Effects of Antiangiogenic Agent Bevacizumab on Over Cancer Cell Culture (Ovcar-3) Alone and Combined with Classic Chemotherapeutics
Received: 20-Sep-2021 / Accepted Date: 05-Oct-2021 / Published Date: 11-Oct-2021 DOI: 10.4172/2476-2024.1000022
Abstract
Background: Cancer is an important problem that has serious effects on the quality of life. Despite advances in diagnosis, it still affects millions of people. Ovarian cancer is the most common cause of gynecological cancer deaths; it has a very important place in gynecological oncology. Combination therapy is a treatment method that combines more than one agent used in treatment. Today it is one of the most important weapons in cancer treatment.
Methods: In this study, the aim was to examine the sole and combined effects of classical chemotherapeutics and Bevacizumab on Ovcar-3 cells in vitro.
Results: To determine the effects of classical chemotherapeutics, and Bevacizumab on Ovcar-3 cells, MTT, DAPI staining, caspase-3 and real-time PCR analyses was performed. In the light of the data obtained from the MTT results, it was determined that bevacizumab did not have any effect on ovcar-3 cells, so DAPI staining and caspase-3 analyses were not conducted with this substance. It was observed that the combination of paclitaxel 2.5 nM+carboplatin 100 μM caused a decrease on IRS, COX-2 and VEGF gene levels. Bevacizumab was not included in the analysis as it was found to have no effect on ovcar-3 cells.
Conclusion: In this study, classical chemotherapeutics sole and in combination showed significant anticancer effects in MTT, DAPI Staining, Caspase-3 and Real-Time PCR analyses in Ovcar-3 cancer cell lines. However, the same effect was not observed with Bevacizumab and this is different from some results in the literature. Researching this subject with new studies will contribute to the literature.
Keywords
Bevacizumab; Cancer therapy; Combination therapy; Ovcar-3 cell lines
Introduction
Ovarian cancer causes more deaths than any other type of cancer diagnosed in the female reproductive system. In ovarian malignancies, which have an extremely important place in terms of general women's health, the main treatment is surgery and subsequent chemotherapy. Cisplatin, carboplatin, cyclophosphamide, and paclitaxel are effective drugs alone against ovarian cancer. Among these drugs, the combination of platinum with taxanes is preferred [1-4].
Advances made in recent years have made it possible for us to examine the complex interactions of numerous genes responsible for cancer. However, despite these studies, success in cancer treatment is low. The biggest challenge we face in cancer treatment today is the lack of anticancer drugs with high efficacy, broad-spectrum and low side effects. In this respect, innovative approaches in drug development are extremely important [5,6].
The advantage of in vitro cell studies is the consistency and reproducibility of the results obtained. Physical/chemical environment and physiological conditions such as cell cultures, temperature, pressure, pH, oxygen and carbon dioxide can be controlled very precisely. Cell culture is most commonly used for examining cell biology, researching disease mechanisms, and drug research [7,8].
Combination therapy is a treatment method that combines two or more therapeutic agents. This method is the cornerstone of today's cancer treatment. The combination of anti-cancer drugs is more effective than single therapy because the effect is achieved on cancer cells synergistically or in an additional way. Thus, drug resistance and metastatic potential decrease, mitotic activity regresses, cancer stem cell density decreases, and apoptosis is activated. The advantage of combination therapy is that cancer cells cannot adapt to the toxic effects of multiple therapeutic agents simultaneously targeting multiple pathways [9,10].
Bevacizumab is a monoclonal antibody that targets VEGF-A. It is the first angiogenesis inhibitor approved for use. VEGF not only controls angiogenesis but also regulates tumor-induced immunosuppression. These recently identified immune-modulatory roles of VEGF have made Bevacizumab an important alternative for combination therapies [11,12].
