Effects of Probiotics on Intermediate Disease Markers in Individuals with Overweight and Obesity: Systematic Review and Meta-Analysis
Received: 15-Apr-2024 / Manuscript No. DPO-24-132292 / Editor assigned: 18-Apr-2024 / PreQC No. DPO-24-132292 (PQ) / Reviewed: 04-May-2024 / QC No. DPO-24-132292 / Revised: 07-Apr-2025 / Manuscript No. DPO-24-132292 (R) / Published Date: 14-Apr-2025
Abstract
Background: Overweight and obesity has become a global health issue with an increasing prevalence worldwide. Probiotics has shown its effectiveness on intermediate disease markers, however, its efficacy remain unclear. This meta-analysis examined the effects of probiotics on intermediate disease markers in individuals with overweight and obesity.
Methods: All randomized controlled trials published in the PubMed, Embase, Cochrane Library, and Web of Science databases between 2013 and 2023 were systematically searched. The Cochrane handbook risk of bias assessment tool was used to assess study quality. 26 studies with 1,884 adults with overweight and obesity were selected for inclusion in our analysis. Data were analyzed using the review manager 5.3 and Stata version 15.1 software.
Results: Probiotics significantly reduced Low-Density Lipoprotein (LDL) (MD=-0.1, 95% CI: -0.20, 0.00, p<0.05) and Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) (MD=-0.17, 95% CI: -0.32, -0.01, p<0.05) and increased High-Density Lipoprotein (HDL) (MD=0.11, 95% CI: 0.01, 0.21, p<0.05). There were no significant changes observed in the levels of triglycerides, total cholesterol, fasting glucose, glycated hemoglobin, blood pressure and C-reactive protein (p>0.05).
Conclusion: Our results of this meta-analysis suggests that adding probiotics may be helpful for improving intermediate disease markers, such as LDL, HOMA-IR and HDL, in overweight and obese individuals. However, more high-quality studies are needed to confirm these findings.
Keywords
Obesity; Overweight; Probiotics; Intermediate disease markers; Meta-analysis
Introduction
Overweight/obesity is a global health issue with an increasing prevalence worldwide. In March 2023 the world obesity federation released the latest edition of the world obesity map report. According to this report it was estimated that in 2035 more than 4 billion people worldwide will be overweight or obese, accounting for more than half of the global population and causing an economic loss of 4 trillion dollars. This is troubling because obesity, as a disease and an established risk factor, contributes to a variety of physical health issues, including diabetes, cardiovascular disease, hypertension, stroke, gout and various types of cancer [1].
Overweight/obesity is often accompanied by abnormal metabolic states such as hyperglycemia, hyperinsulinemia, high Triglycerides (TG) and low High-Density Lipoprotein (HDL), which are manifestations of obesity-induced metabolic disorders. Obesity also leads to increased inflammatory responses, which elevate inflammatory factors that further aggravate its development. Therefore, common intermediate disease markers, such as Low-Density Lipoprotein (LDL), TG, Total Cholesterol (TC), HDL, Fasting Glucose (FG), glycated hemoglobin (HbA1c), Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) and C-Reactive Protein (CRP) are associated with obesity [2].
Currently, patients with obesity can be treated with a combination of dietary modifications, increased exercise, pharmacotherapy and surgery. However, obesity is difficult to control because of unhealthy diet, poor adherence to long-term exercise, adverse effects of medications and trauma from surgery. Therefore, the development of alternative therapies is necessary. At present, many animal experiments and human randomized controlled trials have confirmed that probiotics are beneficial to obesity. The most commonly used probiotic species are from the Lactobacillaceae family, Bifidobacteria spp., and yeasts. Probiotics are most often ingested in the form of food or beverages, such as yogurt or dietary supplements. Probiotic studies have shown promising results in relation to the treatment of hypertension, dyslipidemia, diabetes and cardiovascular disease. However, the efficacy of probiotics in overweight and obesity is not clear [3].
Individuals with overweight and obesity are more likely to develop heart disease, diabetes, and hypertension and may have the greatest potential to benefit from probiotics therapy. However, its efficacy remains unclear. This meta-analysis examined the effects of probiotics on intermediate disease markers among overweight and obese individuals [4].
Materials and Methods
Search strategy, selection and data extraction
To ensure the integrity of this review, it adhered to the guidelines set forth in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. Additionally, the protocol for this study has been registered in the International Prospective Register of Systematic Reviews (PROSPERO) under the identifier CRD42023420984 [5].
In order to gather relevant data, comprehensive searches were conducted in PubMed, Embase, Cochrane Library and Web of Science, covering the period from inception to February 4th, 2023. The search strategy was developed based on the PICOS tool, focusing on the following aspects: (P) Population: individuals classified as overweight or obese; (I) Intervention: probiotics; (C) Comparator: placebo; (O) Outcomes: intermediate disease markers. For more detailed information, please refer to Online Resource 1 [6].
