Effects of Defensive Style Nordic Walking Intervention in Patients with Lumbar and Lower-Limb Osteoarthritis
Received: 08-Mar-2018 / Accepted Date: 13-Mar-2018 / Published Date: 14-Mar-2018 DOI: 10.4172/2165-7025.1000383
Abstract
Regular exercise is effective for improving physical functions and pain relief in patients with lumbar and lowerlimb osteoarthritis. However, some of the patients have difficulty in performing land-based exercises due to disease status. This study focused on defensive style Nordic walking as a land-based exercise the patients can perform and aimed to investigate the effects of defensive style Nordic walking intervention on functional performance and pain in patients with lumbar and lower-limb osteoarthritis with low walking capacity. After 10 weeks of non-intervention, thirteen patients participated in the defensive style Nordic walking intervention for 10 weeks. The sixty-minute Nordic walking exercise was conducted once a week. Self-reported maximum walking distance without a break of the patients was less than 1 km and eight patients usually use a cane while walking. Functional performance measurement was conducted before non-intervention, after non-intervention (before intervention), and after intervention periods. Pain was measured using visual analog scale before and after each exercise session. Indices to estimate mobility, lower-limb muscle strength, and balance ability were improved by the intervention as compared with the non-intervention. Pain score decreased immediately after one session of defensive style Nordic walking and throughout the intervention period. These results suggest that the defensive style Nordic walking is an effective landbased exercise to improve mobility, lower-limb muscle strength, balance, and pain relief in patients with lumbar and lower-limb osteoarthritis with low walking capacity.
Keywords: Nordic walking; Osteoarthritis; Functional performance; Pain
Abbreviations
OA: Osteoarthritis; NW: Nordic Walking; ADL: Ability of Daily Living; SD: Standard Deviation; TUG: Timed Up and Go; FR: Functional Reach; COP: Center of Pressure; VAS: Visual Analog Scale; ANOVA: Analysis of Variance; EIA: Exercise-Induced Analgesia
Introduction
Osteoarthritis (OA) in the lumbar and lower limbs causes impaired physical functions and pain [1,2]. Combined with physiological aging, these physical conditions deteriorate walking capacity and activities of daily living (ADL) [2,3], which may lead to disabilities and need for nursing care [1,4].
Exercise is one of the effective interventions to improve physical functions and reduce pain for patients with OA [5]. Aquatic exercises are recommended for patients with OA, as they can be performed under low-gravity conditions with low impact on the joints. Meanwhile, land-based exercises have the advantage that patients can perform the exercises inside or near patients’ house, which requires less trouble of getting out to pool facilities. However, land-based exercises are often difficult for patients with OA, especially those with severe symptoms, due to multiple factors, such as pain, limited range of motion, low muscle strength, and fear of falling. We focused on walking with two poles, which is called Nordic walking (NW), as a land-based exercise for patients with OA. During NW, arms and legs move alternately: when the left foot is forward, the right hand is also forward and the pole held with the right hand is planted, and vice versa. In most of cases, walkers push the poles obliquely backward and gain propulsive force, which leads to increase in arm muscle activation [6], wider stride length [7], and eventually higher heart rate and energy expenditure [8,9] compared to normal walking. In this technique, using dedicated poles, the arm is outstretched and the palm of the hand is opened to release the pole after pushing it backward (pole backward style NW). Another technique of using the poles in NW is to stretch an arm forward and plant a pole straight in front of the body. This method is similar to using sticks and canes as walking aids for individuals with difficulty in walking. It is called defensive style NW or pole walking.
Most of studies on NW have been conducted using the pole backward style NW and few studies with the defensive style NW. These studies reported that vertical ground reaction forces [7,10], vertical knee joint compressive forces [7], and knee varus moment [10] during defensive style NW were lower than those during normal walking, although there is no consensus about the reduction of joint load during pole backward style NW. Therefore, defensive style NW can be a suitable land-based exercise for patients with OA with reduced load on joints in the lumbar and lower limbs.
To the best of our knowledge, there have been no reports investigating the effects of defensive style NW intervention in patients with OA. With regard to pole backward style NW, a study recently reported that more than 3.0 km NW at an intensity of 12-14 on the Borg scale of perceived exertion (around “somewhat hard”) for 4 months improved functional performance, mental health, and pain relief in patients with hip OA [11]. In general, some OA patients can implement this intervention, but others may not due to impaired walking capacity (e.g., difficulty in walking long distances at moderate intensity). Therefore, the present study aimed to investigate the effects of defensive style NW on functional performance and pain in patients with lumbar and lower-limb OA with low walking capacity.