In this study, the aim was to investigate the alone and combined effects of classical chemotherapy drugs (carboplatin and paclitaxel) and angiogenesis inhibitor bevacizumab on ovcar-3 cells by applying in vitro tests. For this purpose, cytotoxic and apoptotic effects of combined therapy were examined. The examination was carried out with the Tetrazolium Test (MTT assay). Apoptotic effects were examined by DAPI staining and caspase-3 Elisa colorimetric kit test. In addition, the expression of IRS-1 (insulin receptor substrate-1), Ki-67 (kinase inhibitor-67), VEGF (Vascular endothelial growth factor), and COX-2 (cyclooxygenase-2) genes was investigated by Real-Time PCR.
Materials and Methods
Preparation of materials
Glass and plastic materials and liquid solutions used in the studies were kept in an autoclave at 121°C for 20 minutes at 1.5 atm/Hg pressures, and at 180°C for 2 hours in a sterilizer. Some liquid chemicals used were passed through a 0.2 mm spaced cellulose nitrate filter.
Preparation of drug dosages
Drug doses were prepared by dissolving in Dimethyl sulfoxide (DMSO) (1:40 ratio). Doses were used as soon as they were prepared. The doses to be used were determined using the information obtained from literature reviews.
Cells
Ovcar-3 cells were purchased from American Type Culture Collection (ATCC). Ovcar-3 cells were grown in 20% Fetal Bovine Serum, penicillin-streptomycin, sodium bicarbonate, MEM Nonessential amino acid solution, RPMI 1640 medium at 37°C in a medium containing 5% CO2.
MTT assay
After determining the viability of the cells by Trypan Blue staining, the cells were counted with Thoma slide and cultured in 96-well plates at 5 × 104 cells per well for 24 hours. The medium in the wells was emptied and the media containing different concentrations of test substances were placed in the plates. After incubation period, the media were removed from the treated cells for the periods determined by the test substances (24 hours, 24, and 48 hours, 72 hours). The cells were incubated with 5 mg/ml-1 MTT solution for two hours to convert the MTT dye to the water-insoluble formazan salt. MTT dye was removed from the cells. 0.1 ml DMSO was added to each well to dissolve the formazan salts formed by living cells. Optical densities of the cells in the plates were read on an ELISA device (Spectra max 340 PC Molecular Devices, LLC USA) at a wavelength of 570 nm. The viability rates of the test cells are expressed as a percentage, assuming the control cell viability rate not treated with the test substance as 100%. Experiments were repeated three times independently of each other. IC50 doses determined by applying the agents alone were used in combinations.
Apoptosis assay
Morphological examination with fluorescent staining (DAPI staining): Cells were seeded into six-well plates with sterile round lamellas and cultured in a 24-hour CO2 incubator. The medium in the wells was removed. The effective doses of the agents determined as a result of the cytotoxicity tests were applied to the cells adhered on the lamellae for 12 hours. The medium was then removed from the wells, lamellas were washed with sterile phosphate buffer solution (PBS),detected for 15 minutes at 37°C in 3.7% paraformaldehyde solution dissolved in PBS. After detection, lamellas were washed three times with PBS and incubated for 30 minutes at 37°C with 1 mg/ml DAPI in a dark environment. The lamellas were then washed with PBS and capped and photographed under a fluorescent microscope.
Caspase 3 analysis
Cells were seeded into six-well plates with sterile round lamellas and cultured in a 24-hour CO2 incubator. The medium in the wells was removed. The effective doses of the agents determined as a result of the cytotoxicity tests were applied to the cells adhered on the lamellae for 12 hours. The medium was then removed from the wells, lamellas were washed with sterile phosphate buffer solution (PBS), detected for 15 minutes at 37°C in 3.7% paraformaldehyde solution dissolved in PBS. After detection, lamellas were washed three times with PBS and incubated for 30 minutes at 37°C with 1 mg/ml DAPI in a dark environment. The lamellas were then washed with PBS and capped and photographed under a fluorescent microscope.
