Effects of a Single Dose of Methylphenidate on Saccadic Eye Movements in Adults with Attention-Deficit/Hyperactivity Disorder.
Received: 15-Mar-2018 / Accepted Date: 22-Oct-2018 / Published Date: 29-Oct-2018
Abstract
Background: Oculomotor tasks have been used to investigate executive functions and frontal-striatal functioning in humans, but there are relatively few studies on saccadic eye movements (SEMs) in adults with attention-deficit/ hyperactivity disorder (ADHD). Methylphenidate (MPH) is an effective treatment for ADHD symptoms. We therefore evaluated oculomotor performances of ADHD adults before and after administration of a single MPH dose.
Methods: Forty stimulant drug-naive DSM-5 adult ADHD patients participated in this study. Saccade parameters were measured in the morning at baseline, and two weeks later following a single low dose administration of MPH (10 mg orally). Results were compared with 34 healthy control (HC) subjects. Oculomotor performances were determined in automatic attentional tasks (visually-guided-saccades, i.e. gap and step tasks) and voluntary attentional tasks (overlap and antisaccade tasks).
Results: Compared to HCs, ADHDs at baseline showed increased saccade latency (in the gap and antisaccade tasks), increased directional errors in the antisaccade task, decreased average speed (in all tasks), decreased saccade accuracy (in all tasks), increased percentage of anticipatory saccades (in all tasks) and increased percentage of express saccades (in the overlap task). A single low dose of MPH normalized the saccade latencies and the percentage of errors in the antisaccade task, and improved average speed in automatic attentional tasks.
Conclusions: Medication-naive ADHD adults show impairments on motor planning and response inhibition. A single-dose of MPH improves oculomotor performances in these patients. Thus, impaired SEMs could be potential pathophysiologic markers of deficits in frontal-striatal pathways in adults with ADHD.
Keywords: ADHD; Adults; Oculometry; Saccade; Methylphenidate; Inhibition
Introduction
Attention Deficit/Hyperactivity Disorder (ADHD) is a prevalent psychiatric disorder in children characterized by a persistent pattern of inattention and/or hyperactivity-impulsivity that often continues into adulthood [1]. It has been estimated that ADHD impairs approximately 4% to 5% of the adult population; however, less than one third of these adults have been diagnosed for review [2]. Adults with the disorder continue to have attentional problems, deficits in executive functioning and working memory, and poor inhibitory control [3]. These manifestations contribute to impairments across academic, behavioral, social and affective domains, and have neurobiological bases [4]. Hypoactivity of the frontal-striatal circuits, possibly involving dysfunction in catecholamine transmission norepinephrine (NE) and especially dopamine (DA), has been hypothesized to produce these symptoms and lead to deficits in the voluntary control of behavior [5]. Stimulant medications such as methylphenidate (MPH) have been used for decades as a first line treatment option for ADHD [6]. MPH is also a well-established treatment for ADHD adults [2], although prescribed off-label in France like in most European countries. The short-term mean response rate in ADHD adults is about 60% [7]. Eye movements depend on structures implicated in attention and in motor control [8-10]. Saccadic eye movement (SEM) is a short and rapid eye movement that can be voluntary or reflexive (i.e., sensory-triggered). Initiation of visually triggered saccades involves occipital and parietal cortex and their inputs to the superior colliculus (SC), which then projects to the premotor circuit in the brain stem and cerebellum [11-14]. The antisaccade task requires participants to suppress a reflexive saccade towards a target and instead generate a volunteer saccade in the opposite direction. Planning of volitional saccades and suppression of reflexive saccades is under the control of frontoparietal cortex, frontal eye field (FEF), supplementary eye field and basal ganglia, which also project to SC and brain stem premotor circuit [12-15]. Deficits in oculomotor control have been found in adults with ADHD, albeit with varying characteristics from study to study, making it difficult to establish a pattern of response tasks related to ADHD (Table 1). These discrepancies may be explained by the heterogeneity of the populations studied, the heterogeneity of experimental paradigms, and the fact that most patients included in these studies were previously medicated. This latter consideration is of importance since it has been shown differences in brain structure between drug-naive and previously treated ADHD patients [16-19].
| Study | Subjects | Prosaccade Gap task |
Prosaccade Step task |
Prosaccade Overlap task |
Antisaccade Gap task |
Conclusions |
|---|---|---|---|---|---|---|
| Munoz et al. [12] | 38 ADHDs (12 unmedicated for at least 20 hours) 105 healthy controls |
- Longer latencies - Decreased average speed - Normal rates of express saccades |
- Normal rates of express saccades | - Greater error rates - Decreased average speed |
- Longer latencies - Greater error rates - Decreased average speed - Normal rates of express saccades |
Dysfunction in response inhibition, which is modulated by the frontal lobe |
| Feifel et al. [16] |
12 ADHDs (unmedicated for at least 48 hours) 12 healthy controls |
- Normal latencies - Higher rates of anticipatory saccades - Normal accuracy |
- Normal latencies - Decreased average speed |
- Normal latencies - Increased error rates |
Deficit in inhibitory mechanisms, possibly reflecting abnormalities in prefrontal cortex-basal ganglia circuitry | |
| Carr et al. [17] |
72 persistent ADHDs and 20 residual ADHDs (unmedicated for at least 24 hours [short-acting preparation] to 48 hours [long-acting preparation]) 67 healthy controls |
- Extended latencies - Greater error rates (not found in residual ADHDs) - Increased anticipatory saccades (persist in residual ADHDs) |
ADHD is associated with weakness in motor inhibition but not attentional inhibition or filtering | |||
| Hakvoort Schwerdtfeger et al. [18] | 14 ADHDs (unmedicated for at least 20 hours) 20 healthy controls |
- More variable latencies - Greater error rates - Tendency to make more express saccades |
- Slower sacacadic reaction times - More direction errors - Normal rates of express saccades |
Impairments do not result from an inability to execute a saccade (because saccade metrics are normal) but rather stem from the failure to properly prepare the oculomotor network optimally |
Table 1: Summary of main findings in adults with ADHD during execution of saccade paradigms.
Some neuroimaging studies demonstrated that a single dose of MPH resulted in increased activation within frontal lobes, basal ganglia, and cerebellum [20]. These findings indicate that MPH may help to return the brain functioning in ADHD patients to a normal level. It has also been reported in ADHD children that saccadic performances are improved with chronic administration of MPH [21-23], but this was not systematically reproduced [24,25]. However, studies on the acute effect of MPH on SEMs are still lacking in ADHD adults. The main goals of the present study were to examine whether SEMs (i.e., prosaccades and antisaccades) are altered in MPH-naive ADHD adults, and whether they can be modified by an acute stimulant challenge (single-dose of MPH).
