Make the best use of Scientific Research and information from our 700+ peer reviewed, Open Access Journals that operates with the help of 50,000+ Editorial Board Members and esteemed reviewers and 1000+ Scientific associations in Medical, Clinical, Pharmaceutical, Engineering, Technology and Management Fields.
Meet Inspiring Speakers and Experts at our 3000+ Global Conferenceseries Events with over 600+ Conferences, 1200+ Symposiums and 1200+ Workshops on Medical, Pharma, Engineering, Science, Technology and Business
Research Article Open Access
Elimination of Grapevine Bois Noir Phytoplasma by Tissue Culture Coupled or not With Heat Therapy or Hot Water Treatment
| L Chalak1,2*, A Elbitar1, N Mourad1, C Mortada1 and E Choueiri1 | ||
| 1Lebanese Agricultural Research Institute, Tal Amara, P.O. Box 287, Zahlé, Lebanon | ||
| 2Lebanese University, Faculty of Agricultural Sciences, Dekwaneh, Beirut, Lebanon | ||
| Corresponding Author : | L Chalak Lebanese Agricultural Research Institute Tal Amara, P.O. Box 287, Zahlé, Lebanon Fax: + 961 3 211855 E-mail: lamis.chalak@gmail.com |
|
| Received January 14, 2013; Accepted May 10, 2013; Published May 18, 2013 | ||
| Citation: Chalak L, Elbitar A, Mourad N, Mortada C, Choueiri E (2013) Elimination of Grapevine Bois Noir Phytoplasma by Tissue Culture Coupled or not With Heat Therapy or Hot Water Treatment. Adv Crop Sci Tech 1:107. doi: 10.4172/2329-8863.1000107 | ||
| Copyright: © 2013 Chalak L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Advances in Crop Science and Technology
Abstract
Background and Aims: Bois Noir phytoplasma is one of the most widespread yellows in grape industry in the European and Middle Eastern countries causing damages of increasing concern for the growers. So far, traditional before hot water therapy was not totally successfully in controlling this disease as it interfered with the vitality of woody propagation material leading to a weak recovery of grafted vines. This study examined the efficiency of tissue culture techniques to eliminate Bois Noir phytoplasma from grapevines.
Methods: Six tissue culture protocols were tested on Chardonnay grapevine infected by Bois Noir by using stem cuttings and shoot tips associated or not to heat treatment at 38 ± 1°C for 40 days from culture initiation; and stem cuttings combined with a hot water bath at 50°C for 15 and 30 min prior to culture initiation.
Key results: The protocols were all suitable, either for shoot regeneration or for phytoplasma elimination. Stem cutting culture coupled with heat or hot water treatments appeared to be the most effective treatments leading to a correct rate of survival explants and yielding to 100% sanitized shoots.
Conclusion: On the basis of these results, the stem cutting culture coupled with heat or hot water treatment and followed by micropropagation of sanitized explants, appears to be a good candidate to become a routine technique for producing phytoplasma-free vines within certification programs.