To prevent the worsening of symptoms due to exercise, we provided supervised and reduced amount of intervention. Therefore, we did not expect to observe the effects of aerobic exercise, such as increase in aerobic capacity and reduction of body fat and serum lipid concentration, but the effects on functional performance, including mobility, lower-limb muscle strength, and balance ability, and pain relief were investigated.
Previous studies on normal walking intervention for patients with knee OA reported improvement in maximal walking speed [12], stride length [12], lower-limb muscle strength [13,14], postural sway during quiet standing [15], and pain relief [13,16,17]. Another study demonstrated improvement in 50-foot walking time and pain relief after a 12-week normal walking intervention in patients with rheumatoid arthritis and lower-limb OA [18]. Additionally, a review article recommended walking exercise to reduce chronic musculoskeletal pain [19].
Pole backward style NW also improved 8-foot Up and Go score, 30- s chair stand test, and pain relief in patients with hip OA [11]. For patients with Parkinson’s disease, NW intervention improved maximal walking speed [20,21], Timed Up and Go (TUG) score [20,22], balance ability [21,22], and pain relief [21]. Based on these previous studies, although they did not use defensive style NW, we hypothesized that defensive style NW intervention had the potential to improve functional performance and reduce pain in patients with lumbar and lower-limb OA. It is important to improve functional performance and reduce pain for patients with OA because these factors are closely related to ADL [2,3].
Materials and Methods
Participants
Patients were recruited through advertisement at some senior centres, adult day cares, and in local newspapers. The eligibility criteria were patients who had a diagnosis of OA in the knee, hip, or lumbar, had difficulty in walking long distances, and did not have cardiovascular disease. Among 28 applicants, patients who had complications with neurological diseases (post-polio syndrome n=2; contusio cerebri n=1; cerebral infarction n=1) and whose self-reported walking distance without break was more than 2-3 km (n=9) were excluded from our study population. Two patients withdrew their application after they got detailed explanation. Finally, 13 patients (2 males and 11 females) volunteered to participate in this study (lumbar spinal canal stenosis n=4; knee OA n=4; hip OA n=5). Eight of them usually used a cane while walking. The average age, height, and weight of the subjects were 71.0 ± 8.2 years (means ± SD), 152.2 ± 3.8 cm, and 54.9 ± 9.6 kg, respectively. All participants were inexperienced in NW. The study was designed and conducted according to the Helsinki Declaration and was approved by the Research Ethics Committee of the University of Tokyo. Written informed consent was obtained from all subjects prior to the commencement of the study.
Protocol and study design
The defensive style NW intervention was conducted one session per week for 10 weeks. Subjects spent 10 weeks of usual life (control period) before participating in the NW intervention (intervention period). Measurements of functional performance were conducted three times: before the control period, after the control period (before the intervention period), and after the intervention period. The individual disease status and functional performance of patients with OA vary considerably. Therefore, we compared the change of functional performance during the intervention period with that during the control period to verify the effects of NW intervention rather than establishing a control group with same disease.
Defensive style Nordic walking intervention
The ten-week NW intervention was supervised by a professional instructor. One session of exercise lasted for 60 min, consisting of warming up (static and dynamic stretching) for 15 min, NW for 40 min, and cooling down (static stretching) for 5 min. The intervention was conducted in a gymnasium. As the subjects of this study had difficulty walking long distances (their self-reported walking distance without break was less than 1 km), chairs were set and used during the warming up, NW exercise, and cooling down. The instructor mainly taught pole techniques of defensive style NW rather than allowing subjects to walk long distances in the 40-min NW exercise. The pole length was set at 0.63 times as each subjects’ height according to the tutorial by Japan Nordic Walk League. In the defensive style NW, walkers plant a pole upright near the front half of a contralateral forefoot (Figure 1). To walk with this style smoothly, subjects practiced stretching hands forward as if shaking someone’s hand with relaxed and light grasp of the poles. The only instruction for leg movement was to roll the foot from heel to forefoot during foot contact if it was possible for each subject. Total walking distance was 200 m in the first session, which gradually increased each session and finally reached 1,000 m in the tenth (last) session. Three subjects were absent from the intervention once within the regular 10 weeks. Therefore, an additional session was arranged for them to complete 10 sessions.
Self-reported walking distance and pain measurement
Self-reported walking distance without break was investigated using questionnaires with the five-point scale: more than 2-3 km, around 1 km, around 300 m, around 100 m, and around 10 m. For subjects who usually used a cane while walking, we asked them to answer in terms of using the cane. The response was as follows: 5 subjects answered around 1 km, 3 subjects around 300 m, 3 subjects around 100 m, and 2 subjects around 10 m at the first investigation (before the control period). Self-reported walking distance without break was measured again after the NW intervention period.