Methods and Material
Participants
Selection procedure: The investigations adhered to the principle of the Declaration of Helsinki and were approved by the local ethical committee (Comité de Protection des Personnes Est IV, Hôpital Civil Strasbourg). All participants gave their informed and written consent.
Control group: Thirty-four healthy adult volunteers (19 male/15 female, aged 19 to 47 years; mean age ± SD, 31.8 ± 8.4 years) free of psychiatric, neurological and medical illness were selected by SuriCog. They were required to have no current medication use. All were recorded with the EyeBrain’s eye-tracker device.
Patient recruitment and assessment: Forty stimulant drug-naive adult outpatients (22 male/18 female, aged 19 to 53 years; mean age ± SD, 33.7 ± 10.2 years) meeting DSM-5 criteria for ADHD (American Psychiatric Association, 2013) participated in this study. Twenty-eight patients met DSM-5 criteria for the combined presentation (cADHD); and the remaining 12 showed predominantly inattentive presentation (iADHD). Patients were recruited from the ADHD outpatient unit of the Pole 8/9, Psychiatric Hospital of Rouffach (France). They were newly diagnosed as having ADHD on the basis of a medical examination conducted by an experienced research psychiatrist in the field of adult ADHD (F.D. or A.E.). The French versions of the Diagnostic Interview for ADHD in Adults (DIVA 2.0 [26] semistructured interview providing a list of concrete and realistic examples, for both current and retrospective (childhood) behavior and the World Health Organization Adult ADHD Self-Report Scale (ASRS v1.1) [27] were then used to evaluate current ADHD symptoms, although these scales are calibrated to DSM-IV criteria. The 18-item ASRS is a symptom checklist designed to evaluate current manifestation of ADHD symptoms in adults. The frequency of occurrence of symptoms is scored 0=never; 1=rarely; 2=sometimes; 3=often; 4=very often. It has been found that the 6 questions in Part-A are the most predictive of the disorder and are best for use as a screening instrument [27]. All patients were screened using health questionnaire and medical history interview. Exclusion criteria were as follow: serious physical disease, impaired cardiovascular functioning, chronic obstructive pulmonary disease, seizure disorder or encephalopathy, head trauma, CNS tumor, or history of major psychiatric disorders. No patients were currently treated with psychotropic medications. ADHDs in remission or with comorbid severe personality disorder or substance abuse or alcohol dependence (according to DSM-5 criteria), or with a strabismus, and pregnant or breast-feeding women were excluded. All patients had normal cardiovascular evaluation and normal blood tests (including full blood cell count, hematocrit, electrolytes, iron, thyroid hormones levels). Our patients were evaluated under similar oculomotor protocol than the one applied to the control group.
Ocular motor paradigms
All participants had normal or corrected-to-normal vision. Stimuli were presented on a PC screen of 22”; its resolution was 1920 × 1080 and the refresh rate was 60 Hz. Stimuli for saccades were presented randomly on the screen at 20°C or -20°C (saccades toward right or left respectively). Three different paradigms were used to stimulate horizontal visually guided saccades (the gap, the step, and the overlap) and one for antisaccades (Figure 1). The prosaccade task is used to test the ability of subjects to generate reflexive visually triggered saccades. In this task, subjects are required to look from a central fixation point (FP) to an eccentric target (T) stimulus as soon as it appears either on the right or left side of the screen. During antisaccade trials, subjects must suppress the reflexive prosaccade and instead generate a voluntary saccade to the mirror position of the T (where no stimulus appeared).
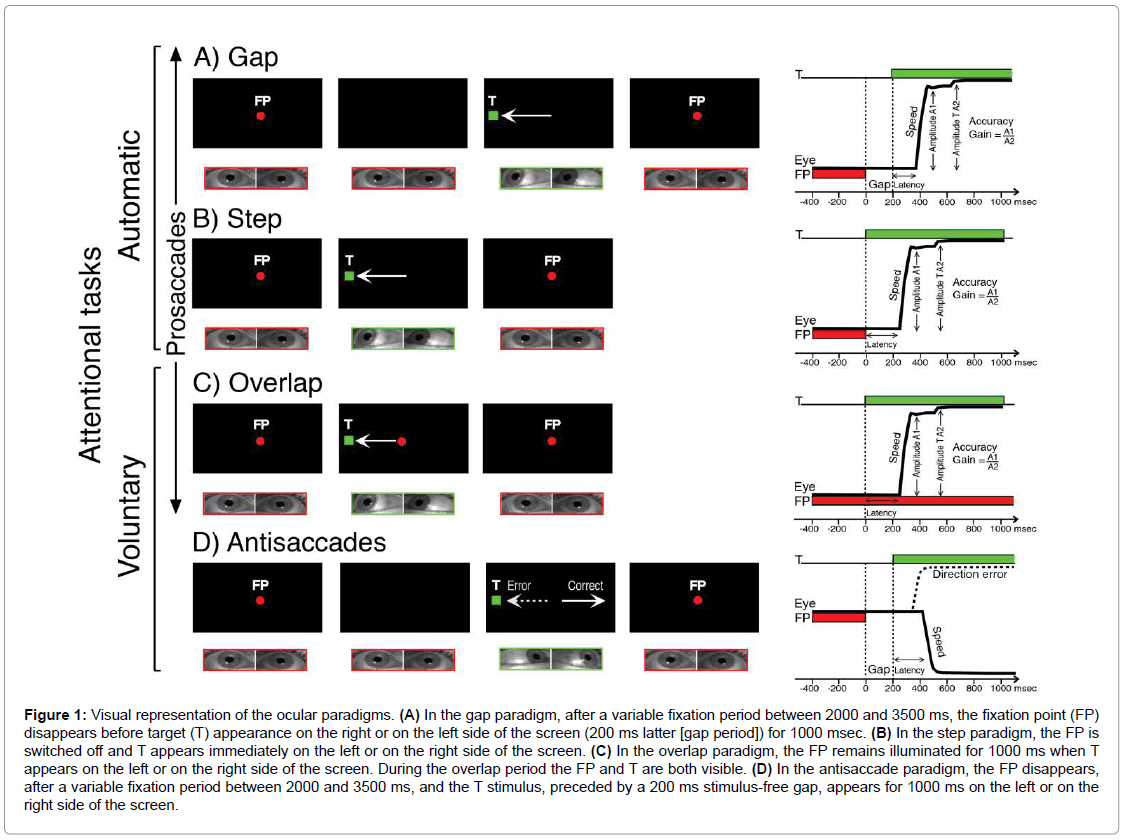
Figure 1: Visual representation of the ocular paradigms. (A) In the gap paradigm, after a variable fixation period between 2000 and 3500 ms, the fixation point (FP) disappears before target (T) appearance on the right or on the left side of the screen (200 ms latter [gap period]) for 1000 msec. (B) In the step paradigm, the FP is switched off and T appears immediately on the left or on the right side of the screen. (C) In the overlap paradigm, the FP remains illuminated for 1000 ms when T appears on the left or on the right side of the screen. During the overlap period the FP and T are both visible. (D) In the antisaccade paradigm, the FP disappears, after a variable fixation period between 2000 and 3500 ms, and the T stimulus, preceded by a 200 ms stimulus-free gap, appears for 1000 ms on the left or on the right side of the screen.