To be eligible for inclusion in this review, studies needed to meet the following criteria:
• Adult participants (18 years or older) with overweight or obesity, as determined by local Body Mass Index (BMI) standards
• Clinical randomized controlled trials
• Control groups receiving placebo interventions
• Experimental groups receiving probiotics as the sole active intervention
• Studies reporting at least one intermediate disease marker, such as Low-Density Lipoprotein (LDL), Triglyceride (TG), Total Cholesterol (TC), High-Density Lipoprotein (HDL), Fasting Glucose (FG), glycated hemoglobin (HbA1c), Homeostasis Model Assessment of Insulin Resistance (HOMA-IR), Systolic Blood Pressure (SBP), Diastolic Blood Pressure (DBP) or C-Reactive Protein (CRP)
The following criteria were used to exclude studies:
• Non-original studies, including letters, reviews and editorials
• Non-randomized and uncontrolled clinical trials
• Studies conducted on non-human subjects
• Studies where probiotics were not administered as the sole intervention
• Studies lacking accessible data for extraction
• Randomized controlled trials involving children, pregnant or lactating women, individuals with gastrointestinal disorders, those using intragastric balloon and/or undergoing gastrointestinal surgery (including bariatric surgery) and patients using medications known to affect body weight and composition
Data extraction was conducted independently by two reviewers. Any disagreements that arose during the extraction process were resolved through discussions with a third reviewer. A pre-designed form was utilized to record the data under the following categories [7]:
• Author
• Country
• Year of publication
• Trial registration number
• Study duration
• Sample size
• Characteristics of the probiotics, including species/strains and vehicle (powder/capsules)
• Outcomes, including LDL, TG, TC, HDL, FG, HbA1c, HOMA-IR, SBP, DBP, CRP
Quality assessment
The included studies were assessed for their quality using the cochrane handbook risk of bias assessment tool. This tool consists of seven domains, which include random sequence generation, allocation concealment, blinding of outcome assessment, blinding of participants and personnel, selective reporting, incomplete outcome data and other sources of bias. Each domain was categorized as low risk of bias, high risk of bias or unclear risk of bias. A study with all domains graded as low risk of bias was considered to have a low risk of bias, while those with one or more domains graded as unclear or high risk of bias were considered to have a high risk of bias. The assessment was performed independently by two reviewers, with any disagreements settled by a third reviewer [8].
Statistical analysis
The meta-analysis utilized the mean and Standard Deviation (SD) changes in outcome variables after treatment in both the intervention and control groups across all selected studies. Data analyses were conducted using Review Manager 5.3 and Stata software version 15.1. For continuous variables, the Standardized Mean Difference (SMD) was employed, while the Odds Ratio (OR) was used for dichotomous variables. All metrics were reported with 95% Confidence Intervals (CIs). Heterogeneity testing involved the Q test and I2 statistic. If the I2 statistic was less than 50%, indicating no significant heterogeneity among the included studies, a fixed-effect model was applied for the meta-analysis. Otherwise, a random-effect model was utilized. Furthermore, one-way sensitivity analyses were conducted to assess the influence of individual studies on the combined results for outcomes exhibiting significant heterogeneity. Publication bias was evaluated using funnel plots and Begg's test. Sensitivity analysis was employed to assess the robustness of the meta-analysis results. Statistical significance was determined by a p-value less than 0.05 [9].
Results
Study selection
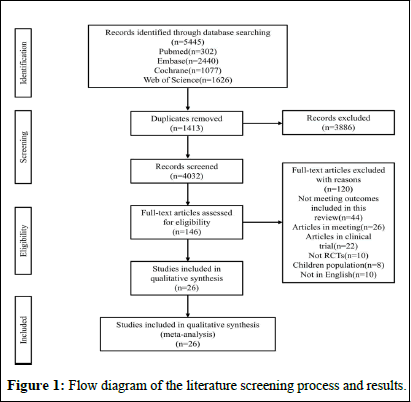
The flowchart of literature search process is presented in Figure 1A total of 5445 relevant articles in PubMed (n=302), Embase (n=2440), Cochrane Library (n=1077) and Web of Science (n=1626) were yielded through systematic literature search. After removing 1413 duplicates, titles and abstracts of the 4032 studies were browsed. Full texts of the remaining 146 articles were read. 26 eligible RCTs with 1884 participants were included for the final analysis [10].
Figure 1: Flow diagram of the literature screening process and results.