Real-time PCR
RNA isolation: Ovcar-3 cells were cultivated in 75 cm2 flasks as 1 × 104 cells and incubated for 48 hours. Following the incubation, cells were exposed to the specified concentrations of substances based on the data obtained from the results of the MTT assay. After the cells were treated with the substances for 24 hours, the cells were collected into the centrifuge tube with the help of PBS, PBS-EDTA, trypsin together with the supernatant. By using Thoma slide, 4 × 106 cells were taken and the supernatant was centrifuged at 1250 rpm for six minutes. Buffer RTL plus+Beta mercaptoethanol mixture was placed on the cells and placed in columns holding genomic DNA and centrifuged at 10,000 rpm for 1.5 minutes. After repeated centrifugation, by throwing the upper column, the underlying RNAs were measured and stored at (-80°C) until the process was performed for real-time PCR. Real-time PCR analysis was performed on BioRad, Hercules, California, USA.
RNA concentration measurement
To determine the amount and purity of the RNA, the isolated RNA was measured in a nanodrop device; DNAse was diluted with RNAse free water.
cDNA synthesis
For cDNA synthesis, the reaction was prepared with a total volume of 20 μl and cDNA synthesis was performed on a palm cycler device.
Relative quantification
In our study, while interpreting our real-time PCR results, the concentration value of our target genes was proportioned to the concentration value of the reference gene, and how much the results obtained differed compared to the control group were examined. The housekeeping or internal control genes encodes proteins involved in cell functioning and is therefore always expressed. The 18S rRNA gene is one of the frequently preferred internal control genes in Real Time-PCR studies related to cancer studies. The 18S rRNA gene was used for this purpose in our study [13].
SPSS program was used in the statistical evaluation of the results. One-way ANOVA and Tukey test as a post-hoc were used. The significance limit was determined as p<0.05.
Results
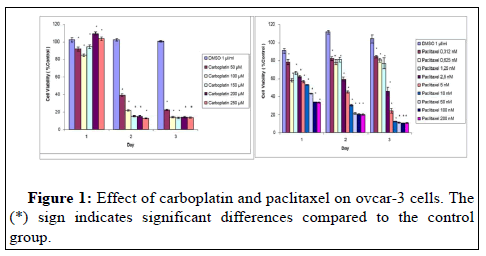
At the end of 72 hours, a significant decrease was observed in all doses of carboplatin according to control. This reduction is 20% at 50 μM and 18% in the range of 100-250. At the dose of 0.312 μM paclitaxel, 82% viability was detected in the cells at the end of 24-48 and 72 hours. In the first 24 hours there was a 67% reduction in cell viability at the doses of 100 μM and 200 μM (Figure 1).
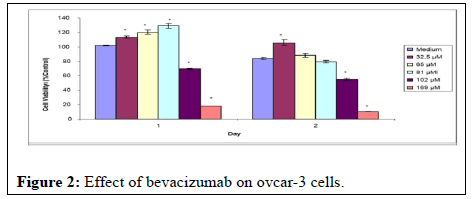
As a result of the MTT assays, it was observed that bevacizumab did not affect ovcar-3 cells. Although the doses of the drug with a concentration of 25 mg/ml used during the experiments were given as high as possible, doses of 32.5, 65, and 81 μM on the cells did not prevent cell proliferation. Although some decrease was observed in doses of 102 and 169 μM, which are quite high compared to the literature, this decrease is thought to be related to the lack of nutrients of the cells during exposure to the substance (Figure 2).
It was observed that the combination of carboplatin 150 μM +Paclitaxel 2.5 nM reduced cell viability by 46% at the end of 24 hours. It was determined that the cell viability was 9% after 48 hours of treatment with the combination of carboplatin 250 μM+paclitaxel 200 nM. The reductions seen with different combination doses are at a similar level (Figure 3). The combination of carboplatin, paclitaxel and bevacizumab did not have a significant effect on cell viability. No cytotoxic effect of bevacizumab was detected in ovcar-3 cells in combination as in treatment alone.
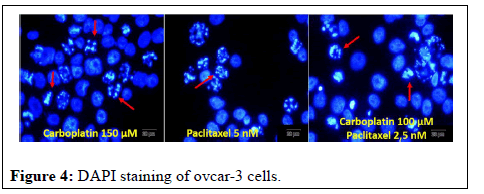
Arrows in photographs of ovcar-3 cells where carboplatin and paclitaxel were applied alone and together indicate apoptotic cells. In apoptotic cells, it has been observed that the apoptotic cell nuclei are fragmented into small pieces; the nuclei are strangulated and condensed. It was determined that bevacizumab did not have any effect on ovcar-3 cells, so DAPI staining was not performed (Figure 4).