Eye movement recordings
Eye movements were recorded using the Mobil EyeBrain Tracker (Mobile EBT®), a CE marked medical eye-tracking device (SuriCog). It benefits from cameras that capture the movements of each eye independently by recording the horizontal and vertical position of the eyes. Recording frequency for both eyes was set up to 300 Hz. The precision of this system is 0.25°C. Calibration was performed at the beginning of eye movement recordings, and consists of following 13 red dots (diameter of 0.5°C) presented on a PC screen, the tasks started immediately after the calibration.
Procedures
In patients, eye movements were recorded in the morning at baseline (BL), and two weeks later following a single low dose administration of MPH (10 mg orally) at 8 AM. Control subjects (HCs) performed only a baseline session. In order to assess the reliability of the ocular performances in patients, 2 recording baseline sessions were performed on the same day at 9 AM (S1-BL), and at 11 AM (S2-BL). Two weeks later, 2 recording sessions were performed following administration of MPH (10 mg orally) at 8 AM: the first one at 9 AM (S1-MPH), and the second one at 11 AM (S2-MPH). Moreover, subjective assessments using a series of 16 self-rating visual analogic scales (VAS) [28] were administered in patients after S1-BL and S1-MPH sessions. Following Bond and Lader [29], the VAS were analyzed using 3 factors (a high score indicating an impairment): alertness, contentedness (well-being) and calmness.
Data Analysis
The MeyeAnalysis© software, provided with the eye tracker, was used to determine automatically the onset and the end of each saccade by using a “built-in saccade detection algorithm”. Saccade parameters extracted were: saccade latencies (time between the onset of the target and the beginning of the eye movements), average speed of the saccade, and saccade accuracy (characterized by the ratio of the amplitude of the first saccade to the amplitude of the target). All detected saccades were verified by the investigator and corrected or discarded as necessary. Eye movements were analyzed using the better signal of both eyes. In the present study, only saccades with latencies of at least 80 msec were considered stimulus-triggered events [30]. Saccades were classified as anticipatory if they were initiated<80 msec after target appearance. Express saccades were characterized by a reaction time between 80 and 130 msec [30]. Anticipatory and express saccades were excluded from analysis of SEM parameters, but were counted given their pathophysiologic significance.
Statistical analysis
Despite logarithmic or other transformations, the distribution of some data remained non-normal (Kolmogorov-Smirnov one-sample test for goodness-of-fit) and it was necessary to use non-parametric statistical methods. Between-group differences were tested for significance with the Kruskal–Wallis one-way analysis of variance by ranks (H-test); and, where the overall effect was significant, by Mann- Whitney two-tailed test (U-test), followed by Bonferroni’s post-hoc test. Within-subject differences were evaluated with the Wilcoxon two-tailed signed-rank test (T test). Correlations between quantitative variables were estimated using the Spearman rank coefficient (ρ). Optimal cut-off values were determined using receiver operating characteristic (ROC) curve analysis [31]. Categorical data were analyzed with chi-square (χ2) test or Fisher’s Exact test (two-tailed). All statistical analyses were computed using StatView (SAS Institute Inc, Cary NC, USA). Results were considered significant when p ≤ 0.05.
Results
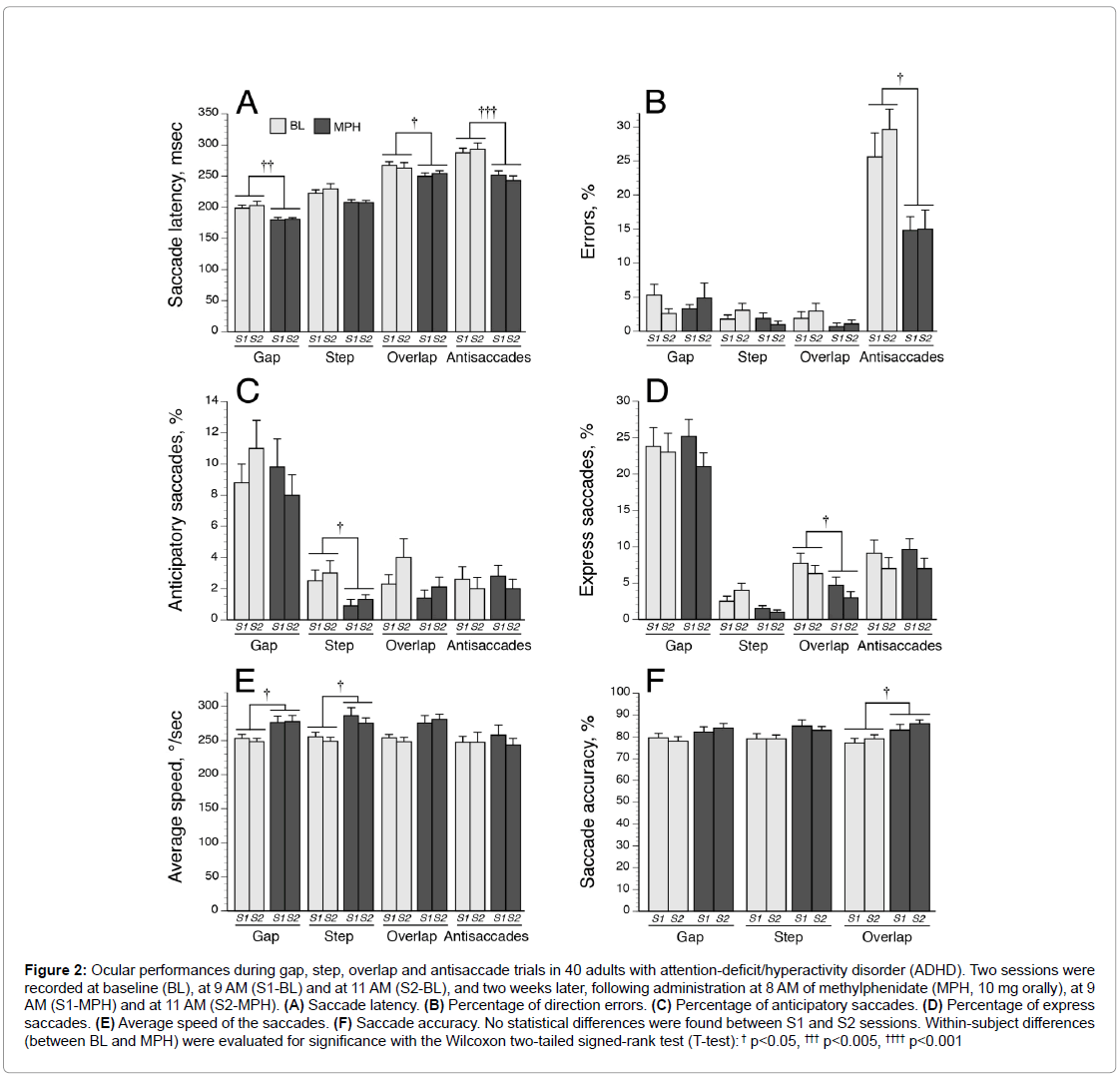
The ADHD and control groups were comparable for age and sex distribution. Figure 2 illustrates the oculomotor performances in ADHDs evaluated during morning sessionsat 9 AM (S1) and 11 AM (S2) at baseline and after a single dose of MPH. No significant differences were observed between S1 and S2 parameters. Thus, inter-group and intra-group comparisons were performed using SEMs recorded during S1-BL and S1-MPH sessions.
