Research Article Open Access
Evaluation of the Ni2+ Phytoextraction Potential in Mesembryanthemum crystallinum (Halophyte) and Brassica juncea
| Amari T1*, Ghnaya T1, Sghaier S1, Porrini M2, Lucchini G2, Attilio Sacchi G2 and Abdelly C2 | |
| 1Laboratoire des Plantes Extrêmophiles, Centre de Biotechnologie de Borj-Cédria, BP 901, 2050 Hammam-lif, Tunisia | |
| 2Department of Agricultural and Environmental Sciences, Università degli Studi di Milano, 20133 Milan, Italy | |
| *Corresponding Author : | Amari T Laboratoire des Plantes Extrêmophiles Centre de Biotechnologie de Borj-Cédria BP 901, 2050 Hammam-lif, Tunisia Tel: +21679325848 Fax: +21679325848 E-mail: taoufik.amari@gmail.com |
| Received January 02, 2016; Accepted February 24, 2016; Published February 29, 2016 | |
| Citation: Amari T, Ghnaya T, Sghaier S, Porrini M, Lucchini G, et al. (2016) Evaluation of the Ni2+ Phytoextraction Potential in Mesembryanthemum crystallinum (Halophyte) and Brassica juncea. J Bioremed Biodeg 7:336. doi: 10.4172/2155-6199.1000336 | |
| Copyright: © 2016 Amari T, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Among heavy metal stressors, nickel (Ni) pollution is one threatening risk to the environment. In this view, the growing concerns about environmental pollution have stimulated the efforts to promote the individuation of phytoextractor plants that are able to tolerate and accumulate toxic metals, including Ni, in the aerial parts. More recently, it has been suggested that halophytes, i.e. native salt-tolerant species, could be more suitable for metal extraction, from saline soils than glycophytes, most frequently used so far. In the framework of this approach, we evaluated here the Ni-phytoextraction ability of the halophyte Mesembryanthemum crystallinum comparatively to the model species Brassica juncea. Plants were maintained for 3 months on a soil containing 0, 25, 50, and 100 ppm NiCl2 . Nickel impaired the growth activity of both species. Interestingly, M. crystallinum was less impacted by NiCl2 addition. The plant mineral nutrition was differently affected by NiCl2 exposure depending on the ion, the species and even the organ. In both species, roots were the preferential sites of Ni2+ accumulation, but the fraction translocated to shoots was higher in M. crystallinum than in B. juncea. The relatively good tolerance of M. crystallinum to Ni suggests that this halophyte is more efficient to extract Ni2+ than B. juncea.
| Keywords |
| Halophyte; Nickel; Phytoextraction; M. crystallinum; Tolerance |
| Introduction |
| Environmental pollution by heavy metals represents a major threat to human, animal and plant health [1,2]. Nowadays, land contamination with heavy metals has become a serious problem in the world. In Tunisia, saline depressions with low population levels, often represent a sink of industrials and urban waste and many of them are contaminated by Cd2+, Pb2+ and Ni2+ [3]. Heavy metals are released into environment by natural and anthropogenic sources. The most significant anthropogenic sources are Human activities, particularly industry, urbanism and agricultural practices [4]. Among heavy metals, Nickel (Ni) is recognized as a dangerous environmental pollutant [5]. It has adverse effects on human health such as Allergic dermatitis, cancer of the lungs, nose and sinuses [6,7]. Cancers of the throat and stomach have also been attributed to its inhalation [8]. In plants, Ni toxicity affects various physiological processes such as water relationship and photosynthesis activity [9,10] nitrogen metabolism and nutrient uptake [11]. In addition, there is increasing evidence that Ni toxicity is associated with oxidative stress [12,13] as reflected by the increase in the concentration of free radicals, which can overwhelm cell’s intrinsic antioxidant defenses and can lead to cell damage or death [7,14]. The growing concerns about environmental pollution have stimulated the efforts to propose new approaches on the remediation of environment. In this way, several physicochemical techniques were used to clean up metal-contaminated soils. Yet, these metal removing processes are quite expensive and can severely inhibit soil fertility with subsequent negative impacts on the ecosystem [15]. Hence, biological treatment, especially phytoremediation, has emerged as a promising technology contributing to reduce the concentrations of Ni in contaminated soils to acceptable levels within a reasonable time frame. This approach based on the capability of selected plants to grow and accumulate metals is an environmental-friendly and relatively cheap technique comparatively to physicochemical methods [16,17]. Phytoremediation includes phytoextraction, phytostabilization, phytovolatization and rhizofiltration [18]. As far as heavy metals