Characteristics of included studies
The 26 studies were all controlled and randomized clinical trials which were published between 2013 and 2023 from Korea, Poland, Australia, Brazil, Canada, Finland, France, Italy, Japan, Indonesia, India, Iran, Tunisia and United Kingdom . The studies’ populations consisted of subjects with overweight and/or obesity, according to BMI values, excluding relevant comorbidities. The intervention duration varied from four weeks to six months. Probiotics vehicle included capsule, powder, milk powder, yogurt, tablet and smoothie. The studies administered either one, two, three or four or more probiotic species. Characteristics of included studies are pictured in Table 1 [11].
|
Author |
Country |
Year |
Trial registration number |
Study duration |
Total |
Intervention | Control | Outcome |
|
Minji Sohn |
Korea |
2023 |
NCT03759743 |
12 weeks |
T:49 |
Capsules with Lactobacillus plantarum strain LMT1-48 | Capsules | LDL,TG,TC, HDL, FG, HOMA-IR, SBP, DBP, CRP |
|
C:50 |
||||||||
|
Rym Ben Othman |
Tunisia |
2022 |
PACTR202210705998795 |
4 weeks |
T:15 |
Capsules with Bifidobacteruim longum, Lactobacillus helveticus, Lactococcus lactis, Streptococcus thermophilus | Capsules | LDL, TG, TC, HDL, FG, HbA1c, HOMA-IR, SBP |
|
C:15 |
||||||||
|
Young Gyu Cho |
Korea |
2022 |
KCT0005861 |
12 weeks |
T:38 |
Capsules with Lactobacillus fermentum MG4231 and MG4244 | Capsules | LDL,TG,TC,HDL,FG,SBP,DBP,CRP |
|
C:37 |
||||||||
|
Sung-Joon Mo |
Korea |
2022 |
NR |
12 weeks |
T:30 |
Capsules with Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 | Capsules | LDL, TG, TC, HDL, CRP |
|
C:29 |
||||||||
|
Minji Sohn |
Korea |
2022 |
KCT0003944 |
12 weeks |
T:41 |
Capsules with Lactobacillus plantarum K50 | Capsules | LDL, TG, TC, HDL, FG, SBP, DBP, CRP |
|
C:40 |
||||||||
|
Maria Magdalena Coman |
Italy |
2022 |
NR |
12 weeks |
T:12 |
Capsules with Lactiplantibacillus plantarum IMC 510 | Capsules | LDL, TG, TC, HDL |
|
C:7 |
||||||||
|
Beatrice SY Choi |
Canada |
2022 |
NR |
12 weeks |
T:58 |
Capsules with Lacticaseibacillus rhamnosus HA-114 | Capsules | LDL, TG, TC, HDL, FG, HOMA-IR, SBP, DBP, CRP |
|
C:53 |
||||||||
|
Takayuki Toshimitsu |
Japan |
2021 |
UMIN000027709 |
12 weeks |
T:46 |
Yogurt with Lactobacillus plantarum OLL2712 | Yogurt | LDL, TG, TC, HDL, FG, HbA1c, HOMA-IR, SBP, DBP, CRP |
|
C:46 |
||||||||
|
Pierre Dechelotte |
France |
2021 |
NCT03657186 |
12 weeks |
T:104 |
Capsules with Hafnia alvei HA4597 | Capsules | LDL, TC, HDL, FG, HbA1c |
|
C:108 |
||||||||
|
Louise Crovesy |
Brazil |
2021 |
NCT02505854 |
8 weeks |
T:10 |
Capsules with Bifidobacterium lactis UBBLa-70 | Capsules | LDL, FG |
|
C:11 |
||||||||
|
Endang Sutriswati Rahayu |
Indonesia |
2021 |
NR |
8 weeks |
T:30 |
Milk powder with Lactobacillus plantarum Dad-13 | Milk powder | LDL, TG, TC, HDL |
|
C:30 |
||||||||
|
Yusuke Tanaka |
Japan |
2020 |
UMIN000030079 |
12 weeks |
T:47 |
Tablet with Lactobacillus plantarum L‑137 | Tablet | LDL, TG, TC, HDL, FG, CRP |
|
C:49 |
||||||||
|
Soo Lim |
Korea |
2020 |
NCT03248414 |
12 weeks |
T:47 |
Powder with Lactobacillus sakei CJLS03 | Powder | LDL, TG, TC, HDL, FG, HbA1c, HOMA-IR, SBP, DBP |
|
C:48 |
||||||||
|
Karolina Majewska |
Poland |
2020 |
NR |
12 weeks |
T:25 |
Powder with Bifidobacterium bifidum W23, Bifidobacterium lactis W51, Bifidobacterium lactis W52, Lactobacillus acidophilus W37, Lactobacillus brevis W63, Lactobacillus casei W56, Lactobacillus salivarius W24, Lactococcus lactis W19 and Lactococcus lactis W58 | Powder | LDL, TG, TC, HDL |
|
C:25 |
||||||||
|
Eun-Ji Song |
Korea |
2020 |
NR |
12 weeks |
T:25 |
Capsules with Bifidobacterium breve CBT BR3 and Lactobacillus plantarum CBT LP3 | Capsules | LDL, TG, TC, HDL, FG, SBP, DBP, CRP |
|
C:25 |