Figure 2: Ocular performances during gap, step, overlap and antisaccade trials in 40 adults with attention-deficit/hyperactivity disorder (ADHD). Two sessions were recorded at baseline (BL), at 9 AM (S1-BL) and at 11 AM (S2-BL), and two weeks later, following administration at 8 AM of methylphenidate (MPH, 10 mg orally), at 9 AM (S1-MPH) and at 11 AM (S2-MPH). (A) Saccade latency. (B) Percentage of direction errors. (C) Percentage of anticipatory saccades. (D) Percentage of express saccades. (E) Average speed of the saccades. (F) Saccade accuracy. No statistical differences were found between S1 and S2 sessions. Within-subject differences (between BL and MPH) were evaluated for significance with the Wilcoxon two-tailed signed-rank test (T-test): † p<0.05, ††† p<0.005, †††† p<0.001
Oculomotor performances in ADHD adults at baseline versus normal control subjects
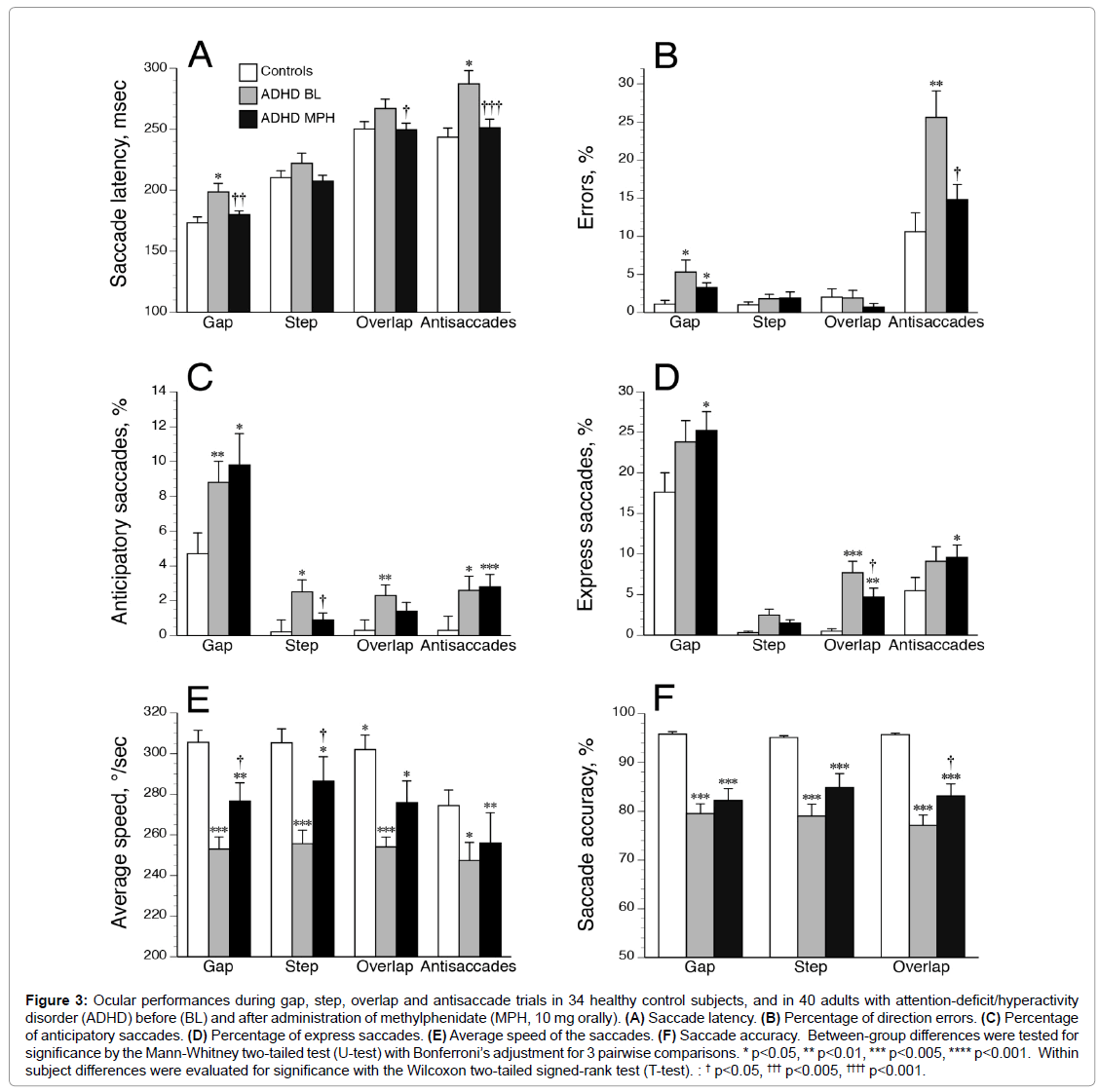
Compared to HCs, drug-naive ADHDs were slower to initiate correct saccades (Figure 3A) and executed a higher percentage of direction errors (Figure 3B) during gap and antisaccade tasks. ADHDs made also more anticipatory saccades in all tasks (Figure 3C), and more express saccades during the overlap paradigm (Figure 3D) than HCs. Using a criterion of greater than 4% (ROC analysis [31], the number of patients showing an increased percentage of abnormal express saccades during overlap paradigm was higher in ADHDs (n=28) compared to HCs (n=1; p<0.00001 by two-tailed Fisher’s Exact Test; specificity, 97%; sensitivity, 70%). Moreover, average speed (Figure 3E) and accuracy values (Figure 3F) were markedly decreased in all tasks for ADHDs as compared with HCs. We found no significant effect of age or gender on the oculomotor performances in both control and ADHD groups (all p>0.10). Saccade impairments were overall relatively comparable between ADHD subtypes (Table 2) except increased saccade latencies in cADHDs compared to iADHDs during step and overlap tasks. Nevertheless, cADHDs were slower than HCs in initiating correct saccades in the gap (mean ± SD, 205.0 ± 58.9 vs. 173.2 ± 28.3 msec, respectively; p=0.004), overlap (277.4 ± 51.4 vs. 250.3 ± 35.1 msec; p=0.01) and antisaccade (293.2 ± 73.6 vs. 243.5 ± 43.7 msec, p=0.001) tasks, while iADHDs were not. Further analyses comparing the mild (n=12), moderate (n=15) and severe (n=13) ADHD groups (according to DSM 5 specifiers) found no influence of severity of ADHD symptoms on SEM characteristics (all p>0.20).