| Keywords | |
| Grapevine; Chardonnay; Phytoplasma; Sanitation; Nodal cuttings; Shoot tips; Heat treatment; Hot water bath; PCR | |
| Introduction | |
| Phytoplasmas are wall-less intracellular bacteria restricted to sieve tubes and transmitted by leafhoppers in which they multiply [1]. They affect a wide range of plants causing severe diseases, generally known as yellows [2,3]. In grapevines (Vitisvinifera L.), Flavescence dorée (FD) caused by an elm yellows-type phytoplasma belonging to the 16Sr-V subgroups C and D [4] and Bois noir (BN) elicited by a stolbur-type phytoplasma belonging to 16SrXII-A group [5] are the most common yellows in Europe [6]. | |
| BN, one of the most widespread yellows in the European grape industry [7], occurs also in several Middle Eastern countries, like Israel [8], Lebanon [9] and Syria [10]. BN is particularly serious in Lebanon, especially in the Bekaa Valley, whose damages are the cause of increasing concern for the growers. The control of FD is based on chemical treatments against the vector (Scaphoideustitanus Ball), a strategy that is not effective for BN containment because of the marked differences in its epidemiology [7]. Propagation of infected plants by nurseries widely contributes to the introduction of the disease to viticulture area previously free from it [11]. Therefore, steps are undertaken towards the development of protocols for the production of phytoplasma-free propagating material. | |
| In vivo hot water treatment has been proposed since 1966 [12] for curing dormant woody plant material from phytoplasma and, more recently, as an efficient way to contain spreading of grapevine yellows [13-16]. However, it has been reported that hot water treatment applied to the dormant buds prior to grafting can interfere with the vitality of woody propagation material leading to a weak recovery of grafted vines [17-19]. | |
| Whereas tissue culture techniques, alone or coupled with heat treatment, have been successfully employed for eliminating viruses from a wide range of hosts [20-25] their potentialities for phytoplasma elimination have been much less explored. Notable examples are the successful use of shoot tip grafting for the elimination of the phloemrestricted huanglongbing liberibacter from citrus [26] and the use of apical meristems and embryogenic callus for knocking out sugarcane yellows phytoplasma from sugarcane [27]. More recently, Candidatus Phytoplasma phoenicium was successfully eliminated from different almond varieties using stem cuttings and shoot tip cultures associated or not with thermotherapy [28]. The main advantage of tissue culture techniques is the possibility of large scale propagation of the sanitized stocks that could enter certification schemes. | |
| Because, as mentioned, vineyards severely touched by BN, particularly of cv. Chardonnay, are widespread in some Middle Eastern countries, the control of this disease through the development of a protocol to produce phytoplasma-free propagating material has become an issue of primary importance. In the present paper, we report on the efficiency of procedures using tissue culture techniques associated or not with heat or hot water treatment for producing phytoplasma-free vines of cv. Chardonnay. | |
| Materials and Methods | |
| In summer 2011, shoots were collected in vineyards in West Bekaa (Lebanon) from cv. Chardonnay vines showing yellows symptoms (i.e. leaf rolling, yellowing of the leaves and incomplete wood ripening) that were affected by BN based on PCR assays, RFLP analysis and rDNA sequence [9]. Young shoots from the current season’s growth (June) were surface sterilized with a 30% solution of Na-hypochloride for 20 min, rinsed 3 times in sterile distilled water, and blotted on filter paper. Single-node stem cuttings (1 cm in length) and shoot tips (0.4-0.5 mm in diameter) were excised in a laminar flow cabinet, and used as explants. The growth medium was MS [29] supplemented with 15 mg.l-1 ascorbic acid and 15 mg.l-1 citric acid, 0.5 mg.l-1 BAP (6-benzylaminopurine) and 0.01 mg.l-1 NAA (naphthalene acetic acid), solidified with 0.8% BactoDifco agar, and autoclaved at 118°C for 20 min. All cultures were placed in a growth cabinet with a photoperiod of 16 h of artificial light and 8 h of darkness at 25 ± 2°C, except for cultures exposed to thermotherapy. | |
| Six treatments were tested: (i) culture of stem cuttings with or without thermotherapy at 38 ± 1°C for 40 days from culture initiation; (ii) culture of stem cuttings after a hot water bath at 50°C for 15 and 30 min prior to culture initiation; (iii) culture of shoot tips with and without thermotherapy, where shoots were exposed or not to 38 ± 1°C for 40 days prior to shoot tip collection. For each treatment, 100 explants were used (20 explants×5 replications) vs. 30 explants for the negative control (10 explants×3 replications) as indicated in Table 1. | |