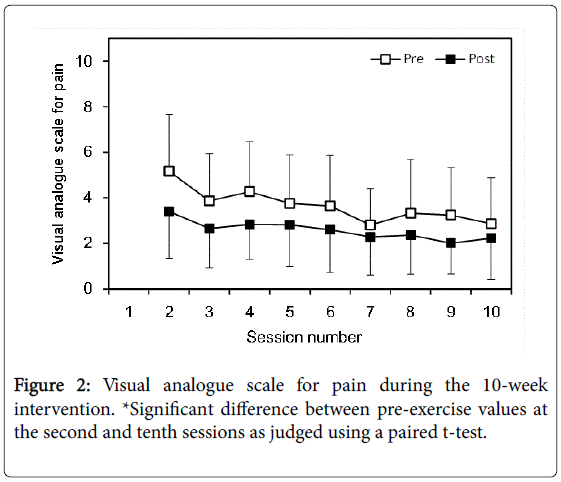
Pain was measured before and after each session using 10-cm visual analogue scale (VAS). The left end of the scale was labelled “no pain” and the right end “very severe pain.” The distance from the left end to the line marked by subjects was measured. In the first session, subjects practiced quantifying their pain through the VAS scale. Therefore, we analyzed the VAS for pain from the second to tenth sessions.
Functional performance assessments
Maximal walking speed and TUG score were measured to evaluate subjects’ mobility. Subjects walked as fast as possible on a 15-m straight pathway and walking time for the middle 10 m of the pathway was measured using a stopwatch. Measurements were conducted twice and the faster speed was adopted for analysis. For TUG test, subjects were asked to stand up from a chair, walk to a marker 3 m away from a chair, turn around the marker, walk back to the chair, and sit down. In this study, subjects were instructed to move as fast as possible and safely. The measurements were conducted twice; in the clockwise as well as counterclockwise directions and the value for the faster speed was adopted for analysis. To prevent the effect of shoes, subjects were asked to wear the same shoes at the pre-control, post-control/preintervention, and post-intervention measurements.
Maximal isometric knee extension and flexion strength were measured for both legs using a dynamometer (MYORET RZ-450, Kawasaki Heavy Industries, Ltd., Tokyo, Japan). Subjects sat on the chair of the dynamometer and their trunks and thighs were fixed to the chair with bands. Maximal isometric knee extension and flexion were performed at 80° of anatomical knee joint angle. Measurements were conducted twice for each leg and the higher value was used for analysis. As the difference in strength between right and left legs was wide in the patient population, the analysis was not performed with values grouped according to the right and left legs, but with legs with lower and higher strengths at the pre-control measurement.
Dynamic and static balance were evaluated using the functional reach (FR) test, one-leg standing time, and centre of pressure (COP) during quiet standing. For the FR test, subjects stood next to and perpendicular to the wall with their feet shoulder-width apart and positioned the arm which was closer to the wall at 90° of shoulder flexion with fingers stretched straight (starting position). After the starting point marked by the middle finger was recorded, subjects were asked to bend and reach forward as far as possible without taking steps. The location of the middle finger at the farthest position was recorded again and the distance between the two positions was calculated. Measurements were conducted for both sides (stretching the right and left hands forward) and twice at one side. The maximal value was adopted as the FR test value.
To measure the one-leg standing time, subjects stood barefoot on one leg for as long as possible with their hands on their waists and eyes open. Standing time was measured using a stopwatch from when one foot lifted off the floor to when the lifted foot landed, the supporting foot moved from the starting position, or the hands moved away from the waist. Maximal measurement time was set at 60 s. Measurements were conducted twice at each side and higher value was used for analysis. Similar to the maximal isometric knee extension and flexion strength, the one-leg standing time was analysed using grouped values by legs with shorter and longer standing times at the pre-control measurement.
COP was measured for 60 s using a force platform (GRAVICODER GS3000, ANIMA Corp., Tokyo, Japan) and sampled at a rate of 20 Hz. Subjects stood barefoot on the force platform with their arms at their sides and eyes looking straight at a point on a wall 3 m ahead. Subjects were instructed to stand as still as possible with their feet together. However, individuals who had difficulty in standing with feet together stood with their feet a little apart. The distance between the feet was recorded for each subject and reproduced in each measurement. Mean velocity (mm/s) and sway area (mm2) were calculated for the COP displacement.
Statistical analysis
To test the effect of the NW intervention, the amount of change between pre- and post-NW intervention values was compared to that between pre- and post-control values. After the normality of the difference between these two amount-of-changes was tested using the Shapiro-Wilk test, a paired t-test was performed to variables with normal distribution and the Wilcoxon signed-rank test was performed to a variable with non-normal distribution (only mean velocity of COP). A paired t-test with Bonferroni correction was performed to compare post- with pre-NW intervention values as well as compare post- with pre-control values after checking for normality using the Shapiro-Wilk test. As the difference between pre- and post-values was not normally distributed for mean velocity of COP in the NW intervention and control periods and knee extension strength with higher initial value in the control period, the Wilcoxon signed-rank test was performed to compare pre- with post-values of these indices. Selfreported walking distance without break at the pre-control measurement was compared with that at the post-intervention measurement using the Wilcoxon signed-rank test.
