Electrochemical Chelation of Heavy Metals by 2-Benzimidazole
Received: 12-Feb-2018 / Accepted Date: 28-Feb-2018 / Published Date: 05-Mar-2018 DOI: 10.4172/2168-9806.1000188
Abstract
The performance of carbon paste electrodes modified with 2-Benzimidazole Thiol (BIT) was investigated as a modified electrode for the determination of lead. 2-Benzimidazole was developed and introduced as a new modifying agent for heavy metal chelation. The physical parameters that could influence the current densities were investigated and optimized. The prepared electrode showed a good linear response to lead ions in the different concentrations, the detection limit was 2,77.10-9 mol/l. The behavior of Pb2+ is studied by Square Wave Voltammetry (SWV), Cyclic Voltammetry (CV) and Electrochemical Impedance Spectroscopy (EIS). Three peaks of oxidation of Pb2+ are observed successively at -0.7 V, -0.5 V and -0.42 V, the peak of reduction occurs at +0.43 V. The interference effect was studied.
Keywords: Lead; 2-Benzimidazole Thiol; Square Wave Voltammetry (SWV); Cyclic Voltammetry (CV); Electrochemical impedance spectroscopy (EIS)
Introduction
Pollution of the environment by heavy metals is mainly due to human activities but also to natural phenomena such as weather and volcanic eruptions [1-5]. Industrial sources include metal processing in refineries, coal in power plants, petroleum combustion, nuclear power plants and power lines, plastics, textiles, microelectronics, wood preservation and paper processing. Lead is a persistent toxic metal that has the facility to accumulate in humans, animals and plants. According, finding the sensitive, fast and simple analytical method for precise determination and chelating of Pb2+ is a priority.
There are some reviews on the use of spectrometric techniques such Atomic Absorption Spectrometry (AAS) [6,7], atomic emission spectrometry (AES) [8] and mass spectrometry (MS). Although, these methods proved their effectiveness and high but bring also some disadvantages such as complicated operation, expensive maintenance, expensive appliance and low electrical power requirement [9]. Recently, it has been demonstrated that the chemically modified carbon paste electrodes have received considerable attention due to their numerous advantages, such as easy manufacture, no poison, low prize [10-12].
In the present study, we proposed a new modified electrode based on carbon paste modified with 2-Benzimidazole Thiol and we also tested its ability to analyze lead, cadmium and mercury.
Experimental Section
Reagents and chemicals
All chemicals were of the highest quality. Graphite powder (spectroscopic grade RWB, Ringsdorff-Werke GmbH, Bonn-Bad Godesberg, Germany) was obtained from Aldrich and was used without further purification. PbSO4 is obtained from Merck chemicals. Deionised water was used to prepare all solution. All organic molecules were synthesized in our laboratory.
Apparatus
Electrochemical experiments were performed using a Volta lab potentiostat (model PGSTAT 100, Eco Chemie B. V., Utrecht, The Netherlands) driven by the general purpose electrochemical systems data processing software (volta lab master 4 software). A conventional three-electrode system consisting of the 2BIT-modified carbon paste working, platinum counter and SCE reference electrodes was used. ThepH-meter (Radiometer Copenhagen, PHM210, Tacussel, French) was used for adjusting pH values.
Electrode preparation
The 2-Benzimidazole Thiol modified carbon paste electrode (BITCPE) was prepared by thoroughly hand-mixing of synthesis BIT and graphite powder (CP) to give BIT/CP ratios of 10% by weight (w/w). The obtained paste was dried at room temperature then a portion of the resulting paste was grounded and packed firmly into homemade PTFE cylindrical tube (geometric area 0.1256 cm2) electrode. Electrical contact was established with a bar of carbon.