Figure 3: Ocular performances during gap, step, overlap and antisaccade trials in 34 healthy control subjects, and in 40 adults with attention-deficit/hyperactivity disorder (ADHD) before (BL) and after administration of methylphenidate (MPH, 10 mg orally). (A) Saccade latency. (B) Percentage of direction errors. (C) Percentage of anticipatory saccades. (D) Percentage of express saccades. (E) Average speed of the saccades. (F) Saccade accuracy. Between-group differences were tested for significance by the Mann-Whitney two-tailed test (U-test) with Bonferroni’s adjustment for 3 pairwise comparisons. * p<0.05, ** p<0.01, *** p<0.005, **** p<0.001. Within subject differences were evaluated for significance with the Wilcoxon two-tailed signed-rank test (T-test). : † p<0.05, ††† p<0.005, †††† p<0.001.
| cADHD (n=28) | iADHD (n=12) | |||
| Age, years* | 33.4 ± 10.3 | 34.6 ± 10.1 | ||
| Gender (M/F) | 17/11 | 5/7 | ||
| Weight | 79.6 ± 16.9 | 74.3 ± 17.5 | ||
| Severity (mild/moderate/severe) | 5/12/11a | 7/3/2 | ||
| ASRS scores A A+B |
17.6 ± 3.8a 45.7 ± 10.4a |
14.5 ± 3.8 38.2 ± 7.5 |
||
| BL | MPH | BL | MPH | |
| VAS scores Factor 1, Alertness Factor 2, Contentedness Factor 3, Calmness |
67.7 ± 12.4 40.5 ± 15.5 39.7 ± 19.8 |
34.9 ± 13.9c 31.6 ± 17.0b 31.1 ± 17.3 |
67.1 ± 11.3 39.4 ± 11.2 44.7 ± 22.9 |
37.2 ± 11.9c 32.0 ± 11.4 c 32.1 ± 18.3 b |
| Saccade latency (msec) | ||||
| - Gap | 205.0 ± 48.9 | 181.5 ± 19.6 c | 183.6 ± 26.3 | 176.1 ± 20.4 |
| - Step | 232.1 ± 56.3a | 212.2 ± 30.0b | 199.1 ± 33.0 | 196.8 ± 23.9 |
| - Overlap | 277.4 ± 51.4a | 255.2 ± 34.7b | 242.9 ± 35.7 | 236.9 ± 29.7 |
| - Antiscaccades | 293.2 ± 73.6 | 255.9 ± 41.7c | 273.4 ± 57.2 | 240.4 ± 48.9c |
| Saccade accuracy (%) | ||||
| - Gap | 78 ± 13 | 83 ± 11 | 82 ± 13 | 84 ± 18 |
| - Step | 80 ± 17 | 83 ± 8 | 77 ± 11 | 81 ± 15 |
| - Overlap | 77 ± 15 | 85 ± 9b | 77 ± 9 | 88 ±12c |
| Average speed (°/sec) | ||||
| - Gap | 252.3 ± 39.1 | 272.1 ± 45.4 | 254.5 ± 35.5 | 291.6 ± 70.6 |
| - Step | 259.4 ± 46.4 | 273.8 ± 39.8 | 246.8 ± 27.1 | 279.7 ± 64.4 |
| - Overlap | 256.1 ± 33.1 | 272.6 ± 37.2 | 249.1 ± 24.1 | 301.2 ± 57.5b |
| - Antiscaccades | 251.6 ± 63.1 | 232.2 ± 45.9 | 237.7 ± 34.2 | 259.1 ± 66.2 |
| Anticipatory saccades (%) | ||||
| - Gap | 8 ± 5 | 9 ± 3 | 12 ± 11 | 12 ± 11 |
| - Step | 2 ± 4 | 1 ± 3 | 4 ± 5 | 0 ± 2 |
| - Overlap | 2 ± 4 | 1 ± 3 | 2 ± 3 | 2 ± 4 |
| - Antiscaccades | 1 ± 3 | 1 ± 4 | 5 ± 7 | 2 ± 4 |
| Express Saccades (%) | ||||
| - Gap | 25 ± 17 | 25 ± 14 | 21 ± 15 | 25 ± 17 |
| - Step | 2 ± 4 | 2 ± 3 | 4 ± 5 | 1 ± 3 |
| - Overlap | 8 ± 8 | 4 ± 6b | 8 ± 11 | 7 ± 8 |
| - Antiscaccades | 9 ± 10 | 9 ± 9 | 10 ± 14 | 10 ± 10 |
| Errors (%) | ||||
| - Gap | 4 ± 6 | 3 ± 4 | 9 ± 17 | 3 ± 4 |
| - Step | 4 ± 7 | 1 ± 2b | 2 ± 3 | 5 ± 14 |
| - Overlap | 1 ± 2 | 0 ± 1 | 4 ± 11 | 2 ± 5 |
| - Antiscaccades | 25 ± 21 | 14 ± 13b | 26 ± 26 | 18 ± 13 |
Table 2: Demographic characteritics and oculomotor performances at baseline and after a single dose of methylphenidate for ADHD patients classified according to DSM-5 subtypes.