||||||||
|
D. R. Michael |
United Kingdom |
2020 |
ISRCTN12562026 |
24 weeks |
T:110 |
Capsules with Lactobacillus acidophilus CUL60, Lactobacillus acidophilus CUL21, Lactobacillus plantarum CUL66, Bifidobacterium bifidum CUL20 and Bifidobacterium animalis subsp. lactis CUL34 | Capsules | LDL, TG, TC, HDL, CRP |
|
C:110 |
||||||||
|
Monika Szulinska |
Poland |
2018 |
NCT03100162 |
12 weeks |
T:23 |
Powder with Bifidobacterium bifidum W23, Bifidobacterium lactis W51, Bifidobacterium lactis W52, Lactobacillus acidophilus W37, Lactobacillus brevis W63, Lactobacillus casei W56, Lactobacillus salivarius W24, Lactococcus lactis W19 and Lactococcus lactis W58 | Powder | LDL, TG, TC, HDL, FG, HOMA-IR |
|
C:24 |
||||||||
|
Monika Szulinska |
Poland |
2018 |
NCT03100162 |
12 weeks |
T:23 |
Powder with Bifidobacterium bifidum W23, Bifidobacterium lactis W51, Bifidobacterium lactis W52, Lactobacillus acidophilus W37, Lactobacillus brevis W63, Lactobacillus casei W56, Lactobacillus salivarius W24, Lactococcus lactis W19 and Lactococcus lactis W58 | Powder | SBP, DBP |
|
C:24 |
||||||||
|
Joohee Kim |
Korea |
2018 |
KCT0000756 |
12 weeks |
T:30 |
Capsules with Lactobacillus gasseri BNR17 | Capsules | LDL, TG, TC, HDL, FG, HbA1c, HOMA-IR, CRP |
|
C:30 |
||||||||
|
Lotta K. Stenman |
Finland |
2016 |
NCT01978691 |
24 weeks |
T:25 |
Smoothie with Bifidobacterium animalis ssp. lactis 420 | Smoothie | CRP |
|
C:36 |
||||||||
|
F Higashikawa |
Japan |
2016 |
NR |
12 weeks |
T:21 |
Powder with Pediococcus pentosaceus LP28 | Powder | LDL, TG, TC, HDL, FG, HbA1c, HOMA-IR |
|
C:20 |
||||||||
|
Jun-ichi Minami |
Japan |
2015 |
NR |
12 weeks |
T:19 |
Capsules with Bifidobacterium breve B-3 | Capsules | FG, HbA1c, CRP |
|
C:25 |
||||||||
|
KL Ivey |
Australia |
2014 |
ACTRN12612000033842 |
6 weeks |
T:40 |
Yogurt with Lactobacillus acidophilus La5 and Bifidobacterium animalis subsp lactis Bb12 | Yogurt | FG, HbA1c, HOMA-IR |
|
C:37 |
||||||||
|
Hemalatha Rajkumar |
India |
2014 |
CTRI/2012/08/002856 |
6 weeks |
T:15 |
capsules with Bifidobacterium longum, Bifidobacterium infantis, Bifidobacterium breve, Lactobacillus acidophilus, Lactobacillus paracasei, Lactobacillus delbrueckii subsp. bulgaricus, Lactobacillus lantarum and Streptococcus salivarius subsp. thermophilus | Capsules | LDL, TG, TC, HDL, FG, CRP |
|
C:15 |
||||||||
|
Seung-Pil Jung |
Korea |
2013 |
NR |
12 weeks |
T:28 |
Capsules with Lactobacillus gasseri BNR17 | Capsules | LDL, TG, TC, HDL, FG, HbA1c, SBP, DBP |
|
C:29 |
||||||||
|
Mitra Zarrati |
Iran |
2013 |
NR |
8 weeks |
T:25 |
Yogurt with Lactobacillus acidophilus La5, Bifidobacterium Bb12, and Lactobacillus casei DN001 | Yogurt | SBP, DBP |
|
C:25 |
||||||||
|
Note: T: Experimental group; C: Control group; LDL: Low-Density Lipoprotein; TG: Triglyceride; TC: Total Cholesterol; HDL: High-Density Lipoprotein; FG: Fasting Glucose; HbA1c: Glycated Hemoglobin; HOMA-IR: Homeostasis Model Assessment of Insulin Resistance; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure; CRP: C-Reactive Protein; NR: Not Reported |
||||||||
Table 1: Characteristic of included studies.
Quality assessment
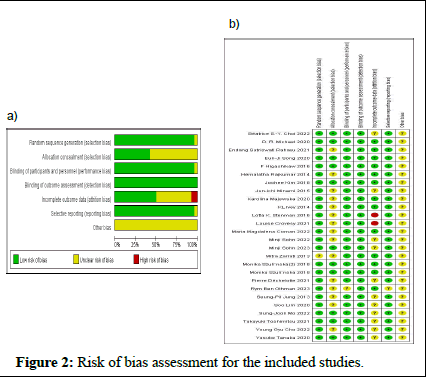
The risk of bias assessment of the included studies is demonstrated in Figure 2. Eleven studies were evaluated as having a low risk of selection bias. Almost all Randomized Controlled Trials (RCTs) performed blinding of the participants and personnel, except for one. Twenty-six RCTs had a low risk of bias for blinding of the outcome assessment. Twenty-five RCTs had a low risk of bias for selective reporting. Overall, two RCTs were rated as having a high risk of bias due to incomplete outcome data. All studies had a low risk of detection bias and an unclear risk of other bias [12].