Procedure
The electrode, after having been washed with bidistilled water and heated at room temperature, was transferred into the sample cell (100 mL) (the sample was purged with pure nitrogen for 10 min). A preconcentration step was necessary; the working electrode was immersed in water sample in open circuit. The initial working procedure consisted of measuring the electrochemical response of BIT modified carbon paste electrode in 0.1 M NaCl electrolyte at fixed concentration of Pb2+. The cyclic voltammetry behavior of proposed modified carbon paste electrode was recorded from -1.5 to 1.5 V.
Results and Discussion
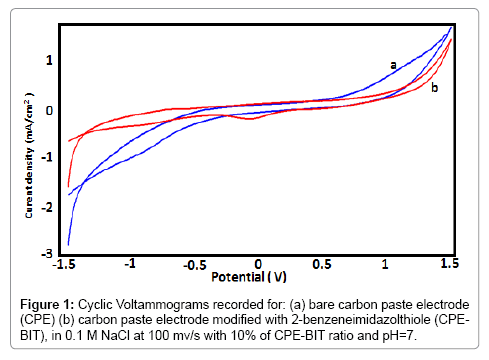
Figure 1 exhibit a cyclic voltammograms (CVs) recorded respectively, at BIT-CPE and CPE, in supporting electrolyte 0.1 M NaCl. The two curves (a and b) are not super imposable which confirms the modification of carbon paste electrode. We can see that the CVs recorded at BIT-CPE shows two will defined peaks, the first in the sense of anodic scanning at about -0.69 V corresponded of the oxidation of 2-Benzimidazole Thiol and the second is located at -0,07 V.
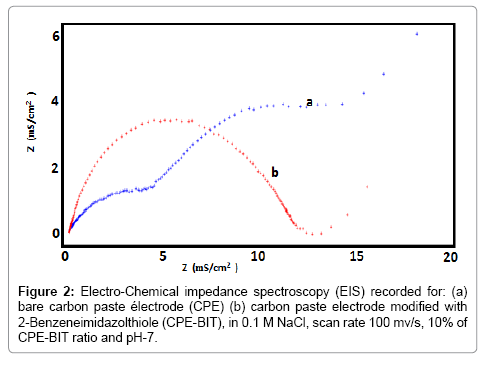
Electrochemical impedance spectroscopy (EIS) corresponding to CPE and BIT-CPE can be seen in Figure 2. In the case of the carbonpaste electrode, the impedance diagram shows three separate time constants, while the one recorded for the BIT-CPE electrode shows an unsymmetrical half circle.
Lead detection
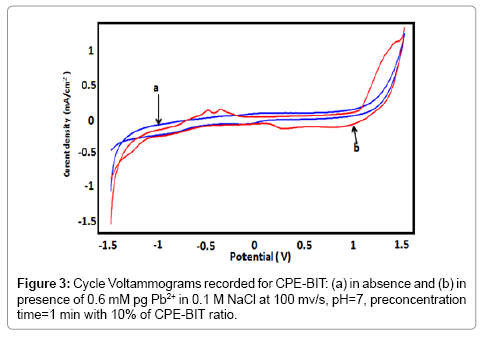
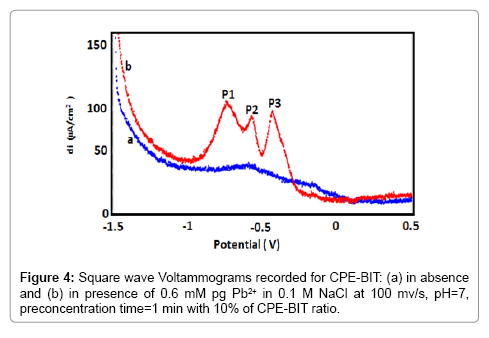
The determination of lead was investigated by the Cyclic Voltammetry (CVs) (Figure 3) and the Square Wave Voltammetry (SWVs) (Figure 4) recorded for BIT-CPE before (curve a) and after 1 min of accumulation in a solution containing 0.6 mM of Pb2+ (curve b). In the anodic scanning direction, two oxidation peaks appear at Epa1=- 0.7 V and Epa2=-0.5 V, whereas in the opposite direction of scanning, we observe one peak at Ep3=-0.42 V.