The effects of paclitaxel and carboplatin alone and together on the caspase-3 activities of ovcar-3 cells after 24 and 12 hours of treatment are shown in Figure 5. After twelve hours of treatment, it was observed that 2.5 and 5 nM doses of paclitaxel caused a greater increase in caspase-3 activity compared to the combination (Figure 5). It was determined that bevacizumab did not have any effect on ovcar-3 cells, so caspase-3 experiments were not conducted with this substance.
Ovcar-3 cells were treated with different doses and combinations of paclitaxel and carboplatin for 24 hours. After treatment, analysis was performed on IRS, Ki-67, COX-2 and VEGF genes by rt-PCR technique in the cell line. According to the results, 50 μM, 100 μM and 150 μM doses of carboplatin and 1.25 nM, 2.5 nM and 5 nM doses of paclitaxel caused a decrease in COX-2 level. It was observed that the combination of paclitaxel 2.5 nM+carboplatin 100 μM caused a decrease on IRS, COX-2 and VEGF gene levels. The data obtained in the combined applications showed that they are not as effective on gene activations as single applications. (Bevacizumab was not included in the analysis as it was found to have no effect on ovcar-3 cells.
Discussion
Drug candidates that fail in the clinical development process cause serious financial investment, resource and time losses. For this reason, studies conducted in cell culture can increase the chance of success as well as prevent waste of resources. In drug development studies, pharmacological activities of drug candidates are almost always tested in cell culture [14,15].
Apoptosis is involved in many biological processes and is one of the most frequently used areas of research on cell biology. Apoptosis can be accurately detected with the MTT staining method. DAPI staining method is frequently used to determine the apoptotic effect morphologically. Caspase chain has an important role in the regulation of apoptosis. The released cytochrome C activates caspase-3 [16].
Paclitaxel acts by preventing cell division and polymerization through microtubules, thus apoptosis occurs. Paclitaxel also has angiogenic inhibitory effects by suppressing VEGF expression. Carboplatin is an anticancer drug it triggers apoptosis. Apoptosis acts through the activation of a family of cysteine proteases called caspases. Apoptosis occurs with the activation of caspases such as caspases 3 and 7 [17-20].
In our study, carboplatin reduced the viability of ovcar-3 cells at all doses on the second and third days. In similar studies in the literature, the results are similar to those obtained in our study. In some randomized clinical studies, Paclitaxel combination with cisplatin or carboplatin was compared in patients with advanced ovarian cancer. It has been observed that the treatment regimen in which carboplatin is used in combination is more tolerable. According to the results of another study, the viability of cells treated with Docetaxel +carboplatin, docetaxel+PNP-GDEPT and carboplatin+PNP-GDEPT and the combination of three agents was examined. The greater reduction occurred in the case of the combination of all three. Increased sensitivity of resistant ovcar-3 cells to docetaxel and carboplatin has been observed when included in PNP-GDEPT combination regimens. It has been found that this occurs through increased apoptosis as a result of down-regulation of genes responsible for the drug resistance mechanism [21,22].
In our study, paclitaxel reduced the viability of ovcar-3 cells at all doses on the first, second and third days. The results obtained in similar studies are similar to the results of our study. In a study, MCTS (multicellular tumor spheroids) were used because conventional cell culture methods may not fully reflect the clinical features of the disease. Paclitaxel showed a much weaker effect in MCTS compared to 2D cultures. Following this process, licofelone, which is predicted to have a synergistic effect with paclitaxel, was combined with paclitaxel and applied to the cells. The combination has been found to have a significant synergistic effect [23].
In our study, it was observed that the combination of paclitaxel +carboplatin showed the most significant effect in the first 24 hours at low doses (2.5 nM and 50 μM, respectively).
