Research Article Open Access
Effective Strategies for Selection of Suitable Clay Stabilizers to Control Clay Swelling
Jie Xiao*, Jianghong Wang and Xin SunHolding Energy Petroleum Engineering, (BeiJing) Co. Ltd., China
- *Corresponding Author:
- Jie Xiao
Consultant, Holding Energy Petroleum Engineering
(BeiJing) Co., Ltd, China
Tel: + 010-88593266-817
E-mail: info@smartpetrochem.com
Received Date: December 16, 2016; Accepted Date: December 22, 2016; Published Date: January 02, 2017
Citation: Xiao J, Wang J, Sun X (2017) Effective Strategies for Selection of Suitable Clay Stabilizers to Control Clay Swelling. Oil Gas Res 3: 124. doi: 10.4172/2472-0518.1000124
Copyright: © 2017 Xiao J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Oil & Gas Research
Abstract
This paper discussed the mechanisms and processes in clay swelling. Various parameters used to predict the clay swelling processes were discussed. This study also shows how to select the suitable clay stabilizer for clay swelling control under different circumstances. Selection of clay stabilizers depends on the types of formation, the intrinsic the treatments. Different treatments require different types of clay stabilizers. Before applying any chemical treatment for clay swelling, knowing the mineralogy of clay minerals and locations of clay minerals is always being the first priority
Keywords
Clay swelling; Clay stabilizer; Crystalline swelling; Osmotic swelling
Introduction
Clay minerals can be classified into kaolinite group, smectite group, and illite group. Different types of clay minerals maintain different properties and should be addressed separately in terms of the formation damage issues caused by clay minerals [1]. Kaolinite tends to break apart, then migrates and accumulates in the critical pore throat areas. The consequences may be severe formation plugging and loss of permeability. Chlorite is very sensitive to acid or oxygenated waters and will precipitate Fe(OH)3. Illite will leach potassium ions to increase the expandable clay and then migrates with other fines. In most cases, illites are interlayered, which makes them hard to stabilize and more easily to be dispersed in the formation. Smectite is the most sensitive expandable clay mineral to brine salinity, which can cause severe loss of permeability and micro porosity [2-5]. The formation damage issues caused by clay minerals can be due to chemical reactions or physical processes, which are determined by many factors, including mineralogy and chemical composition, mineral abundance, mineral size, etc. To design an effective chemical treatment for formation damage by clay minerals, the mineralogy and the location of minerals must be known because different minerals act differently with chemicals [6]. In this paper, the mechanisms of clay swelling were analyzed and summarized. Then various methods to predict clay swelling were present. Based on that analysis, effective strategies for selection of suitable clay stabilizers were presented [7].
Mechanism of Clay Swelling
Clay swelling is the result of d-spacing increase and volume increase whenever the exchangeable cations are hydrated. According to Norrish [8], the main processes of clay swelling involve main two steps: the crystalline and osmotic swelling processes.
Crystalline swelling
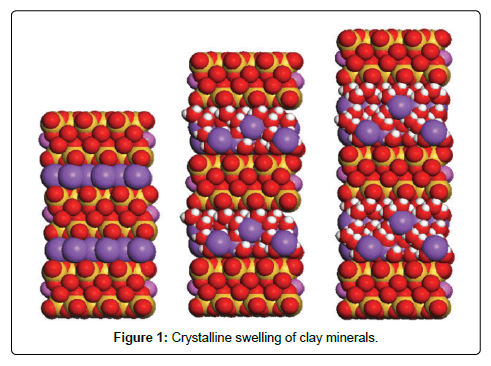
Crystalline swelling occurs in the presence of concentrated brines or brines that contain high concentration of divalent or multivalent cations. The formation of water layers on the surfaces of clay minerals causes crystalline swelling, with the concentrations below critical salt concentration. Figure 1 shows a molecular structure of 2:1 ratio sheet with exchangeable cations between layers. The left part is the anhydrous structure with no hydrations of water. The middle picture demonstrates the changes in the dimensions with four hydrated water molecules. The right part shows the changes in the dimensions with eight hydrated water molecules [9,10].
Osmotic swelling
Osmotic swelling occurs when clay minerals are exposed to solutions that contain large quantity of sodium cations. The formation of an electric double layer on surfaces of clay minerals caused clay swelling. In this case, more clay swelling and more formation damage would be expected. Different from crystalline swelling, osmotic swelling occurs at low concentrations above critical salt concentrations [11].