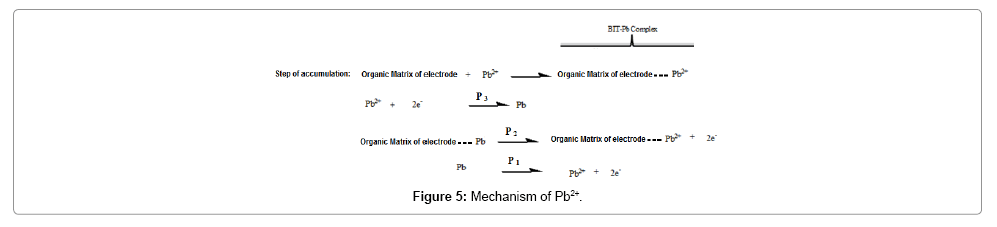
The square-shaped voltammetry clearly shows these peaks which could be attributed according to the following mechanism (Figure 5):
At first, the Pb2+ ions are accumulated in the organic film of the electrode; it is the accumulation step then reduced in situ or in the solution. In the anodic scanning direction the lead formed is oxidized either in the solution or in the organic matrix, resulting in the appearance of two oxidation peaks.
Optimization of experimental conditions
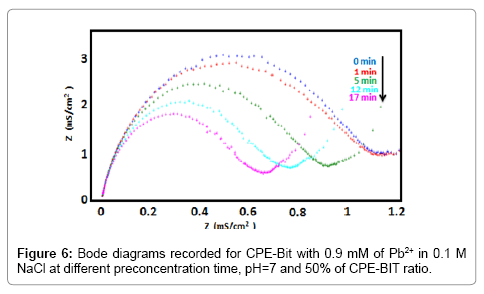
In order to reveal the performance of BIT-CPE to detect lead, we carried out a series of manipulations which aim at the determination of the parameters influencing the electrochemical responses like BIT/CPE ratio, pH, and accumulation time (Figure 6).
Influence of accumulation time
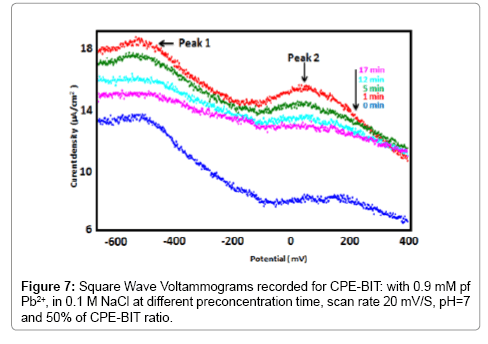
The influence of preconcentration time was examined in 0.9 mM lead solution by SWV and EIS. From the SWVs obtained (Figure 7), we can observe that after only one minute of preconcentration the peak current reaches a maximum value, then begins to decrease gradually until 17 min preconcentration where its becomes stable. The EIS (Figure 8) has the form of a half circle which appears at high frequencies and corresponds to electron exchange phenomena. Then, the preconcentration time was fixed at 1 min for all further experiments.