Figure 2: Risk of bias assessment for the included studies.
Efficacy outcomes
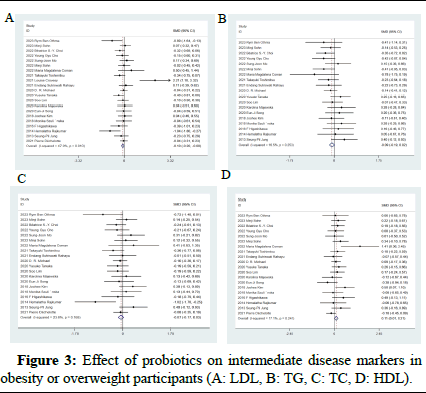
Effect on LDL: Twenty-one studies, with a total sample of 1,605 individuals with overweight and obesity, estimated differences on LDL. The meta-analysis demonstrated that probiotics alleviated LDL (MD: -0.1 mg/dL; 95% CI: -0.20, 0.00) (p<0.05) compared with control groups. The forest plot of this result is pictured in Figure 3A. A moderate level of heterogeneity was observed (I2=44%; p=0.02).
Effect on TC: Twenty studies demonstrated the effect of probiotics on TC levels among 1,586 subjects. No statistically significant difference was observed on TC among individuals supplementing probiotics (MD: -0.07 mg/dL, 95% CI: -0.16, 0.03) (p=0.19) with no significant heterogeneity (I2=21%, p=0.20; Figure 3C).
Effect on HDL: Twenty studies (1,584 participants) assessed HDL. Probiotics interventions significantly decreased serum HDL levels (MD: 0.11 mg/dL, 95% CI: 0.01, 0.21) (p<0.05) with no significant heterogeneity (I2=14%, p=0.29; Figure 3) [13].
Figure 3: Effect of probiotics on intermediate disease markers in obesity or overweight participants (A: LDL, B: TG, C: TC, D: HDL).
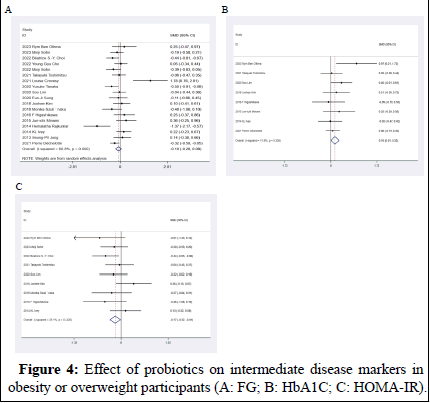
Effect on FG: Eighteen RCTs included in our study reported the effects of probiotics on the intermediate disease marker FG. The data showed that there was no statistically significant difference on FG with the intervention of probiotics compared to control (MD: -0.1 mg/dL, 95% CI: -0.28, 0.08, p=0.27; Figure 4A). The heterogeneity test revealed an I2 value of 59%; therefore, moderate statistical heterogeneity was observed and the random effects model was adopted.
Effect on HbA1c: Eight studies measured the effect of probiotics on HbA1c among 651 subjects. HbA1c levels were not significantly reduced in the intervention group compared with the control group (MD: 0.15%, 95% CI: -0.01, 0.30) (p=0.06) with no significant heterogeneity (I2=14%, p=0.32; Figure 4B).
Effect on HOMA-IR: Nine studies, with 649 participants, assessed differences on HOMA-IR. The meta-analysis revealed a statistically significant difference in HOMA-IR in participants in the intervention group (MD: -0.17 mg/dL, 95% CI: -0.32, -0.01) (p<0.05) with no significant heterogeneity (I2=23%, p=0.24; Figure 4) [14].
Figure 4: Effect of probiotics on intermediate disease markers in obesity or overweight participants (A: FG; B: HbA1C; C: HOMA-IR).
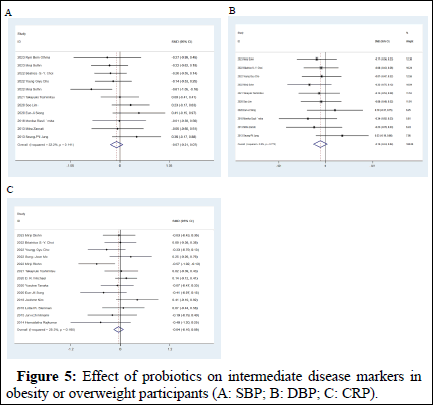
Effect on blood pressure: Eleven studies measured the effect of probiotics on SBP among 831 adults and ten studies measured the effect of probiotics on DBP among 801 adults between the intervention and control groups. There was no statistically significant difference observed on SBP and DBP between the two groups (MD: -0.07 mmHg, 95% CI: -0.21, 0.07 in SBP, p=0.31 and MD: -0.1 mmHg, 95% CI: -0.24, 0.04 in DBP, p=0.17). Heterogeneity in both studies was not statistically significant (I2=31%, p=0.15, Figure 5A and I2=0%, p=0.79, Figure 5).