Predict clay swelling
The regions for crystalline or osmotic swelling can be differentiated by the value of interplanar spacing. At critical salt concentration, a discontinuity in the inter planar space can be observed. This is the transition point between crystalline and osmotic swelling. Charts can be constructed under various salt concentrations to predict the transition point. In the other ways, x-ray diffraction method can be used to construct the clay swelling charts under various salt concentrations. Then the compatibility of various clay minerals with various electrolyte solutions can be determined from the chart. From this chart, the clay swelling process can be determined whether it is crystalline swelling or osmotic swelling [12-15]. Osmotic repulsive pressure exists between clay particles when the total amount of ions in the double layer of clay minerals is more than that of in the solution. In this case, water in the pore space will enter into double to dilute the concentration [16]. As the electric filed acts as a semi-permeable membrane, water can enter into the double layer but the exchangeable cations cannot leave the layer. As a result, the double layer expands and the inter-particle distance increases. This leads to expansion of clay minerals and the result is clay swelling. Lots of researchers have proposed models and equations to calculate the values of osmotic repulsive pressure, thus predict the extent of clay swelling [17-20]. Researchers also proposed some other important parameters used to predict clay swelling, such as, water absorption rate, clay swelling coefficient, water content during clay swelling, time-dependent clay expansion coefficient, porosity reduction by clay swelling, and permeability reduction by clay swelling. All those parameters are very helpful in predicting the extent of clay swelling, with the region charts and osmotic repulsive pressure being the most effective ones [21].
Selection of Clay Stabilizers
Application of clay stabilizers has been proved to be the most effective way to control clay swelling. Table 1 lists the different stages in the development process of clay stabilizers [22-26]. Clay stabilizers can be categorized into two groups: inorganic clay stabilizers and organic stabilizers. Inorganic clay stabilizers include simple inorganic compounds, cationic inorganic polymers and steam additives. Organic clay stabilizers include simple organic compounds, cationic organic polymers, anionic organic polymers and non-ionic organic polymers [27-29]. The simple inorganic compounds are easy to apply and cheap, compared with other types of clay stabilizers. Examples are KCl, CaCl2, and they are widely used for water flooding or drilling fluids. They can also be used as a pretreatment for EOR. However, they are usually weak and temporary. They cannot be applied with steam either [30-32]. The cationic inorganic polymers, on the other hand, usually are used as a pretreatment for EOR or as a post treatment following acidizing treatments. Compared with the simple inorganic compounds, they can last longer and cannot be reversible. However, the shortcomings of applying cationic inorganic polymers are they require a shut period prior to application and they are very pH sensitive. Therefore, their effectiveness are limited under certain circumstances. Steam additives are developed to overcome the disadvantages of simple inorganic compounds and cationic inorganic polymers. They are cheap and acted as a pH buffer. They are applied for pH and salinity control during steam injection and can be used to treat the whole formation. However, they are less effective compared with the other types of clay stabilizers [33-36]. Simple organic compounds are similar to simple inorganic compounds and are used in water flooding, workovers or pretreatment. They are easy to apply but with limitations of temporary effects and not applicable in oil-wet formations. Cationic organic polymers are used mainly in acidizing or fracturing treatments, well completion or pre-treatment for EOR [37,38]. They are tolerant to carrier fluids and are irreversible. However, they are not suitable for low permeability formation. Anionic organic polymers are mainly used in drilling and completion fluids. They are also used as viscosity control agents. Shortcomings of those additives are acid and temperature sensitive and they are less effective compared with cationic polymers. Non-inoic organic polymers act similar to anionic organic polymers and examples are polycrylamide and vinyl pyrrolidine. Cationic polymer clay stabilizers are better in controlling clay swelling as they cannot be replaced by simple cations and can irreversibly stabilize clay swelling. While on the other hand, anionic or non-ionic organic polymers control clay swelling by bridging or encapsulating clay particles. As discussed above, clay swelling is most affected by the surface properties of clay minerals. The bulk properties of clay minerals control the hydrothermal stability of clay minerals. Normally, it is hard to change the hydrothermal properties of clay minerals [39]. However, solution properties, especially the pH value, have a profound effect on hydrothermal reaction processes through affecting reaction rate and reaction path. Adjustment of solution properties can control the hydrothermal properties of clay minerals to some extent.
| Development Stages | Types of Clay Stabilizers |
|---|---|
| 1960s | Monovalent salts |
| 1970s | Polyvalent salts |
| 1980s | High molecule with cationic organic polymers cationic surfactants |
| 1990s | Low molecule weight cationic organic polymers, organic cations |
| 2000s | Hydrophobic polymers |
| 2010s | Ultra low molecular weight cationic organic polymers |
Table 1: Development stages of clay stabilizers.