Impact of a single low dose of MPH on oculomotor performances in ADHD adults
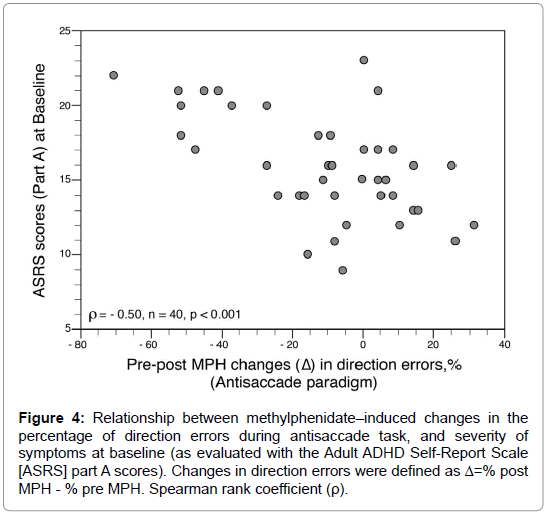
After MPH administration (10 mg orally), latency for saccades recorded with the gap, overlap, and antisaccade paradigms decreased significantly (Figure 3A). Antisaccade errors decreased also and were normalized with MPH, while gap errors were unaffected (Figure 3B). Except during the step paradigm, anticipatory saccades did not decrease significantly with MPH (Figure 3C). The percentage of express saccades in the overlap paradigm decreased significantly with MPH (but remained higher than in HCs), while in the other paradigms express saccades showed no improvement (Figure 3D). The average speed increased after MPH significantly only in automatic tasks (i.e., gap and step conditions) but remained slower (in all tasks) than in HCs (Figure 3E). Accuracy values after MPH did not change compared to baseline and remained impaired in ADHDs (Figure 3F). The 3 factor subscores of the VAS were improved after MPH: alertness (p<0.00001); contentedness (well-being) (p<0.003); and calmness (p=0.007). However, no significant relationship was found between VAS subscores and oculomotor performances, neither at BL nor after MPH. The effect of MPH on SEMs was not influenced by age, sex, or weight (all p>0.20). However, some differences emerged between cADHD and iADHD patients (Table 2). While both groups showed decreased saccade latencies with MPH in the antisaccade task, cADHDs exhibited more pronounced enhancements in their oculomotor performances than iADHD especially for prosaccade latencies and antisaccade errors. In addition, the percentage of direction errors during antisaccade task decreased markedly after MPH in ADHDs with severe symptoms (from 36 ± 28% to 9 ± 10%; p=0.005 by T test) while in ADHDs with moderate and mild symptoms this percentage remained unchanged (BL, 20 ± 17%; post-MPH, 18 ± 14%). The changes in direction errors (expressed as Δ=%post MPH - %pre MPH) were higher in ADHDs with severe symptoms (Δ=-27 ± 24%) than in ADHDs with moderate and mild symptoms (Δ=-3 ± 20%) (p=0.005 by U test). The MPH-induced changes in thepercentage of direction errors during antisaccade task were negatively correlated with the severity of symptoms at baseline (as evaluated with the ASRS part A scores) (Figure 4).
Figure 4: Relationship between methylphenidate–induced changes in the percentage of direction errors during antisaccade task, and severity of symptoms at baseline (as evaluated with the Adult ADHD Self-Report Scale [ASRS] part A scores). Changes in direction errors were defined as Δ=% post MPH - % pre MPH. Spearman rank coefficient (ρ).
Discussion
The main findings from the present study are as follows: (1) MPHnaive ADHDs exhibit an overall deficit in oculomotor ability compared to HCs, suggesting impairments on motor planning and response inhibition; (2) a single low dose of MPH normalizes the saccade latencies and the percentage of errors in the antisaccade paradigm, and improves average speed (in automatic attentional tasks only). To our knowledge, this is the first study that illustrates the acute effect of a single-dose of MPH on oculomotor performances in ADHD adults.
Impaired oculomotor performances in methylphenidatenaive ADHD adults
Our findings that latencies and direction errors are significantly increased during gap tasks (i.e., pro- and anti-saccade paradigms) among ADHD participants confirm and extend previous reports describing deficits in eye-movement control in adults with ADHD [12,17,18]. When studying SEMs, a stimulus-free 200 msec-gap facilitates prosaccade execution-since the eye is “disengaged” from the central fixation point-inducing shorter latencies compared to the step and overlap paradigms [10]. However, a gap makes more difficult the execution of an antisaccade, which requires a voluntary inhibition of a reflexive saccade and a generation of a voluntary saccade in the opposite direction. Given that the gap prepares the eye for a saccade execution, direction errors during the antisaccade task are increased since it is difficult to counter the reflexive prosaccade towards the target [10]. Our antisaccade data suggest that ADHDs exhibit impairments in regulating the processes of saccade initiation and saccade suppression leading to the generation of more reflexive saccades and more direction errors than HCs. These findings are in agreement with previous studies [12,16-18], while the finding that 70% of ADHD adults exhibit an increased rate of abnormal expressed saccades in the prosaccade overlap paradigm is new. Taken together, our results are consistent with inhibitory failure possibly reflecting deficits in frontostriatal pathways. It has been shown that the dorsolateral prefrontal cortex (DLPFC), which projects directly to the FEF and SC, and the substantia nigra pars reticulate are required for saccadic suppression [32,33]. However, in our ADHD population inhibitory deficit seems to concern not only voluntary but also automatic control of saccade generation. Indeed, ADHDs make also more anticipatory saccades in the prosaccade gap and step paradigms suggesting a disinhibition of saccade generating neurons in the FEF and SC. Overall, our findings are in line with the disinhibition theories of ADHD [34,35], which posit that most symptoms of ADHD are secondary to underactivity of the behavioral inhibition system. Furthermore, accuracy and average speed of correct pro- and anti-saccades are markedly reduced in all tasks in our population of drug-naive ADHD adults as compared with control subjects. These alterations are compatible with frontostriatal, brain stem, and cerebellar dysfunctions [12,36]. However, our findings contrast with previous studies reporting that saccade metrics are normal [16,18] or near normal [12] in ADHDs. One must nevertheless note that most patients enrolled in those previous studies were chronically treated with stimulant drugs (despite a short washout period before testing), and it is possible that oculomotor performances are different in participants who had been previously prescribed MPH. Although most oculomotor performances do not differentiate cADHDs from iADHDs-both subtypes are associated with more direction errors (in the gap tasks), more anticipatory and express saccades (in the non-gap tasks), reduced accuracy of correct pro- and anti-saccades and slower average speed-cADHDs are slower than HCs in initiating correct saccades (in the gap, overlap, and antisaccade tasks), while iADHDs are not. This could indicate some degree of additional difficulty in motor planning, and/or inadequate attention and/or motivation in the cADHD group possibly reflecting greater deficits in executive functions.