| Newly developed shoots derived from the different treatments underwent subculturing for a period of 30 days before being tested for the first time by nested PCR. Then, explants were multiplied for two other successive subcultures to undergo a second PCR testing before being rooted on MS medium supplemented with 1 mg.l-1 NAA. One positive and one negative controls taken from infected and healthy grapevine samples and assayed for the presence of BN were also used. | |
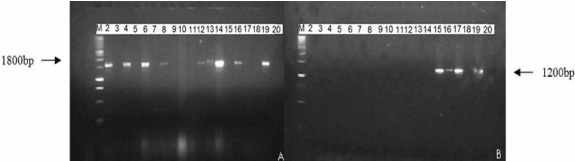
| Total nucleic acids were extracted from portions of whole shoots using the CTAB (cethyl-trimethyl-ammmonium bromide) extraction protocol described by Maixner et al. [3]. Phytoplasma universal PCR primers P1/P7 based on the sequencing of the 16S-23S gene [30] were used for amplification of ribosomal DNA, followed by nested PCR using R16F2n/R16R2 [31]. The amplification of a 1800 bp band for the first PCR and of a 1200 bpamplicon for the nested PCR were expected. PCR products were analyzed by agarose gel electrophoresis, stained with ethidium bromide and visualized under a UV transilluminator. The percentage of explants regenerating new shoots and phytoplasmafree shoots was determined. Mean differences among treatmentswere evaluated by Duncan test (General Linear Models Procedure, SAS Institute, Cary, USA). | |
| Results | |
| Viability and regeneration ability | |
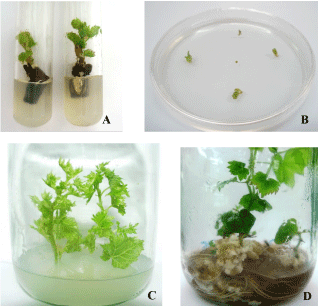
| Figure 1 shows the successive stages of culture evolution for the different explants of cv. Chardonnay while table 1 reports the number of shoots regenerated from the explants after been submitted to the various sanitation treatments. According to the treatments, a certain number of explants did not survive due to an oxidation problem that developed 2-3 days after cultures initiation. Oxidation was particularly evident in stem cuttings and shoot tips that were subjected to heat therapy or hot water bath which led to the loss of a significant number of explants ranked between 22 and 50%. | |
| The survival rate of explants observed 20 days after the initiation of culture varied with the type of explant and treatment applied between 50 and 100%. The best results were obtained with stem cuttings (Figure 1A and 1B), but their treatment with hot water significantly reduced the survival from 100 to 50%. The average number of regenerated shoots per explant observed 30 days after the initiation of culture varied between 1.8 and 2.3. Of all the treatments, was the stem cuttings treated with hot water that gave the lowest number of regenerated plantlets with an average of 1.8 new shoots per explant. | |
| With time, these differences among treatments became lighter and the proliferation ability of the explants issued from heat treatment increased progressively during the successive subcultures (Figure 1C), reaching 2.2 to 2.5 new shoots per explant. These results were comparable to those obtained from control (healthy) explants. Moreover, shoots regenerated from infected material developed normally and similarly to the healthy ones. About a week after the transfer of new shoots to the rooting medium, whitish callus began to appear at the base of the stems and first roots appeared from day 15 (Figure 1D). The rate of rooting recorded after 40 days varied between 84 and 90% without any significant differences among the treatments from which the shoots were issued (not shown). | |
| Effect on sanitation | |
| Table 2 reports the proportion of phytoplasma-free shoots as determined by nested PCR. The expected DNA fragments of 1800 bp and 1200 bp, respectively, were clearly amplified for the positive control samples (Figure 2) confirming their infection with BN while all healthy samples tested were PCR negative. Here it is worthy noted the reliability of results obtained by nested PCR compared to the direct one since, on the whole shoots tested, only three samples found negative in direct PCR (Lanes 15 and 17) were positive with nested PCR, while all the samples found positive in the first test remained positive by nested PCR. | |
| Regarding the various treatments, the nested PCR operated at the end of the first subculture showed sanitation rates varying between 36 and 100%. All tested shoots regenerated from the stem cuttings treated in hot water bath at 50°C for 15 or 30 min were phytoplasma-free, whereas this value dropped to 76% for the shoots regenerated from stem cuttings exposed to heat treatment. As to shoots regenerated from shoot tips, heat treatment increased the sanitation rate from 36% to 76%. | |