Conclusions
Based on above discussion, the following conclusions can be drawn:
• Clay swelling involves two main steps: the crystalline and osmotic swelling processes. Different from crystalline swelling, osmotic swelling occurs at low concentrations above critical salt concentrations.
• Clay swelling can be predicted through various parameters with the region charts and osmotic repulsive pressure being the most effective ones.
• Selection of clay stabilizers depends on the types of formation, the intrinsic the treatments. Different treatments require different types of clay stabilizers. Before applying any chemical treatment for clay swelling, knowing the mineralogy of clay minerals and locations of clay minerals is always being the first priority.
References
- Clarke TA, Nasr HA (2015) Application of a Novel Clay Stabilizer to Mitigate Formation Damage Due to Clay Swelling.Society of Petroleum Engineers.
- Monier EA,Nasr HA (2011) A Study of Several Environmentally Friendly Clay Stabilizers, Society of Petroleum Engineers.
- Hayatdavoudi A (1999) Changing Chemo physical Properties of Formation and Drilling Fluid Enhances Penetration Rate and Bit Life. Society of Petroleum Engineers.
- He J (2011) Calcium Sulphate Formation and Mitigation When Seawater Was Used to Prepare HCl-Based Acids Texas A&M University
- He J (2015) An Innovative Closed Fracture Acidizing Technique for Deep Carbonate Reservoirs Using GLDA.
- Blomquist G,Portigo J (1962) Moisture Density and Volume Change Relationships of Clay Soils Expressed as Constants of Proportionality Highway Research Board Bulletin.
- Civan F (1996) A Multi-Purpose Formation Damage Model Society of Petroleum Engineers
- Civan F (1991) Predictability of Formation Damage: An Assessment Study and Generalized Models. Oklahoma University, Norman.
- Civan F (1997) Model for Interpretation and Correlation of Contact Angle Measurements. Journal of colloid and Interface science 192: 500-502.
- He J,Arensman D,Nasr H (2013) Effectiveness of Calcium Sulphate Scale Inhibitors in Spent Hydrochloric Acid/Seawater System. Journal of Petroleum & Environmental Biotechnology, Vol-4.
- He J,Arensman D,Nasr H (2013) Mitigation of Calcium Sulphate Precipitation in Spent Hydrochloric Acid/Seawater System OTC Brazil.
- Kumar R, He J,Nasr H (2014) New Insights on the Effect of Oil Saturation on the Optimum Acid Injection Rate in Carbonate, Acidizing In SPE Improved Oil Recovery Symposium: Society of Petroleum Engineers.
- Kumar RP, He J,Nasr H (2014) Effect of Oil Saturation on Acid Propagation During Matrix Acidization of Carbonate Rocks. In SPE Latin America and Caribbean Petroleum Engineering Conference: Society of Petroleum Engineers.
- Laux H, Meese E (2008) Multidimensional Simulations of Multiphase Flow for Improved Design and Management of Production and Processing Operation.Offshore Technology Conference.
- He J, Mohamed IM, Nasr H(2011) Mixing Hydrochloric Acid and Seawater for Matrix Acidizing: Is It a Good Practice? In SPE European Formation Damage Conference: Society of Petroleum Engineers.
- He J, Mohamed IM,Nasr H(2012) Mitigation of Calcium Sulphate Scale Formation When Seawater Is Used to Prepare Hcl-Based Acids. In SPE International Symposium and Exhibition on Formation Damage Control: Society of Petroleum Engineers.
- He J, Mohamed IM,Nasr H(2013) Potential Formation Damage Due to Preparing Hcl Acids Using Seawater Canadian Energy. Technology and Innovation Journal 1: 56-63.
- He J, Nasr H (2013)Petro chemistry and Chemical Engineering.
- Khilar KC,Fogler HS (2005) Water Sensitivity of Sandstones.