Methylphenidate challenge-induced changes in oculomotor performances in ADHD
The most striking finding is that direction errors and saccade latencies are fully normalized in ADHD adults during the antisaccade paradigm after a single-low dose of MPH (10 mg orally). Previous studies of ADHD suggested that MPH might reduce antisaccade latencies [11,21,37] and errors [11,24,25,37], although results are divided. It has been showed that MPH boosts catecholamine signaling by blocking the DA and NE transporters increasing extracellular DA and NE levels with a time course matching behavioral effects [38]. Biochemical studies demonstrated that low doses of MPH elicit more potent effects on hippocampal NE than on striatal DA, while increasing both DA and NE release in the prefrontal cortex [39]. In our study the MPH challenge dose was low (ranging from 0.1 to 0.16 mg/kg), but was sufficient to increase inhibitory oculomotor control of voluntary eye movements in ADHDs by restoring suppression signals on saccade neurons in the FEF and SC, probably via an activation of the prefrontal cortex and/or the basal ganglia [12], and by elevating the arousal level or the general tonic level of the attention system, consequently increasing the inhibitory power of the system [40]. While a single 10 mg dose of MPH allows a better saccadic performance, it appears, nevertheless, insufficient to cause the recovery of normal inhibitory oculomotor control of involuntary eye movements, since during the prosaccade gap task the rates of direction errors, anticipatory and express saccades do not improve. In addition, while average speed and accuracy of SEMs are enhanced with MPH (albeit not always significantly) they remain abnormal in ADHDs, suggesting that frontostriatal, brain stem, and cerebellar dysfunctions are only partially reversed. Although the response to MPH is dependent of the dose administered [41] and the ongoing task (e.g., without stimulation, a 20 mg dose of MPH does not significantly increase DA in the striatum [42], the effect of MPH in ADHD is highly influenced by the initial level of performance [43]. Our results are in line with this view, since the higher the symptom severity at baseline, the more pre-post MPH changes in direction errors during the antisaccade paradigm.
Limitations of the present study
Some shortcomings in our study require discussion. First, we did not compare the effects of MPH against placebo in ADHDs, neither the effects of MPH on oculomotor performances in HCs. To date only one study [44] using a single dose of 20 mg of MPH found decreased saccade latency in the visually-guided saccade but no effect on antisaccade performance in HCs. Given the high test–retest reliability, as illustrated in Figure 2, it seems unlikely that improvement in oculomotor performances with MPH in ADHDs may be attributed to a simple placebo effect or a learning effect. Second, as in the vast majority of studies, we did not measure plasma concentration of MPH, and consequently no link can be drawn between plasma MPH concentration and improvement in oculomotor performances. Third, although the primary findings appear to be statistically robust, they must be considered preliminary until replicated in a larger patient population. In conclusion, our pilot study suggests that a single low dose of MPH normalizes direction errors and saccade latencies during the antisaccade paradigm in drug-naive adults with ADHD. Overall our data support the hypothesis that impaired SEMs could be potential pathophysiologic markers of deficits in frontal-striatal pathways in adults with ADHD, although the genetic network underlying hyperactivity and inattention does not appear limited to the fronto-striatal pathway [45,46]. Future studies are needed to determine whether SEMs could be a predictive marker of subsequent MPH efficiency in ADHD.
Acknowledgments
The authors express their gratitude to the nurses and the neuropsychologists of the ADHD outpatient unit of the Pole 8/9, Psychiatric Hospital of Rouffach (France). We also gratefully acknowledge the valuable comments of Cécile Calleja, MD, PhD, Pole 8/9, Psychiatric Hospital of Rouffach (France) who kindly reviewed the manuscript.
Financial disclosures
No conflict of interest is declared
References
- American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Publishing.
- Kooij JJS, Francken MH (2010): Diagnostic interview for ADHD in adult (DIVA 2.0) Diagnostisch Interview Voor ADHD bij volwassenen. DIVA Foundation, The Netherlands.
- Roberts W, Milich R, Fillmore MT (2016) The effects of preresponse cues on inhibitory control and response time in adults with ADHD. J Atten Disord. 20: 317-324.
- De La Fuente A, Xia S, Branch CA, Li X (2013) A review of attention-deficit/hyperactivity disorder from the perspective of brain networks. Front Hum Neurosci 7: 192.
- Del Campo N, Chamberlain SR, Sahakian BJ, Robbins TW (2011) The roles of dopamine and noradrenaline in the pathophysiology and treatment of attention- deficit/hyperactivity disorder. Biol Psychiatry 69:145-157.
- Volkow ND, Swanson JM (2003) Variables that affect the clinical use and abuse of methylphenidate in the treatment of ADHD. Am J Psychiatry 160:1909-1918.
- Wilens TE, Morrison NR, Prince J (2011) An Update on the Pharmacotherapy of Attention-deficit/Hyperactivity Disorder in Adults. Expert Rev Neurother 11:1443-1465.
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, et al. (1998) A common network of functional areas for attention and eye movements. Neuron 21:761-773.
- Schall JD (2004) On the role of frontal eye field in guiding attention and saccades. Vision Res 44:1453-1467.
- Marendaz C, Guyader N, Malsert J (2007) What the eye tells us about the brain. Executive functions, ocular saccades and neuropsychology-neuropsychiatry. Rev Neurol 17:1-35.
- Driscoll GA, Alpert NM, Matthysse SW, Levy DL, Rauch SL, et al. (1995) Functional neuroanatomy of antisaccade eye movements investigated with positron emission tomography. Proc Natl Acad Sci 92:925-929.
- Munoz DP, Armstrong IT, Hampton KA, Moore KD (2003) Altered control of visual fixation and saccadic eye movements in attention-deficit hyperactivity disorder. J Neurophysiol 90:503-514.
- Mc Peek RM, Keller EL (2004) Deficits in saccade target selection after inactivation of superior colliculus. Nat Neurosci 7:757-763.
- McDowell JE, Dyckman KA, Austin BP, Clementz BA (2008) Neurophysiology and neuroanatomy of reflexive and volitional saccades: evidence from studies of humans. Brain Cogn 68:255-270.