In another study, paclitaxel was combined with Silibinin and applied to ovarian cancer cells. MTT analysis was performed. The results showed that cell proliferation was sharply inhibited by paclitaxel. Combined therapy, on the other hand, had a greater effect than drugs alone. The doses used in the combination are IC50 dose determined for paclitaxel and lower than IC50 for Silibinin. This means that the drugs are used in combination at low doses. The P53 and P21 gene expression at different concentrations of the combination of Silibinin and paclitaxel showed a significant difference compared to control cells [24].
In our study, it was determined that paclitaxel and carboplatin increase the activity of caspase-3 alone or in combination. It has been observed that this increase is more pronounced at the end of 12 hours than at the end of 24 hours. After twelve hours, the most significant increase was noted in paclitaxel at a dose of 5 nM. At the end of 24 hours, it was observed in 50 μM dose of carboplatin. This increase in paclitaxel and carboplatin is more pronounced than combined applications.
In our study, it was determined that paclitaxel and carboplatin increase the activity of caspase-3 alone or in combination. After twelve hours, the most significant increase was noted in paclitaxel at a dose of 5 nM. At the end of 24 hours, it was observed in 50 μM dose of carboplatin. This increase in paclitaxel and carboplatin is more pronounced than combined applications.
In a study in the literature, the results of MTT and caspase-3 analysis were examined by combining carboplatin and paclitaxel with another drug. The results demonstrated the superiority of combination therapy over individual treatments at each dose in cell viability. The interesting finding found here was that the greatest reduction in cell viability occurred at lower doses compared to higher doses. Similar determinations were made in our research, an example of this is that in the caspase analysis, carboplatin at a dose of 100 μM and paclitaxel at a dose of 5 nM give more effective results than combinations. In the same study, expression of annexin V and caspase 3 activity were investigated. Significant increases in annexin V expression and caspase-3 activity were detected after carboplatin and paclitaxel treatment. When the agent which is the third member of the combined therapy was added to the combination, a decrease in the increase in caspase-3 activity was observed. In the analysis of gene expressions of our study, it was observed that the decrease effect on gene activations recorded in single applications was more than combined applications [25].
Bevacizumab is an anti-VEGF monoclonal IgG 1 antibody. In combination with paclitaxel, it is indicated in metastatic breast cancer. Today, it is approved for use in the combined treatment of ovarian cancer [26-29].
There are limited studies on the use of antiangiogenic agents and chemotherapy in ovarian cancer cell culture. A study examined the effects of a combination of topotecan and bevacizumab on topotecanresistant ovarian cancer cell cultures. Seventeen ovarian cancer cell cultures were treated with topotecan at different concentrations and times. Six of them were treated with the combination of topotecan +bevacizumab. It was observed that resistance to topotecan treatment was prevented by bevacizumab combined with topotecan at the end of 48 and 96 hours of application [30].
There are also studies showing resistance to bevacizumab. The specific molecular mechanisms that cause resistance are not fully understood yet. Studies have shown that EphB4 overexpression is responsible for resistance. According to another study, bevacizumab resistance can be induced by activating Akt phosphorylation in ovarian cancer cells. In another study, it was reported that high hypoxia-inducible factor-1α (HIF1α) upregulates bevacizumab resistance genes, limiting the efficacy of bevacizumab targeting the VEGF pathway [31-33].
It is known that angiogenesis induced by VEGF plays a key role in cancer development. IRS-1 supports tumor growth, but the mechanism is not fully understood. Ki-67 expression is associated with common histopathological parameters. COX-2, catalyzes the first step in the formation of prostaglandins [34-37].
The results obtained in our study showed that paclitaxel and carboplatin, when used alone, affect the factors mentioned above to decrease. These results are in line with the findings obtained in previous studies on the subject. In our study, different from other studies, various combinations of paclitaxel and carboplatin did not show the expected effect on the factors.
Conclusion
In our study, bevacizumab showed no anticancer effects in MTT, DAPI Staining, caspase-3 and Real-Time PCR analyses in ovcar-3 cancer cell lines alone and combined. This is different from some results in the literature. Researching this subject with new studies will contribute to the literature.
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, et al. (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68: 394-424.