A two-way repeated-measures analysis of variance (ANOVA) was performed (single session [pre-session, post-session] × session number [second-tenth session]) to estimate the effects of a single NW session and NW intervention on pain. To prevent the decrease in the number of data due to missing values, pain data were analysed including additional session after regular 10-week intervention for the absentees. Therefore, all subjects had pain data of 10 sessions and “session number” indicated the number of sessions for each subject. As the non-sphericity was confirmed using the Mauchly test, the Greenhouse- Geisser correction was applied to correct the degrees of freedom. After checking for the normality, a paired t-test was performed to compare pre-session value at the second session with pre-session value at the tenth session.
Effects size was analysed by calculating Cohen’s d for a paired ttest and the Wilcoxon signed-rank test, and by calculating partial η 2 for the two-way repeated-measures ANOVA. Cohen’s d of 0.2, 0.5, and 0.8 were considered as small, medium, and large effect sizes, respectively [23].
As we failed to save COP data for one person at one measurement, COP velocity and sway area were analyzed for 12 subjects. Data were expressed as means ± SD, and considered statistically significant if p<0.05. In the paired t-test with Bonferroni correction, statistical significance was set at p<0.025. Statistical analyses were performed using IBM SPSS Statistics 21.0 (SPSS Japan Inc., an IBM Company, Tokyo, Japan) except for the calculation of Cohen’s d value using G*Power 3 Ver.3.1.9.2 [24].
Results
Table 1 shows the results of measured indices at the pre-control, post-control/pre-NW intervention, and post-NW intervention periods. Maximal walking speed showed no significant difference between the amount of changes during the NW intervention and control periods. Post-NW intervention value was significantly different from the pre-NW intervention value (p=0.022, Cohen’s d=0.730). There was no significant difference between pre- and post-control values. With regard to TUG score, there was a significant difference between the amount of changes during the NW intervention and control periods (p=0.041, Cohen’s d=0.636).
| Leg | Pre-CON | Post-CON/ | Post-NW | CON vs. NW |
|
|---|---|---|---|---|---|
| pre-NW | |||||
| Mobility | |||||
| Maximal walking speed (m/s) | 1.21 ± 0.42 | 1.17 ± 0.39 | 1.26 ± 0.34 * | ||
| Timed Up and Go (s) | 8.89 ± 3.64 | 10.1 ± 5.07 | 7.88 ± 2.82 * | † | |
| Lower-limb muscle strength | |||||
| Knee extension (Nm/kg) | low | 1.04 ± 0.49 | 1.05 ± 0.43 | 1.25 ± 0.58 * | † |
| Knee extension (Nm/kg) | high | 1.42 ± 0.64 | 1.4 ± 0.68 | 1.62 ± 0.67 * | † |
| Knee flexion (Nm/kg) | low | 0.381 ± 0.126 | 0.365 ± 0.112 | 0.438 ± 0.159 * | † |
| Knee flexion (Nm/kg) | high | 0.476 ± 0.165 | 0.432 ± 0.187 | 0.505 ± 0.183 * | † |
| Balance | |||||
| Functional reach (cm) | 25.6 ± 8.1 | 25.9 ± 6.1 | 28.6 ± 4.8 | ||
| One-leg standing (s) | low | 12.5 ± 8.7 | 11.8 ± 8.1 | 23.1 ± 17 * | † |
| One-leg standing (s) | high | 21.7 ± 16.3 | 23.1 ± 16.4 | 33.2 ± 22.1 | |
| COP velocity (mm/s) | 19.5 ± 10.2 | 20.3 ± 12.4 | 9.2 ± 9.3 | ||
| COP area (mm2) | 521 ± 248 | 553 ± 269 | 449 ± 202 | ||
Data are expressed as means ± SD.
CON: Control Period; NW: Nordic Walking Intervention Period; COP: Centre of Pressure.
*Significant difference between post-CON/pre-NW and post-NW values as judged using a paired t-test with Bonferroni correction.
†Significant difference between the amount of changes in the control and NW intervention periods as judged using a paired t-test.
Table 1: Functional performance before and after control and intervention periods.
Post-NW intervention value was significantly different from the pre-NW intervention value (p=0.023, Cohen’s d=0.722). There was no significant difference between pre- and post-control values. Selfreported walking distance without break did not show a significant difference between pre-control and post-NW intervention values.