Influence of scan rate
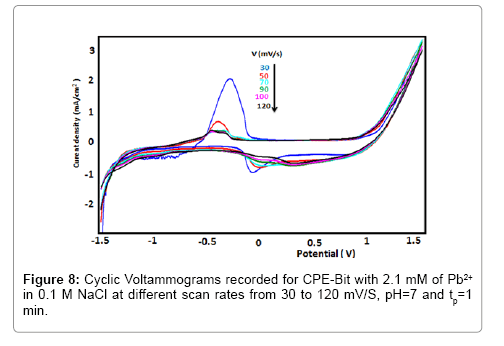
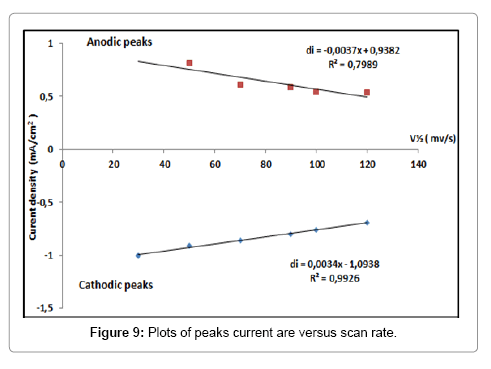
The influence of the scan rate on the redox peaks of Pb2+ was studied for BIT-CPE, at immersed time (1 min) in 2.1 mmol.l-1 of lead. The Figure 8 shows both the anodic and the cathodic peak currents linearly decreases with the scan rate over the range from 30 to 120 mv/s, indicating that the electron transfers for Pb2+ at the CPEBIT is adsorption controlled reaction. The Figure 9 shows the linear relationship between the scan rate anodic peak and cathodic peak currents of Pb2+ at the CPE-BIT.
Influence of pH
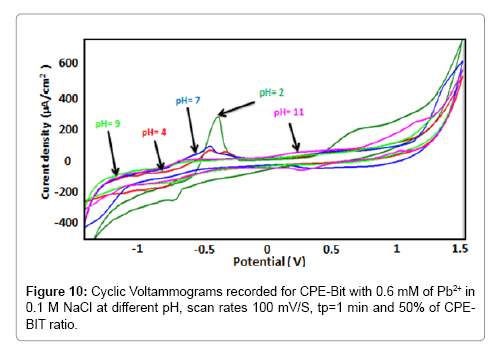
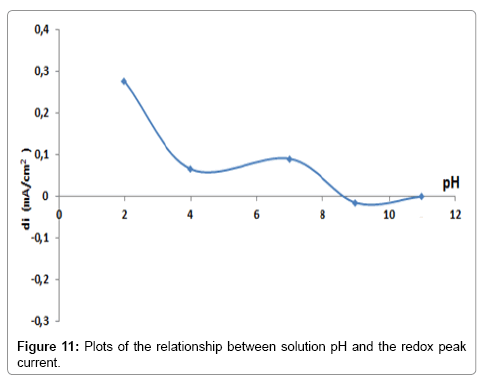
The pH effect on the BIT-CPE peak currents of lead ions was studied in the pH range of 4 to 11. The results obtained (Figure 10 and 11) show that the oxidation peak current increased when the pH value decreases (Figure 12). The decrease in peak currents at higher pH values could be due to the formation of lead hydroxide complexes at higher pH values, which hinders the accumulation of Pb2+ [13].
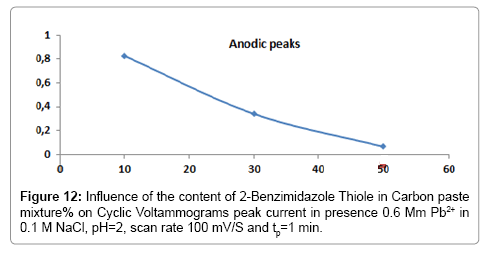
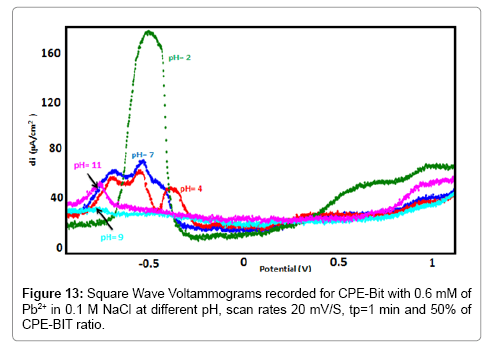
Influence of the BIT loading
The influence of the amount of 2-Benzimidazole Thiol on the sensitivity of the electrode is studied. The results obtained (Figure 13) showed that the peak current decreased with increasing the BIT concentration. This decrease is probably due to the decrease of the conductivity of the surface of the electrode.