Effect on CRP: Twenty studies (a total sample of 1,071 participants) assessed differences in CRP levels. The result of our meta-analysis revealed that there was no statistically significant difference in CRP level (MD: -0.04 mg/dL, 95% CI: -0.16, 0.09, p=0.55) with no significant heterogeneity (I2=32%, p=0.13; Figure 5C) [15].
Figure 5: Effect of probiotics on intermediate disease markers in obesity or overweight participants (A: SBP; B: DBP; C: CRP).
Publication bias: The data of Egger’s test (Table 2) support the absence of publication bias. Furthermore, we constructed separate funnel plots for all intermediate disease markers to examine possible publication bias. Visual inspection of the funnel plots did not reveal any significant publication bias [16].
| Variable | Studies, n | t | 95% CI | p |
| LDL | 21 | 0.57 | -1.44, 2.51 | 0.576 |
| TG | 19 | 0.57 | -1.44, 2.52 | 0.573 |
| TC | 20 | 0.07 | -1.77, 1.89 | 0.947 |
| HDL | 20 | 1.59 | -0.402. 2.89 | 0.13 |
| FG | 18 | 1.69 | -0.54, 4.81 | 0.111 |
| HOMA-IR | 9 | -0.44 | -5.7, 3.91 | 0.674 |
| HbA1c | 8 | 1.29 | -1.37, 4.44 | 0.243 |
| SBP | 11 | 0.74 | -2.87, 5.67 | 0.477 |
| DBP | 10 | 0.42 | -2.93, 4.26 | 0.682 |
| CRP | 13 | -1.41 | -4.60, 1.01 | 0.186 |
Table 2: Egger’s test.
Discussion
Our meta-analysis included 26 RCTs involving 1,884 patients with overweight and obesity from Korea, Poland, Australia, Brazil, Canada, Finland, France, Italy, Indonesia, India, Iran, Japan, Tunisia and the United Kingdom [17].
This meta-analysis comprehensively analyzed the effects of probiotics on intermediate disease markers (LDL, HDL, TC, TG, FG, HbA1c, HOMA-IR, SBP, DBP and CRP). Our findings showed that the consumption of probiotics caused a significant decrease in LDL and HOMA-IR levels and a significant increase in HDL levels.
Probiotics supplementation significantly decreased serum LDL levels which are consistent with other meta-analyses. However, the mechanism by which probiotics reduce cholesterol by allowing the unwinding of bile salts in the gut, partly by promoting the production of bile salt hydrolase activity, is currently not well defined. This unwinding of bile salts contributes to decrease the reabsorption of bile salts, with more cholesterol being converted during de novo bile salt synthesis. A previous meta-analysis studied the effects of probiotics on lipid metabolism-related intermediate disease markers (TG and TC) compared to controls and found no difference between interventions, which is consistent with our findings. However, improvement in TG and TC levels in patients with higher TG and TC levels at baseline was statistically significant after supplementation with probiotics. HDL cholesterol is a defense molecule and its plasma levels are inversely associated with cardiovascular disease. Our study revealed that supplementation with probiotics significantly increased HDL levels, which is consistent with the findings of other meta-analyses.
We also found that probiotics had no significant effect on FG and HbA1c levels, whereas other studies have reported probiotics supplementation could reduce FG and HbA1c levels. This may be because the characteristics of the enrolled participants in these meta-analyses were different from ours; for instance, patients with diabetes were included in these studies. Most of the individuals included in our study were obese subjects without diabetes and with normal blood sugar; however, one study was excluded. Therefore, probiotics may be beneficial in reducing blood glucose and HbA1c levels in patients with blood glucose levels above baseline values, but they may have no significant effect in individuals with normal blood glucose and HbA1c levels. However, our results showed a significant decrease in HOMA-IR which is an indicator of insulin resistance in diabetes. Although some meta-analyses did not find significant effects of probiotics on blood glucose due to the different characteristics of the included participants, cumulatively, these observations suggest that probiotics may be beneficial for blood glucose control in diabetic patients. Although many drugs are used to maintain blood glucose control, they may cause adverse reactions. These analyses recommend that probiotics supplementation can be used as an adjunct therapy for patients with type 2 diabetes mellitus for promoting better blood glucose control, ultimately contributing to the reduction of drug usage and drug-associated adverse reactions.
In our study, moderate statistical heterogeneity of FG was observed; however, a sensitivity analysis showed the results were stable. Different types or combinations of probiotic strains may be the source of heterogeneity. Moreover, the timing of probiotics administration in different studies may be another source of heterogeneity.