- Bittencourt J, Velasques B, Teixeira S, Basile LF, Salles JI, Nardi AE, et al. (2013) Saccadic eye movement applications for psychiatric disorders. Neuropsychiatr Dis Treat 9:1393-1409.
- Feifel D, Farber RH, Clementz BA, Perry W, Anllo-Vento L (2004) Inhibitory deficits in ocular motor behavior in adults with attention-deficit/hyperactivity disorder. Biol psychiatry 56:333-339.
- Carr LA, Nigg JT, Henderson JM (2006) Attentional versus motor inhibition in adults with attention-deficit/hyperactivity disorder. Neuropsychology 20: 430-441.
- Schwerdtfeger RMH, Alahyane N, Brien DC, Coe BC, Stroman PW, et al. (2012) Preparatory neural networks are impaired in adults with attention-deficit/hyperactivity disorder during the antisaccade task. Neuroimage Clin 2:63-78.
- Frodl T, Skokauskas N (2012) Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects. Acta Psychiatr Scand 125: 114-126.
- Czerniak SM, Sikoglu EM, King JA, Kennedy DN, Mick E, et al. (2013) Areas of the brain modulated by single-dose methylphenidate treatment in youth with ADHD during task-based fMRI: a systematic review. Harv Rev Psychiatry 21:151-162.
- Klein C, Fischer B, Fischer B, Hartnegg K (2002) Effects of methylphenidate on saccadic responses in patients with ADHD. Exp Brain Res 145: 121-125.
- Bucci MP, Seassau M, Larger S, Bui-Quoc E, Gerard CL (2014) Effect of visual attention on postural control in children with attention- deficit/hyperactivity disorder. Res Dev Disabil 35: 1292-1300.
- Bucci MP, Stordeur C, Septier M, Acquaviva E, Peyre H, et al.(2017) Oculomotor Abnormalities in Children with Attention-Deficit/Hyperactivity Disorder Are Improved by Methylphenidate. J Child Adolesc Psychopharmacol 27: 274-280.
- Aman CJ, Roberts RJ, Pennington BF (1998) A neuropsychological exam- ination of the underlying deficit in attention deficit hyperactivity disorder: Frontal lobe versus right parietal lobe theories. Dev Psychol 34: 956-969.
- Mostofsky SH, Lasker AG, Cutting LE, Denckla MB, Zee DS (2001) Oculomotor abnormalities in attention deficit hyperactivity disorder: A preliminary study. Neurology 57: 423-430.
- Kooij JJS, Francken MH (2010): Diagnostic interview for ADHD in adult (DIVA 2.0) Diagnostisch Interview Voor ADHD bij volwassenen. DIVA Foundation, The Netherlands.
- Kessler RC, Adler L, Ames M, Demler O, Faraone S, et al. (2005) The World Health Organization Adult ADHD Self-Report Scale (ASRS): A short screening scale for use in the general population. Psychological Medicine 35: 245-256.
- Norris H (1971) The action of sedatives on brain stem oculomotor systems in man. Neuropharmacol 10: 181-191.
- Bond A, Lader M (1974): The use of analogue scales in rating subjective feelings. Br J Med Psychol 47: 211-218.
- Klein CH, Raschke A, Brabenbush A (2003) Development of pro- and antisaccades in children with attention-deficit hyperactivity disorder (ADHD) and healthy HCs. Psychophysiology 40:17-28.
- Metz CE (1978) Basic principles of ROC analysis. Semin Nucl Med 8: 283-298.
- Munoz DP, Everling S (2004) Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci 5: 218-228.
- Jantz JJ, Watanabe M, Everling S, Munoz DP (2013) Threshold mechanism for saccade initiation in frontal eye field and superior colliculus. J Neurophysiol 109: 2767-2780.
- Nigg JT, Butler KM, Huang-Pollock CL, Henderson JM (2002) Inhibitory processes in adults with persistent childhood onset ADHD. J Consul Clin Psychol 70: 153–157.
- Patel VR, Zee DS (2015) The cerebellum in eye movement control: nystagmus, coordinate frames and disconjugacy. Eye (Lond) 2:191-195.
- Munoz D, Hampton K, Moore K, Goldring JE (1999) Control of purposive saccadic eye movements and visual fixation in children with Attention-Deficit Hyperactivity Disorder. Current Oculomotor Research 415-423.
- Jenson D, Yang K, Acevedo-Rodriguez A, Levine A, Broussard JI, et al. (2015) Dopamine and norepinephrine receptors participate in methylphenidate enhancement of in vivo hippocampal synaptic plasticity. Neuropharmacology 90:23-32.
- Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, et al. (2006) Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry 60:1111-1120.
- Fried M, Tsitsiashvili E, Bonneh YS, Sterkin A, Wygnanski-Jaffe T, et al. (2014) ADHD subjects fail to suppress eye blinks and microsaccades while anticipating visual stimuli but recover with medication. Vision Res 101: 62-72.
- Castells X, Ramos-Quiroga JA, Rigau D, Bosch R, Nogueira M, et al. (2012) Efficacy of methylphenidate for adults with attention-deficit hyperactivity disorder: a meta-regression analysis. CNS Drugs 25:157-169.
- Volkow ND, Wang GJ, Fowler JS, Telang F, Maynard L, Logan J, et al. (2004) Evidence that methylphenidate enhances the saliency of a mathematical task by increasing dopamine in the human brain. Am J Psychiatry 161: 1173-1180.
- Volkow ND, Fowler JS, Wang G-J, Telang F, Logan J, Wong C, et al. (2008) Methylphenidate decreased the amount of glucose needed by the brain to perform a cognitive task. PLoS One 3: e2017.
- Allman AA, Ettinger U, Joober R, O'Driscoll GA (2012) Effects of methylphenidate on basic and higher-order oculomotor functions. J Psychopharmacol 26:1471-1479.
- Mignogna P, Viggiano D (2010) Brain distribution of genes related to changes in locomotor activity. Physiol Behav 99: 618-626.
- Viggiano D, Travaglio M, Cacciola G, Di Costanzo A. (2014) Effect of backward walking on attention: possible application on ADHD. Transl Med UniSa.11:48-54.
Citation: Duval F, Mokrani MC, Erb A, Seassau M, Carcangiu R, et al. (2018) Effects of a Single Dose of Methylphenidate on Saccadic Eye Movements in Adults with Attention-Deficit/Hyperactivity Disorder. Neurosci Psychiatry 1:105.
Copyright: © 2018 Duval F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 4815
- [From(publication date): 0-2018 - Nov 30, 2025]
- Breakdown by view type
- HTML page views: 3809
- PDF downloads: 1006