent Res 62: 783-788.
- Sano H, Nakashima S, Songpaisan Y, Phantumvanit P (2007) Effect of a xylitol and fluoride containing toothpaste on the remineralization of human enamel in vitro. J Oral Sci 49: 67-73.
- American Academy of Pediatric Dentistry (2012) Guideline on xylitol use in caries prevention. Pediatr Dent 34: 166-169.
- Nakai Y, Shinga-Ishihara C, Kaji M (2010) Xylitol gum and maternal transmission of mutans streptococci. J Dent Res 89: 56-60.
- Milgrom P, Ly KA, Tut OK (2009) Xylitol pediatric topical oral syrup to prevent dental caries. Arch Pediatr Adolesc Med 163: 601-607.
- Loesche WJ, Grossman NS, Earnest R, Corpron R (1984) The effect of chewing xylitol gum on the plaque and saliva levels of Streptococcus mutans. J Am Dent Assoc 108: 587-592.
- Holgerson PL, Sjöström I, Stecksén-Blicks C, Twetman S (2007) Dental plaque formation and salivary mutans streptococci in school children after use of xylitol-containing chewing gum. Int J Paediatr Dent 17: 79-85.
- Isokangas P, Söderling E, Pienihäkkien K, Alanen P (2000) Occurrence of dental decay in children after maternal consumption of xylitol chewing gum, a follow-up from 0 to 5 years of age. J Dent Res 79: 1885-1889.
- Scheinin AK, Pienihäkkien K, Tiekso J (1985) Collaborative WHO xylitol field studies in Hungary. VII. Two year caries incidence in 976 institutionalized children. Acta Odontol Scand 43: 381-387.
- Mäkinen KK, Hujoel PP, Bennett CA (1998) A descriptive report of the effects of a 16-month xylitol chewing-gum programme subsequent to a 40-month sucrose gum programme. Caries Res 32: 107-112.
- Isokangas P, Söderling E, Pienihäkkien K, Alanen P (2000) Occurrence of dental decay in children after maternal consumption of xylitol chewing gum, a follow-up from 0 to 5 years of age. J Dent Res 79: 1885-1889.
- Thorild I, Lindau B, Twetman S (2006) Caries in 4-year-old children after maternal chewing of gums containing combinations of xylitol, sorbitol, chlorhexidine and fluoride. Eur Arch Paediatr Dent 7: 241-245.
Relevant Topics
- About the Journal
- Birth Complications
- Breastfeeding
- Bronchopulmonary Dysplasia
- Feeding Disorders
- Gestational diabetes
- Neonatal Anemia
- Neonatal Breastfeeding
- Neonatal Care
- Neonatal Disease
- Neonatal Drugs
- Neonatal Health
- Neonatal Infections
- Neonatal Intensive Care
- Neonatal Seizure
- Neonatal Sepsis
- Neonatal Stroke
- Newborn Jaundice
- Newborns Screening
- Premature Infants
- Sepsis in Neonatal
- Vaccines and Immunity for Newborns
Recommended Journals
Article Tools
Article Usage
- Total views: 5708
- [From(publication date):
specialissue-2016 - Sep 03, 2025] - Breakdown by view type
- HTML page views : 4581
- PDF downloads : 1127