Unlike previous meta-analysis, we found that BP (including SBP, DBP) was not significantly changed by probiotics which may be resulted in the exclusion of RCTs conducted on patients with hypertension, as probiotics seem to have a greater effect in reducing BP in these patients. This discrepancy in findings may also be attributed to the fact that the specific probiotic strains targeted by multiple meta-analyses were different; for instance, one meta-analysis focused exclusively on a single probiotic strain, namely Lactobacillus plantarum. The results demonstrated the intake of Lactobacillus plantarum supplements significantly reduced SBP and DBP, whereas the multi-strain supplements did not reduce BP significantly. However, the mechanisms underlying the potential antihypertensive effects of probiotics are not really clear. Several recent studies have suggested that hypertension may be associated with gut microbiota dysbiosis and that probiotics might be considered as a novel therapeutic method for hypertensive patients. Probiotics, which modulate Th17 cells to improve salt-sensitive hypertension, may be another mechanism. Nevertheless, more basic experiments and clinical trials are needed to verify the effect of probiotics on BP [18].
The occurrence of obesity is closely related to symbiosis and the damage of intestinal barrier, which could trigger inflammatory response. Previous meta-analyses had found that supplementation with probiotics can significantly reduce CRP levels, however, our data show no significant change in CRP levels after the use of probiotics. This was probably because the subjects included were highly heterogeneous, including individuals with diseases that are highly related to inflammation like rheumatoid arthritis, whereas the inclusion proportion of obese patients was small [19].
There are several strengths associated with our manuscript. First, our study is the first to explore the effect of probiotics supplementation on intermediate disease markers of obesity or overweight. Second, all included studies were RCTs; the included RCTs were recent studies published in the last decade and the sample size was relatively adequate. Third, the findings of our meta-analysis are highly applicable. The results of this study and those of other relevant reviews are similar, showing partially beneficial effects of probiotics supplementation. Furthermore, a comprehensive literature review was conducted to reduce publication bias. In our study, standardized templates were used for independent data extraction and two reviewers were invited for quality assessment to ensure the accuracy of the data. Furthermore, sensitivity analyses were performed on results with high heterogeneity to reduce heterogeneity and to analyze possible sources of heterogeneity. Inevitably, however, this study also has some limitations that should be acknowledged. Firstly, although we applied a comprehensive search strategy we only included RCTs published in English, which can contribute to a language bias. Secondly, owing to the small heterogeneity of treatment time among the included RCTs, a subgroup analysis was not employed to evaluate the treatment effect by treatment time. Moreover, the results of the present study were based only on currently published literature. To this end, a majority of studies have only reported the effects of probiotic mixtures, we were unable to analyze the effects of specific strains on intermediate disease markers [20].
Conclusion
Based on our findings, it can be suggested probiotics may have a safety profile in obese patients that merits consideration by clinicians. However, the low quality of the relevant studies and the small sample size made it difficult to draw definitive conclusions. The imbalance of body function can partly be attributed to the imbalance of gut microbiota, because obesity alters beneficial microbes. Therefore, the supplementation of probiotics may be of great significance in the treatment of obesity to improve the related metabolic parameters such as blood glucose, blood lipids. Thus, the supplementation of probiotics can be taken as a complementary therapy.
In conclusion, this meta-analysis suggests that probiotics has a beneficial effect on some of intermediate disease markers among individuals with overweight and obesity, which indicates a potential complementary therapeutic approach. However, our knowledge on this topic is actively progressing.
Authorship Contributions
Qu L: Conceptualization, methodology, resources, investigation, data curation, formal analysis, validation, software, writing-original draft, writing-review and editing, visualization, supervision, project administration, funding acquisition; Xian W: Conceptualization, methodology, resources, investigation, data curation, formal analysis, validation, software, writing-original draft, writing-review and editing, visualization; Cai P: Resources, investigation, data curation, software, formal analysis; Hao G: Conceptualization, methodology, data curation, software, formal analysis.
Availability of data and materials
This review does not contain any studies with human participants or animals performed by any of the authors. All analyses are based on previously published papers.
Funding
None.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bray GA, Kim KK, Wilding JP (2017) Obesity: A chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev 18: 715-723.
[Crossref] [Google Scholar] [PubMed]
- Blüher M (2019) Obesity: Global epidemiology and pathogenesis. Nat Rev Endocrinol 15: 288-298.
[Crossref] [Google Scholar] [PubMed]
- Cerdó T, García-Santos JA, G Bermúdez M, Campoy C (2019) The role of probiotics and prebiotics in the prevention and treatment of obesity. Nutrients 11: 635.
[Crossref] [Google Scholar] [PubMed]
- Vella CA, Allison MA (2018) Associations of abdominal intermuscular adipose tissue and inflammation: The multi-ethnic study of atherosclerosis. Obes Res Clin Pract 12: 534-540.