- Devis-Jauregui L, Eritja N, Davis ML, Matias-Guiu X, Llobet-Navàs D (2020) Autophagy in the physiological endometrium and cancer. In: Autophagy: 1-19.
- Moro Visconti R, Morea D (2019) Big data for the sustainability of healthcare project financing. Sustain 11: 1-17.
- McMullen M, Karakasis K, Rottapel R, Oza AM (2021) Advances in ovarian cancer, from biology to treatment. Nat Cancer 2: 6-8.
- Kumar BS, Maria S, Shejila CH, Udaykumar P (2018) Drug utilization review and cost analysis of anticancer drugs used in a tertiary care teaching hospital. Indian Journal of Pharmaceutical Sciences 80: 686-693.
- Chakraborty S, Rahman T (2012) The difficulties in cancer treatment. Ecancermedicalscience 6: 1-5.
- Jaroch K, Jaroch A, Bojko B (2018) Cell cultures in drug discovery and development: The need of reliable in vitro-in vivo extrapolation for pharmacodynamics and pharmacokinetics assessment. J Pharm Biomed Anal 147: 297-312.
- Segeritz CP, Vallier L (2017) Cell culture: Growing cells as model systems in vitro. In: Basic Science Methods for Clinical Researchers.
- Mokhtari RB, Homayouni TS, Baluch N, Morgatskaya E, Kumar S, et al. (2017) Combination therapy in combating cancer. Oncotarget 8: 38022.
- Çetin B (2019) Sağlık Hizmetlerinde Nesnelerin İnterneti Uygulamalarının Kullanımı.
- Costantini L, Molinari R, Farinon B, Merendino N (2020) Retinoic acids in the treatment of most lethal solid cancers. J Clin Med 9: 360.
- Garcia J, Hurwitz HI, Sandler AB, Miles D, Coleman RL, et al. (2020) Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat Rev 86: 102017.
- Kuchipudi SV, Tellabati M, Nelli RK, White GA, Perez BB, et al. (2012) 18S rRNA is a reliable normalisation gene for real time PCR based on influenza virus infected cells. Virol J 9: 1-7.
- Ghanemi A (2015) Cell cultures in drug development: Applications, challenges and limitations. Saudi Pharm J SPJ 23:453.
- Lovitt CJ, Shelper TB, Avery VM (2014) Advanced cell culture techniques for cancer drug discovery. Biology (Basel) 3: 345-367.
- Kumar N, Afjei R, Massoud TF, Paulmurugan R (2018) Comparison of cell-based assays to quantify treatment effects of anticancer drugs identifies a new application for Bodipy-L-cystine to measure apoptosis. Sci Rep 8: 1-11.
- Boyd LR, Muggia FM (2018) Carboplatin/paclitaxel induction in ovarian cancer: The finer points. Oncol 32: 418-424.
- Kampan NC, Madondo MT, McNally OM, Quinn M, Plebanski M (2015) Paclitaxel and its evolving role in the management of ovarian cancer. Biomed Res Int 2015: 1-21.
- Fleming GF, Brunetto VL, Cella D, Look KY, Reid GC, et al. (2004) Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: A gynecologic oncology group study. J Clin Oncol 22: 2159-2166.
- Thigpen JT, Blessing JA, Homesley H, Creasman WT, Sutton G (1989) Phase II trial of clsplatin as first-line chemotherapy in patients with advanced or recurrent endometrial carcinoma: A gynecologic oncology group study. Gynecol Oncol 33: 68-70.
- Singh PP, Joshi S, Russell PJ, Nair S, Khatri A (2011) Purine nucleoside phosphorylase mediated molecular chemotherapy and conventional chemotherapy: A tangible union against chemoresistant cancer. BMC Cancer 11: 1-15.
- Bicaku E, Xiong Y, Marchion DC, Chon HS, Stickles XB, et al. (2012) In vitro analysis of ovarian cancer response to cisplatin, carboplatin, and paclitaxel identifies common pathways that are also associated with overall patient survival. Br J Cancer 106: 1967-1975.