| Our sanitation rates are indeed similar or even higher than those previously reported for the elimination of BN from different grapevine genotypes by in vivo treatment with hot water at 50°C for 30 and 45 min of dormant buds prior to grafting [14,15,18,19,32]. By converse, the in vitro hot water treatment of vegetating material proved to be more effective than previously reported in terms of viability and growth ability. A hot water bath for the duration of 15 min only, which proved to be efficient in our study, has apparently not been tested on vines before. | |
| The second nested PCR conducted at the end of the third subculture (after 120 days of culture) confirmed the absence of phytoplasmas in the shoots that were negative in the first test. Among 35 shoots regenerated from the different treatments and found phytoplasma-positive in the first test, only one originating from shoot tip culture after four months of culture proved to be phytoplasma-free during the second PCR (not shown). | |
| Discussion | |
| The survival rate of explants used in the different sanitation treatments was influenced by the type of explants and of the treatment (moderately hot air or hot water). Oxidation was the major drawback, resulting in the loss of explants, especially in the case of heat therapy applied to both shoot tips and stem cuttings. Nevertheless, the explant survival rates remained broadly acceptable to make worthwhile the used sanitation techniques, especially since the ability to proliferate was progressively normalized during successive subcultures. It is worth noting that previous studies involving thermotherapy treatment reported its negative impact on the vitality and growth of woody propagation material which led to a weak recovery of the vines either in the nursery or at later stages [17-19]. | |
| The various protocols of in vitro culture tested in this study for the sanitation of cv. Chardonnay from BN were all effective, with rates varying between 36 and 100%. In general, however, the BN agent appeared to be more difficult to eradicate than phytoplasma infecting other fruits species. Indeed, Candidatus Phytoplasma Phoenicium was completely knocked out from two almond varieties using both stem cutting cultures coupled with thermotherapy and shoot tip culture with or without thermotherapy [28]. | |
| On the other hand, our study showed the potential of successive subcultures for getting rid of phytoplasma from infected vines without any particular treatment. Indeed, the elimination of both FD and BN through the simple culture of nodal sections has previously been reported with percentages varying between 50 and 100%, after 9 months of subculturing [33]. | |
| Even though our results are encouraging, they may be confirmed by histological and ultra-structural studies. Furthermore, it remains to be assessed whether the sanitized shoots will remain symptomless after the hardening stage. Among the different techniques assayed in this study, stem cutting culture coupled with heat treatment seemed to be the most practical and easier way for regenerating phytoplasma-free shoots, with a 78% explants survival rate. Nevertheless, and despite the lower explants survival rate at the beginning, the hot water treatment might be the most efficient sanitation technique as it yielded 100% BN-free shoots. Thus, a protocol based on tissue culture followed by micropropagation of sanitized explants, as shown also by other authors [25,28,34] seems to be a good candidate to become a routine technique for producing phytoplasma-free vines within certification programmes. | |
| Acknowledgements | |
| The authors thank Prof. Giovanni Paolo Martelli and Dr. IvanaGribaudo for their critical review of the manuscript. The kind assistance of Ms. A. Kadri and Mr. A. Chehade at LARI is gratefully acknowledged. | |
| References | |
References
|
|
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
|
| Figure 1 | Figure 2 |
Post your comment
Relevant Topics
- Agricultural science
- Agronomy
- Climate impact on crops
- Crop Productivity
- Crop Sciences
- Crop Technology
- Field Crops Research
- Hybrid Seed Technology
- Irrigation Technology
- Organic Cover Crops
- Organic Crops
- Pest Management
- Plant Genetics
- Plant Breeding
- Plant Nutrition
- Seed Production
- Seed Science and Technology
- Soil Fertility
- Weed Control
Recommended Journals
Article Tools
Article Usage
- Total views: 16417
- [From(publication date):
June-2013 - Sep 02, 2025] - Breakdown by view type
- HTML page views : 11681
- PDF downloads : 4736