- Maley D,Farion G,Giurea-Bica G (2013) Non-Polymeric Permanent Clay Stabilizer for Shale Completions,Society of Petroleum Engineers.
- Mohamed I, He J, NasrHA (2013) Effect of Brine Composition on CO2/Limestone Rock Interactions During CO2 Sequestration,Journal of Petroleum Science Research.
- Mohamed IM, He J, Mahmoud M (2010) Effects of Pressure CO2 Volume and the CO2 to Water Volumetric Ratio on Permeability Change During CO2 Sequestration, In Abu Dhabi International Petroleum Exhibition and Conference: Society of Petroleum Engineers.
- Mohamed IM, He J,Nasr H (2011)Sulphate Precipitation during CO2 Sequestration in Carbonate Rock, In SPE Project and Facilities Challenges Conference at METS: Society of Petroleum Engineers.
- Mohamed IM, He J,Nasr H(2012) Carbon Dioxide Sequestration in Sandstone Aquifers: How Does It Affect the Permeability? In Carbon Management Technology Conference: Carbon Management Technology Conference.
- Mohamed IM, He J,Nasr H(2013) Experimental Analysis of CO2 Injection on Permeability of Vuggy Carbonate Aquifers, Journal of Energy Resources Technology 135: 13-301.
- Mohamed IM, He J,Nasr H(2012) Permeability Reduction during CO2 Injection in Sandstone Aquifers: Lab and Simulation Studies. Canadian Energy Technology and Innovation Journal 1: 36-44.
- Mohan KK,Fogler HS (1997)Colloid ally Induced Semiotic Fines Migration: Existence of Micro quakes AICHE Journal 43: 565-576.
- Nasr H Mahmoud, De Wolf CA (2012) Process to Fracture a Subterranean Formation Using a Chelating Agent In: US Patent.
- Ohen HA,Civan F (1993) Simulation of Formation Damage in Petroleum Reservoirs,SPE Advanced Technology Series 1: 27-35.
- Mohamed IM, He J,Nasr H(2011a) Carbon Dioxide Sequestration in Dolomite Rock.International Petroleum Technology Conference.
- Xuejun H, He J,Tenfei S (2015b) Analysis on Design of Coiled Tubing Sliding Drilling Electronic Control Tractor with Hydraulic-Driven in Micro hole. Electronic Journal of Geotechnical Engineering20: 4333-4347.
- Zhou Z (1997) Construction and Application of Clay-Swelling Diagrams by Use of Xrd Methods.
- Zhou Z, Gunter W,Kadatz B (1996) Effect of Clay Swelling on Reservoir Quality, Journal of Canadian Petroleum Technology, Vol-35.
- Zhou ZJ, Cameron S,Kadatz B (1997) Clay Swelling Diagrams: Their Applications in Formation Damage Control. SPE Journal 2: 99-106.
- Mohamed IM, He J,Nasr H(2011) Permeability Change During CO2 Injection in Carbonate Aquifers: Experimental Study In SPE Americas E&P Health Safety Security and Environmental Conference: Society of Petroleum Engineers.
- Reed M (1977) Formation Permeability Damage by Mica Alteration and Carbonate Dissolution.Journal of Petroleum Technology 29: 1056-1060.
- Weaver JD, Nguyen PD,Loghry R (2011) Stabilizing Fracture Faces in Water-Sensitive Shale FormationsSociety of Petroleum Engineers.
- Jiafu Y, Haiwen W,Jia He, Xiaomeng H, Huixin L (2009) Fracturing string packer packer Comparison Analysis Well Testing 18: 6-11.
- Xuejun H, He J,Tenfei S (2015) Analysis of the Critical Buckling Loads and Contact Loads on Coiled Tubing String in a Vertical Micro hole. Journal of Chemistry and Technology of Fuels and Oils 51: 308-319.
Relevant Topics
Recommended Journals
- Oil & Gas Research Journal
- Renewable Energy and Applications Journal
- Oceanography Journal
- Industrial Pollution Control Journal
- Coastal Zone Management Journal
- Climatology & Weather Forecasting Journal
- Geoinformatics & Geostatistics Journal
- Engineering and Technology Journal
- Petroleum & Environmental Biotechnology Journal
- Polymer Sciences Journal
Article Tools
Article Usage
- Total views: 8739
- [From(publication date):
March-2017 - Aug 30, 2025] - Breakdown by view type
- HTML page views : 7584
- PDF downloads : 1155