Maximal isometric knee extension and flexion strength of both legs with lower and higher initial values showed significant differences between the amount of changes during the NW intervention and control periods (knee extension of leg with lower initial value, p=0.047, Cohen’s d=0.613; knee extension of leg with higher initial value, p=0.013, Cohen’s d=0.807; knee flexion of leg with lower initial value, p=0.018, Cohen’s d=0.760; knee flexion of leg with higher initial value, p=0.006, Cohen’s d=0.921). Additionally, all indices showed significant differences between pre- and post-NW intervention values (knee extension of leg with lower initial value, p=0.009, Cohen’s d=0.867; knee extension of leg with higher initial value, p<0.001, Cohen’s d=0.807; knee flexion of leg with lower initial value, p=0.024, Cohen’s d=0.718; knee flexion of leg with higher initial value, p=0.005, Cohen’s d=0.944). There were no significant differences between pre- and postcontrol values in all the lower-limb muscle strength indices.
One-leg standing time of leg with lower initial value showed a significant difference between the amount of changes during the NW intervention and control periods (p=0.020, Cohen’s d=0.741), although that with higher initial value did not. A significant difference between pre- and post-NW intervention values was found for the leg with lower initial value (p=0.009, Cohen’s d=0.971). There was no significant difference between pre- and post-NW intervention values in leg with higher initial value. FR, mean velocity of COP, and sway area of COP showed no significant differences between the amount of changes or the pre- and post-NW intervention values. All the balance indices showed no significant differences between pre- and post-control values.
Significant single session effect (p=0.001, partial η2=0.616) and session number effect (p=0.013, partial η2=0.232) were found in VAS pain score (Figure 2). There was no significant interaction effect. Presession value at the tenth session was significantly smaller than presession value at the second session (p=0.002, Cohen’s d =1.007).
Discussion
We hypothesized that defensive style NW intervention could improve functional performance and reduce pain in patients with lumbar and lower-limb OA with low walking capacity. TUG score, knee extension and flexion muscle strength for both legs with lower and higher initial values, and one-leg standing time in leg with lower initial value showed significant differences in the amount of change in NW intervention from that in control period as well as significant differences between pre- and post-NW intervention values. Pain score decreased immediately after one session of NW and throughout the intervention period. These results suggest that defensive style NW intervention improved mobility, lower-limb muscle strength, balance, and pain relief in patients with lumbar and lower-limb OA with low walking capacity, although the improvement was not found for all indices measured in this study.
Most of NW intervention studies have been conducted using the pole backward style. Although the exercise form was not strictly the same as the defensive style NW used in the present study, previous studies on NW intervention for healthy elderly populations demonstrated improvement in maximal walking speed [25], TUG score [26], and stride length [27], that is, improvement in mobility. NW interventions were also conducted for patient populations. Eightfoot Up and Go test score improved in patients with hip OA [11], while maximal walking speed [20,21], stride length [21], and TUG score [20,22] improved in patients with Parkinson’s disease after NW intervention. Thus, it has been confirmed that NW intervention can improve mobility in healthy elderly individuals and patients with diseases that affect walking. The present study additionally demonstrated that defensive style NW could improve mobility, evaluated using TUG, in patients with lumbar and lower-limb OA with low walking capacity. Meanwhile, no improvement was found in selfreported walking distance without break in the present study. One of the reasons may be small training volume. Bieler et al. [11] who conducted pole backward style NW in patients with hip OA for 4 months observed improvement in 8-foot Up and Go test as well as 6- min walk test in the post-intervention measurement and even in the intermediate measurement (at the second month). The training volume in the present study was less than that of Bieler et al. [11]: less walking distance per session (less than 1.0 km in the present study versus more than 3.0 km in the study of Bieler et al.), less session numbers per week (one session in the present study versus three sessions in the study of Bieler et al.), and probably less exercise intensity (defensive style NW in the present study versus pole backward style NW at moderate intensity in the study of Bieler et al.), which may have led to lack of improvement in the self-reported walking distance without break in the present study.
Effects of NW on lower-limb muscle strength have often been examined using the 30-s chair stand test. Studies which examined effects of pole backward style NW intervention for healthy elderly populations reported improvement in 30-s chair stand test [26,28]. Similar result was confirmed in a study on patients with hip OA [11]. Normal walking intervention also improved lower-limb muscle strength of patients with knee OA: isometric knee extension [14] and isokinetic knee flexion [13] muscle strengths increased after intervention. Based on these NW and normal walking intervention studies, we hypothesized the possibility of improvement in muscle strength by defensive style NW and obtained the expected results, i.e., increases in isometric knee extension and flexion strength for both legs with lower and higher initial values, in patients with lumbar and lowerlimb OA. It is noteworthy that lower-limb muscle strength significantly increased with a small training volume in the present study. We did not set a large training volume so as not to worsen symptoms and pain in patients with OA. Nevertheless, both knee extension and flexion muscle strength in both legs increased. This may be because subjects were patients with low walking capacity and low initial value of lowerlimb muscle strength. Underlying mechanisms could be neuromuscular adaptations [29,30] rather than muscle hypertrophy.