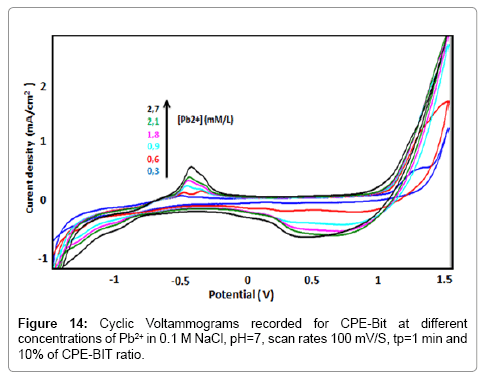
Influence of lead concentration
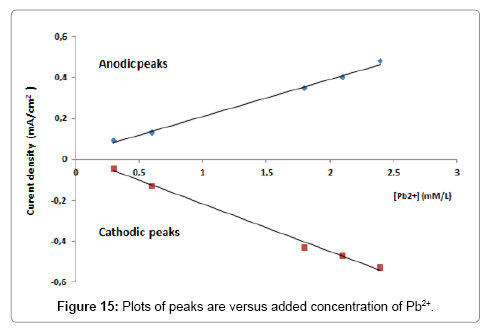
Figure 14 shows the CV curves recorded at BIT-CPE, after pre concentration in solutions containing different concentrations of Pb2+, in the range of 0.3 mM to 2.7 mM. Under optimized conditions the elaborated electrode showed a typical linear response (Figure 15), which can be expressed according to the following equation:
The detection limit (DL, 3s) and quantification limit (QL, 10s) were, respectively, 2,77.10-9 mol/l and 9,24.10-9 mol/l. The two regression lines correspond to the oxidation peaks and reduction peaks are represented by the following two equations (I is expressed in mA and the concentration in mmol/L):
Ioxydation=0.1823[Pb2+] +0.0278 R2=0.9957
Ireduction=-0.2332[Pb2+] +0.0151 R2=0.9947.
Interferences studies
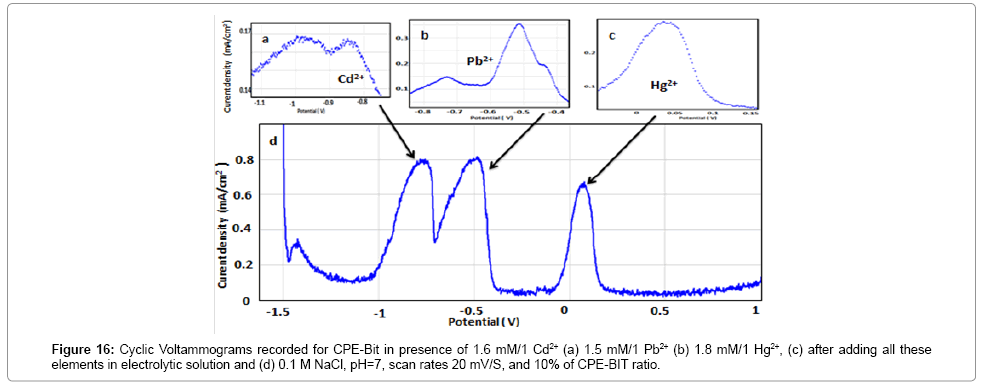
Natural samples such as tap water, plasma, river water, etc. have several chemical elements that affect the health of living beings, which presents a great challenge for chemical analysis laboratories. For this reason, we thought to test the capacity of BIT-CPE to identify with precision three elements at a time such Pb2+, Cd2+ and Hg2+.