[Crossref] [Google Scholar] [PubMed]
- Noce A, Marrone G, Di Daniele F, Ottaviani E, Wilson Jones G, et al. (2019) Impact of gut microbiota composition on onset and progression of chronic non-communicable diseases. Nutrients 11: 1073.
[Crossref] [Google Scholar] [PubMed]
- Gomaa EZ (2020) Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek 113: 2019-2040.
[Crossref] [Google Scholar] [PubMed]
- McFarland LV (2015) From yaks to yogurt: The history, development and current use of probiotics. Clin Infect Dis 60: S85-S90.
[Crossref] [Google Scholar] [PubMed]
- Khalesi S, Sun J, Buys N, Jayasinghe R (2014) Effect of probiotics on blood pressure: A systematic review and meta-analysis of randomized, controlled trials. Hypertension 64: 897-903.
[Crossref] [Google Scholar] [PubMed]
- Cardoso Umbelino Cavallini D, Jovenasso Manzoni MS, Bedani R, Roselino MN, Celiberto LS, et al. (2016) Probiotic soy product supplemented with isoflavones improves the lipid profile of moderately hypercholesterolemic men: A randomized controlled trial. Nutrients 8: 52.
[Crossref] [Google Scholar] [PubMed]
- Mohamadshahi M, Veissi M, Haidari F, Shahbazian H, Kaydani GA, et al. (2014) Effects of probiotic yogurt consumption on inflammatory biomarkers in patients with type 2 diabetes. Bioimpacts 4: 83.
[Crossref] [Google Scholar] [PubMed]
- Ettinger G, MacDonald K, Reid G, Burton JP (2014) The influence of the human microbiome and probiotics on cardiovascular health. Gut Microbes 5: 719-728.
[Crossref] [Google Scholar] [PubMed]
- Higgins JP (2011) The cochrane collaboration’s tool for assessing risk of bias in randomised trials. The cochrane collaboration. BMJ 343: d5928.
[Crossref] [Google Scholar] [PubMed]
- Sohn M, Jung H, Lee WS, Kim TH, Lim S (2023) Effect of Lactobacillus plantarum LMT1-48 on body fat in overweight subjects: A randomized, double-blind, placebo-controlled trial. Diabetes Metab J 47: 92-103.
[Crossref] [Google Scholar] [PubMed]
- Cho YG, Yang YJ, Yoon YS, Lee ES, Lee JH, et al. (2022) Effect of MED-02 containing two probiotic strains, Limosilactobacillus fermentum MG4231 and MG4244, on body fat reduction in overweight or obese subjects: A randomized, multicenter, double-blind, placebo-controlled study. Nutrients 14: 3583.
[Crossref] [Google Scholar] [PubMed]
- Mo SJ, Lee K, Hong HJ, Hong DK, Jung SH, et al. (2022) Effects of Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 on overweight and the gut microbiota in humans: Randomized, double-blinded, placebo-controlled clinical trial. Nutrients 14: 2484.
[Crossref] [Google Scholar] [PubMed]
- Sohn M, Na GY, Chu J, Joung H, Kim BK, et al. (2022) Efficacy and safety of Lactobacillus plantarum K50 on lipids in Koreans with obesity: A randomized, double-blind controlled clinical trial. Front Endocrinol (Lausanne) 12: 790046.
[Crossref] [Google Scholar] [PubMed]
- Lim S, Moon JH, Shin CM, Jeong D, Kim B (2020) Effect of Lactobacillus sakei, a probiotic derived from kimchi, on body fat in Koreans with obesity: A randomized controlled study. Endocrinol Metab (Seoul) 35: 425-434.
[Crossref] [Google Scholar] [PubMed]
- Song EJ, Han K, Lim TJ, Lim S, Chung MJ, et al. (2020) Effect of probiotics on obesity-related markers per enterotype: A double-blind, placebo-controlled, randomized clinical trial. EPMA J 11: 31-51.
[Crossref] [Google Scholar] [PubMed]
- Jung SP, Lee KM, Kang JH, Yun SI, Park HO, et al. (2013) Effect of Lactobacillus gasseri BNR17 on overweight and obese adults: A randomized, double-blind clinical trial. Korean J Fam Med 34: 80.
[Crossref] [Google Scholar] [PubMed]
- Majewska K, Kręgielska-Narożna M, Jakubowski H, Szulińska M, Bogdański P (2020) The multispecies probiotic effectively reduces homocysteine concentration in obese women: A randomized ssdouble-blind placebo-controlled study. J Clin Med 9: 998.
[Crossref] [Google Scholar] [PubMed]
Citation: Wu X, Peng C, Gou H, Le Q (2025) Effects of Probiotics on Intermediate Disease Markers in Individuals with Overweight and Obesity: Systematic Review and Meta-Analysis. Diagnos Pathol Open 10: 252.
Copyright: &Copy; 2025 Wu X, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 245
- [From(publication date): 0-0 - Dec 09, 2025]
- Breakdown by view type
- HTML page views: 176
- PDF downloads: 69