Patients with lumbar and lower-limb OA have lower balance ability than healthy subjects [31-33]. Few studies have examined the effects of walking interventions, including normal walking and NW, on postural balance in such patients. One of the few studies reported improvement in postural sway during quiet standing of patients with knee OA by normal walking intervention [15]. For healthy elderly populations, pole backward style NW intervention improved balance ability as evaluated by one-leg standing time [28,34] and FR [27]. The present study showed that defensive style NW intervention significantly increased one-leg standing time in leg with lower initial value. Although other indices evaluating balance ability, such as FR and COP-based measures, did not show significant improvement, it was shown that balance ability could be improved through defensive style NW intervention in patients with lumbar and lower-limb OA. The mean value of the one-leg standing time in leg with higher initial value increased by approximately 10 s after intervention; however, the change was not statistically significant. One of the reasons may be that the maximal test time was set at 60 s and there was a limit to increase.
The present study showed decrease in pain for each session, and throughout the intervention period. It has been reported that pain is reduced by regular exercise intervention for patients with OA [35,36]. Normal walking is one of the interventions [13,19] and pain reduction by normal walking was confirmed even in the absence of improvement on the radiogram in a study of patients with knee OA [13]. For patients with low back pain, there are studies which reported decrease in pain by using treadmill walking with partial body weight support [37,38]. Pole backward style NW intervention was reported to decrease pain in patients with hip OA [11] and chronic low back pain [39]. The present study additionally demonstrated that regular defensive style NW intervention can decrease pain in patients with lumbar and lower-limb OA.
Positive effect of one session of exercise on pain was also demonstrated in the present study. It is known that pain sensitivity becomes reduced immediately following exercise and this reduction is called “Exercise-Induced Analgesia (EIA)” [40,41]. Both aerobic and resistance exercises can elicit EIA in healthy individuals [40,41]. Meanwhile, there has not yet been a consensus on EIA in patients with OA. One session of exercise including aerobic and resistance components did not change pain in patients with severe hip and knee OA [42], or increased pain in patients with knee OA [43]. Roper et al. [44] reported that pain in patients with knee OA was reduced by walking on an aquatic treadmill but did not change by walking on land treadmill. These results suggest that water-based exercises have the potential to reduce pain in patients with OA but land-based exercises do not. As the reduction in pain after one session of exercise was observed in the present study, it can be said that defensive style NW, even though it is a land-based exercise, had the potential to reduce pain acutely in patients with OA. It is also suggested that the effect of EIA may depend on which limbs patients move during the exercise. In the study of patients with knee OA, EIA effect was not observed in either lower and upper limbs after resistance exercise in painful lower limbs, but pain sensitivity was reduced in both lower and upper limbs after resistance exercise in upper limbs [45]. The EIA’s dependence on exercising limbs was also confirmed in a study of patients with shoulder myalgia [46]: low-intensity isometric contraction in painless lower limbs reduced pain sensitivity of painful shoulder muscles, but the same exercise in painful upper limbs did not change it. In addition to reducing load on painful lower limbs [7,10] defensive style NW activates painless upper limbs by using poles, which may induce EIA in patients with lumbar and lower-limb OA of the present study.
Conclusion
The present study showed that defensive style NW intervention improved mobility, lower-limb muscle strength, balance, and pain relief in patients with lumbar and lower-limb OA with low walking capacity. Additionally, one session of defensive style NW can reduce pain acutely. Land-based exercises which can be performed readily and safely by patient populations are essential to improve physical functions and pain relief, and thus to improve ADL of the patients. The results of the present study suggest that defensive style NW is one of the most effective and feasible land-based exercise for patients with lumbar and lower-limb OA. Further studies are necessary to investigate the long-term effects. We expect an improvement of pathological conditions of OA by the long-term intervention of defensive style Nordic walking.
Acknowledgements
This study was funded by Japan Health Academy. The authors are grateful to all participants.
References
- Lin EHB, Tang L, Katon W, Hegel MT, Sullivan MD, et al. (2006) Arthritis pain and disability: response to collaborative depression care. Gen Hosp Psychiatry 28: 482-486.
- van Dijk GM, Dekker J, Veenhof C, van den Ende CHM, Carpa Study Group (2006) Course of functional status and pain in osteoarthritis of the hip or knee: a systematic review of the literature. Arthritis Rheum 55: 779-785.
- Whitehurst M, Brown LE, Eidelson SG, D'Angelo A (2001) Functional mobility performance in an elderly population with lumbar spinal stenosis. Arch Phys Med Rehabil 82: 464-467.