- Hirst J, Pathak HB, Hyter S, Pessetto ZY, Ly T, et al. (2018) Licofelone enhances the efficacy of paclitaxel in ovarian cancer by reversing drug resistance and tumor stem-like properties. Cancer Res 78: 4370-4385.
- Pashaei-Asl F, Pashaei-Asl R, Khodadadi K, Akbarzadeh A, Ebrahimie E, et al. (2018) Enhancement of anticancer activity by silibinin and paclitaxel combination on the ovarian cancer. Artif Cells Nanomedicine Biotechnol 46: 1483-1487.
- Wahba J, Natoli M, Whilding LM, Parente-Pereira AC, Jung Y, et al. (2018) Chemotherapy-induced apoptosis, autophagy and cell cycle arrest are key drivers of synergy in chemo-immunotherapy of epithelial ovarian cancer. Cancer Immunol Immunother 67: 1753-1765.
- Kazazi-Hyseni F, Beijnen JH, Schellens JHM (2010) Bevacizumab. Oncologist 15: 819.
- Chappell NP, Miller C, Barnett J, Fielden A (2016) Is FDA approved Bevacizumab cost-effective in the setting of platinum-resistant recurrent ovarian cancer? Obstet Gynecol 127: 6S-7S.
- Summers J, Cohen MH, Keegan P, Pazdur R (2010) FDA drug approval summary: Bevacizumab plus interferon for advanced renal cell carcinoma. Oncologist 15: 104.
- Arora S, Balasubramaniam S, Zhang H, Berman T, Narayan P, et al. (2021) FDA approval summary: Olaparib monotherapy or in combination with Bevacizumab for the maintenance treatment of patients with advanced ovarian cancer. Oncologist 26: e164-172.
- Cui S, Lancaster JM, Chan G, Wenham RM (2006) Combination topotecan and bevacizumab causes growth inhibition of ovarian cancer cells that express VEGF. Cancer Res 66: 1237.
- Guerrouahen BS, Pasquier J, Kaoud NA, Maleki M, Beauchamp M-C, et al. (2014) Akt-activated endothelium constitutes the niche for residual disease and resistance to bevacizumab in ovarian cancer. Mol Cancer Ther 13: 3123-3136.
- Pham E, Birrer MJ, Eliasof S, Garmey EG, Lazarus D, et al. (2015) Translational impact of nanoparticle-drug conjugate CRLX101 with or without bevacizumab in advanced ovarian cancer. Clin Cancer Res 21: 808-818.
- Li L, Nan F, Guo Q, Guan D, Zhou C (2019) Resistance to bevacizumab in ovarian cancer SKOV3 xenograft due to EphB4 overexpression. J Cancer Res Ther 15: 1282-1287.
- Li X, Gao Y, Li J, Zhang K, Han J, et al. (2018) FOXP3 inhibits angiogenesis by downregulating VEGF in breast cancer. Cell Death Dis 9: 1-12.
- Inwald EC, Klinkhammer-Schalke M, Hofstädter F, Zeman F, Koller M, et al. (2013) Ki-67 is a prognostic parameter in breast cancer patients: Results of a large population-based cohort of a cancer registry. Breast Cancer Res Treat 139: 539-552.
- Ghosh N, Chaki R, Mandal V, Mandal SC (2010) COX-2 as a target for cancer chemotherapy. Pharmacol reports 62: 233-244.
- Reiss K, Del Valle L, Lassak A, Trojanek J (2012) Nuclear IRS-1 and cancer. J Cell Physiol 227: 2992-3000.
Citation: Gursoy OO, Eren CY, Gurer HG, Koparal AT, Ozalp SS (2021) Effects of Antiangiogenic Agent Bevacizumab on Over Cancer Cell Culture (Ovcar-3) Alone and Combined with Classic Chemotherapeutics. Diagn Pathol Open S6: 022. DOI: 10.4172/2476-2024.1000022
Copyright: © 2021 Gursoy OO, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 2490
- [From(publication date): 0-2021 - Dec 11, 2025]
- Breakdown by view type
- HTML page views: 1780
- PDF downloads: 710