Figure 16 shows the Square Wave Voltammograms recorded at BIT-CPE pre concentered respectively, in solution containing, 1.6 mmol/L Cd2+ (curve a), 1.5 mmol/L Pb2+ (curve b), 1,8 mmol/L Hg2+ (curve c) and after adding all these elements in the same solution (curve d) in 0.1 M NaCl while respecting the optimal conditions. From the negative potential range, the appearance of the oxidation peak of Cd2+ at -0.84 V immediately followed the oxidation peak of Pb2+ at -0.7 V, but as soon as we slightly exceed the negative field potential, we are facing the oxidation peak of Hg2+ exactly at +0.03 V.
Conclusion
In this study, we proposed a new modified electrode BIT-CPE for the simultaneous determination of Pb2+, Cd2+ and Hg2+. We have seen through this work that the realized electrode is very sensitive and very selective with regard to several chemical elements which give it the opportunity to analyze them without disruption or overlap. The prepared electrode is not soluble in water, non-toxic and non-polluting.
References
- Fergusson JE (1990) Heavy elements: chemistry, environmental impact and health effects, Pergamon.
- He ZL, Yang XE, Stoffella PJ (2005) Trace elements in agro ecosystems and impacts on the environment. Journal of Trace Elements in Medicine and Biology 19: 125-140.
- Bradl H (2005) Heavy metals in the environment: origin, interaction and remediation. Elsevier 6.
- Shallari S, Schwartz C, Hasko A, Morel JL (1998) Heavy metals in soils and plants of serpentine and industrial sites of Albania. Science of the Total Environment 209: 133-142.
- Nriagu JO (1989) A global assessment of natural sources of atmospheric trace metals. Nature 338: 47.
- Å mirjákova S, OndraÅ¡oviÄová O, KaÅ¡ková A, Lakticova K (2005) The effect of cadmium and lead pollution on human and animal health. Folia Veterinaria 49: 31-32.
- McGaw EA, Swain GM (2006) A comparison of boron-doped diamond thin-film and Hg-coated glassy carbon electrodes for anodic stripping voltammetric determination of heavy metal ions in aqueous media. Analytica Chimica Acta 575: 180-189.
- Baldwin RP, Christensen JK, Kryger L (1986) Voltammetric determination of traces of nickel (II) at a chemically modified electrode based on dimethylglyoxime-containing carbon paste. Analytical Chemistry 58: 1790-1798.
- El Mhammedi MA, Bakasse M, Chtaini A (2007) Square Wave Voltammetric Determination of Paraquat at Carbon Paste Electrode Modified with Hydroxyapatite. Electroanalysis 19: 1727-1733
- Kalcher K, Kauffmann JM, Wang J, Švancara I, Vytřas K, et al. (1995) Sensors based on carbon paste in electrochemical analysis: a review with particular emphasis on the period 1990-1993. Electroanalysis 7: 5-22
- Svancara I, Vytras K, Barek J, Zima J (2001) Carbon paste electrodes in modern electro analysis. Critical Reviews in Analytical Chemistry 31: 311-345.
- Laghlimi C, Smaini MA, Maallah R, Touzara S, El Qouatli S, et al. (2017) Organic Sensor for the Detection of Ammonium. J Biosens Bioelectron 8: 239.
- Chamjangali MA, Kouhestani H, Masdarolomoor F, Daneshinejad H (2015) A voltammetric sensor based on the glassy carbon electrode modified with multi-walled carbon nanotube/poly (pyrocatechol violet)/bismuth film for determination of cadmium and lead as environmental pollutants. Sensors and Actuators B: Chemical 216 : 384-393.
Citation: Charaf L, Madiha E, Hind S, Amine SM, Jihane E, et al. (2018) Electrochemical Chelation of Heavy Metals by 2-Benzimidazole. J Powder Metall Min 7: 188. DOI: 10.4172/2168-9806.1000188
Copyright: © 2018 Charaf L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5773
- [From(publication date): 0-2018 - Nov 12, 2025]
- Breakdown by view type
- HTML page views: 4758
- PDF downloads: 1015