- Nakamura K, Ogata T (2016) Locomotive syndrome: definition and management. Clin Rev Bone Miner Metab 14: 56-67.
- Bennell KL, Hall M, Hinman RS (2016) Osteoarthritis year in review 2015: rehabilitation and outcomes. Osteoarthritis Cartilage 24: 58-70.
- Schiffer T, Knicker A, Montanarella M, Strüder HK (2011) Mechanical and physiological effects of varying pole weights during Nordic walking compared to walking. Eur J Appl Physiol 111: 1121-1126.
- Willson J, Torry MR, Decker MJ, Kernozek T, Steadman JR (2001) Effects of walking poles on lower extremity gait mechanics. Med Sci Sports Exerc 33: 142-147.
- Rodgers CD, Vanheest JL, Schachter CL (1995) Energy expenditure during submaximal walking with exerstriders®. Med Sci Sports Exerc 27: 607-611.
- Porcari JP, Hendrickson TL, Walter PR, Terry L, Walsko G (1997) The physiological responses to walking with and without Power PolesTM on treadmill exercise. Res Q Exerc Sport 68: 161-166.
- Ota S, Nakanishi A, Sato H, Akita S, Hase K, et al. (2013) Differences in knee joint kinematics and kinetics during level walking and walking with two types of poles - focus on knee varus moment. J Musculoskelet Res 16: 1350018.
- Bieler T, Siersma V, Magnusson SP, Kjaer M, Christensen HE, et al. (2017) In hip osteoarthritis, Nordic Walking is superior to strength training and home-based exercise for improving function. Scand J Med Sci Sports 27: 873-886.
- Peterson MGE, Kovar-Toledano PA, Otis JC, Allegrante JP, Mackenzie CR, et al. (1993) Effect of a walking program on gait characteristics in patients with osteoarthritis. Arthritis Care Res 6: 11-16.
- Ettinger WH, Burns R, Messier SP, Applegate W, Rejeski WJ, et al. (1997) A randomized trial comparing aerobic exercise and resistance exercise with a health education program in older adults with knee osteoarthritis - the Fitness Arthritis and Seniors Trial (FAST). JAMA 77: 25-31.
- Talbot LA, Gaines JM, Huynh TN, Metter EJ (2003) A home-based pedometer-driven walking program to increase physical activity in older adults with osteoarthritis of the knee: a preliminary study. J Am Geriatr Soc 51: 387-392.
- Messier SP, Royer TD, Craven TE, O'Toole ML, Burns R, et al. (2000) Long-term exercise and its effect on balance in older, osteoarthritic adults: results from the Fitness Arthritis and Seniors Trial (FAST). J Am Geriatr Soc 48: 131-138.
- Kovar PA, Allegrante JP, MacKenzie CR, Peterson MGE, Gutin B, et al. (1992) Supervised fitness walking in patients with osteoarthritis of the knee - a randomized, controlled trial. Ann Intern Med 116: 529-534.
- Messier SP, Thompson CD, Ettinger WH (1997) Effects of long-term aerobic or weight training regimens on gait in an older, osteoarthritic population. J Appl Biomech 13: 205-225.
- Minor MA, Hewett JE, Webel RR, Anderson SK, Kay DR (1989) Efficacy of physical conditioning exercise in patients with rheumatoid arthritis and osteoarthritis. Arthritis Rheum 32: 1396-1405.
- O’Connor SR, Tully MA, Ryan B, Bleakley CM, Baxter GD, et al. (2015) Walking exercise for chronic musculoskeletal pain: systematic review and meta-analysis. Arch Phys Med Rehabil 96: 724-734.
- van Eijkeren FJM, Reijmers RSJ, Kleinveld MJ, Minten A, ter Bruggen JP, et al. (2008) Nordic walking improves mobility in Parkinson’s disease. Mov Disord 23: 2239-2243.
- Reuter I, Mehnert S, Leone P, Kaps M, Oechsner M, et al. (2011) Effects of a flexibility and relaxation programme, walking, and Nordic walking on Parkinson’s disease. J Aging Res 2011: 232473.
- Cugusi L, Solla P, Serpe R, Carzedda T, Piras L, et al. (2015) Effects of a Nordic Walking program on motor and non-motor symptoms, functional performance and body composition in patients with Parkinson’s disease. NeuroRehabilitation 37: 245-254.
- Cohen J (1988) Statistical power analysis for the behavioral sciences. 2nd edn, Lawrence Erlbaum Associates: Hillsdale.
- Faul F, Erdfelder E, Lang AG, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39: 175-191.
- Figueiredo S, Finch L, Mai J, Ahmed S, Huang A, et al. (2013) Nordic walking for geriatric rehabilitation: a randomized pilot trial. Disabil Rehabil 35: 968-975.
- Parkatti T, Perttunen J, Wacker P (2012) Improvements in functional capacity from Nordic walking: a randomized controlled trial among older adults. J Aging Phys Act 20: 93-105.
- Kocur P, Wiernicka M, Wilski M, Kaminska E, Furmaniuk L, et al. (2015) Does Nordic walking improves the postural control and gait parameters of women between the age 65 and 74: a randomized trial. J Phys Ther Sci 27: 3733-3737.
- Lee HS, Park JH (2015) Effects of Nordic walking on physical functions and depression in frail people aged 70 years and above. J Phys Ther Sci 27: 2453-2456.
- Moritani T, deVries HA (1980) Potential for gross muscle hypertrophy in older men. J Gerontol 35: 672-682.
- Sale DG (1988) Neural adaptation to resistance training. Med Sci Sports Exerc 20: S135-S145.
- Mientjes MIV, Frank JS (1999) Balance in chronic low back pain patients compared to healthy people under various conditions in upright standing. Clin Biomech (Bristol, Avon) 14: 710-716.
- Masui T, Hasegawa Y, Yamaguchi J, Kanoh T, Ishiguro N, et al. (2006) Increasing postural sway in rural-community-dwelling elderly persons with knee osteoarthritis. J Orthop Sci 11: 353-358.
- Giemza C, Ostrowska B, Matczak-Giemza M (2007) The effect of physiotherapy training programme on postural stability in men with hip osteoarthritis. Aging Male 10: 67-70.
- Kukkonen-Harjula K, Hiilloskorpi H, Manttari A, Pasanen M, Parkkari J, et al. (2007) Self-guided brisk walking training with or without poles: a randomized-controlled trial in middle-aged women. Scand J Med Sci Sports 17: 316-323.
- Jordan JL, Holden MA, Mason EEJ, Foster NE (2010) Interventions to improve adherence to exercise for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev 1: CD005956.
- Beumer L, Wong JN, Warden SJ, Kemp JL, Foster P, et al. (2016) Effects of exercise and manual therapy on pain associated with hip osteoarthritis: a systematic review and meta-analysis. Br J Sports Med 50: 458-463.
- Joffe D, Watkins M, Steiner L, Pfeifer BA (2002) Treadmill ambulation with partial body weight support for the treatment of low back and leg pain. J Orthop Sports Phys Ther 32: 202-215.
- Whitman JM, Flynn TW, Childs JD, Wainner RS, Gill HE, et al. (2006) A comparison between two physical therapy treatment programs for patients with lumbar spinal stenosis - a randomized clinical trial. Spine (Phila Pa 1976) 31: 2541-2549.
- Hartvigsen J, Morso L, Bendix T, Manniche C (2010) Supervised and non-supervised Nordic walking in the treatment of chronic low back pain: a single blind randomized clinical trial. BMC Musculoskelet Disord 11: 30.
- Koltyn KF (2000) Analgesia following exercise - a review. Sports Med 29: 85-98.
- Naugle KM, Fillingim RB, Riley JL (2012) A meta-analytic review of the hypoalgesic effects of exercise. J Pain 13: 1139-1150.
- Ageberg E, Link A, Roos EM (2010) Feasibility of neuromuscular training in patients with severe hip or knee OA: the individualized goal-based NEMEX-TJR training program. BMC Musculoskelet Disord 11: 126.
- Focht BC, Ewing V, Gauvin L, Rejeski WJ (2002) The unique and transient impact of acute exercise on pain perception in older, overweight, or obese adults with knee osteoarthritis. Ann Behav Med 24: 201-210.
- Roper JA, Bressel E, Tillman MD (2013) Acute aquatic treadmill exercise improves gait and pain in people with knee osteoarthritis. Arch Phys Med Rehabil 94: 419-425.
- Burrows NJ, Booth J, Sturnieks DL, Barry BK (2014) Acute resistance exercise and pressure pain sensitivity in knee osteoarthritis: a randomised crossover trial. Osteoarthritis Cartilage 22: 407-414.
- Lannersten L, Kosek E (2010) Dysfunction of endogenous pain inhibition during exercise with painful muscles in patients with shoulder myalgia and fibromyalgia. Pain 151: 77-86.
Citation: Fukusaki C, Leetawesup K, Kato N, Kadokura Y, Nakazawa K, et al. (2018) Effects of Defensive Style Nordic Walking Intervention in Patients with Lumbar and Lower-Limb Osteoarthritis. J Nov Physiother 8: 383. DOI: 10.4172/2165-7025.1000383
Copyright: © 2018 Fukusaki C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 8338
- [From(publication date): 0-2018 - Dec 21, 2025]
- Breakdown by view type
- HTML page views: 7183
- PDF downloads: 1155