are concerned, phytoextraction is especially suitable since those pollutants could not be degraded. Among the Ni-accumulating plants, there is a discrete group of the hyper accumulators that accumulate metal in the shoots to the level of over 1000 μg/g dry weight [19]. All of these plants are typical glycophytes lacking salt-tolerance mechanisms and can therefore not be used to extract metals from salt-affected soils. Recently, it has been suggested that halophytes species, i.e. native salt tolerant species could be more suitable for heavy metal phytoremediation than glycophytes, most frequently used so far [20,21]. Interestingly, literature indicates that halophytes may be useful for phytoremediation [22,23] increasing the interest for halophytic plant utilization to extract several toxic metals [24,25]. Information regarding Ni-phytoextraction using halophytes is scarce. M. crystallinum is a dicotyledonous halophyte from the Aizoaceae family (order: Caryophyllales), commonly known as ice plant, and naturally present in environments characterized by an excess of toxic ions. It has been established as an extremely stress-tolerant model system [26]. This halophyte can yield 20 and 30 t ha−1 biomass and has been shown to accumulate up to 40% NaCl on a dry weight basis. In order to better characterize the Ni-phytoextraction capacity of this halophyte, the plant behavior upon exposure to nickel was compared to B. juncea, a typical glycophyte species commonly used for this purpose [27]. We paid a particular attention to plant growth parameters, and to Ni2+ distribution between roots and shoots. |
| Materials and Methods |
| Soil characteristics and treatments |
| The soil used in this study was collected from the horizon 0–20 cm depth from Borj-Cedria region (30 km north of Tunis). The following soil properties were determined: pH (in water) 7.6; K+ (0.38 μequiv. g−1soil); Na+ (1.31 μequiv. g−1soil); Ca2+ (255.59 μequiv. g−1soil); electric conductivity EC (86.66 μs cm−1); organic matter content (0.47%). The sandy-loam soil was distributed into 24 large plastic pots, each containing 5 kg of air-dried soil. For Ni treatments, the soil was artificially contaminated with 25, 50 and 100 μg Ni g−1soil. Ni was added as aqueous solution of NiCl2 in one dose at the beginning of the experiment. After adding Ni2+, the soil was equilibrated for 21 days during three cycles of saturation with tap water and was thereafter air dried. |
| Culture condition |
| Seeds of Mesembryanthemum crystallinum and Brassica juncea were sown directly in soil, in order to obtain uniform seedlings. Four weeks-old seedlings were selected and transplanted into each pot (3 plants per pot). The experiment was conducted for a period of threemonths and it carried out in an open-air area under natural light and ambient temperature, in order as to keep all plants under conditions as similar as possible to those in the field. |
| Plant growth |
| At harvest, shoots were harvested and successively rinsed three times with cold water and blotted between two layers of filter paper. Roots were carefully removed from the substrate and dipped in a cold solution of HCl (0.01 M) during 5 min to eliminate heavy metals adsorbed at the root surface, and then washed three times with cold distilled water and blotted dry with filter paper. The fresh weight was immediately estimated, and the dry weight was measured after 48 h of desiccation in an oven at 60°C. |
| Nutrient concentrations and nickel accumulation |
| Dried samples (c.a. 300 mg) were ground to a fine powder using a stain-less mill and digested by concentrated HNO3 (10 ml) in a microwave digester (ETHOS D, milestone, Italy) at 100°C. Thereafter, Ni and nutrients concentrations were measured by inductively coupled plasma mass spectrometry (ICP-MS; Perkin Elmer, Sciex-Elan 5000). |
| Bioconcentration factor: The Ni2+ uptake, was depicted by a bioconcentration factor (BCF), provides an index of the ability of the plant to accumulate Ni2+ with respect to the concentration of this pollutant in the soil [28]. It is calculated as follows: |
| Pigment content |
| Pigments were extracted by placing 50 mg of fresh leaf in 2 mL of 100% acetone. The samples were incubated in darkness until complete chlorophyll extraction. Chlorophyll and carotenoids contents in supernatants were analyzed spectrophotometrically at 644.8, 661.6 and 470 nm [29]. |
| Statistical analysis |
| Analyses of variance (ANOVA) with orthogonal contrasts and mean comparison procedures were used to detect differences between treatments. Mean separation procedures were conducted using the multiple range tests with Fisher’s least significant difference (LSD) (P < 0.05). |
| Results |
| Plant morphology and growth |
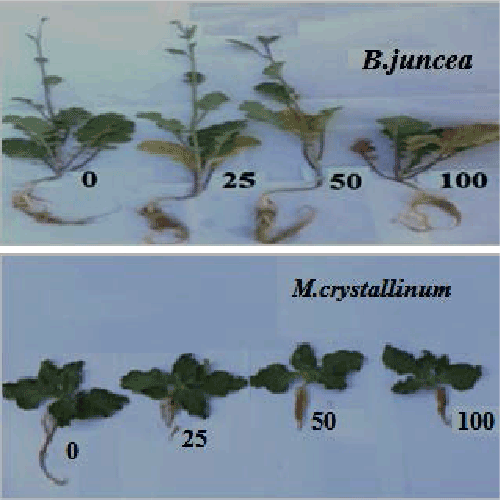
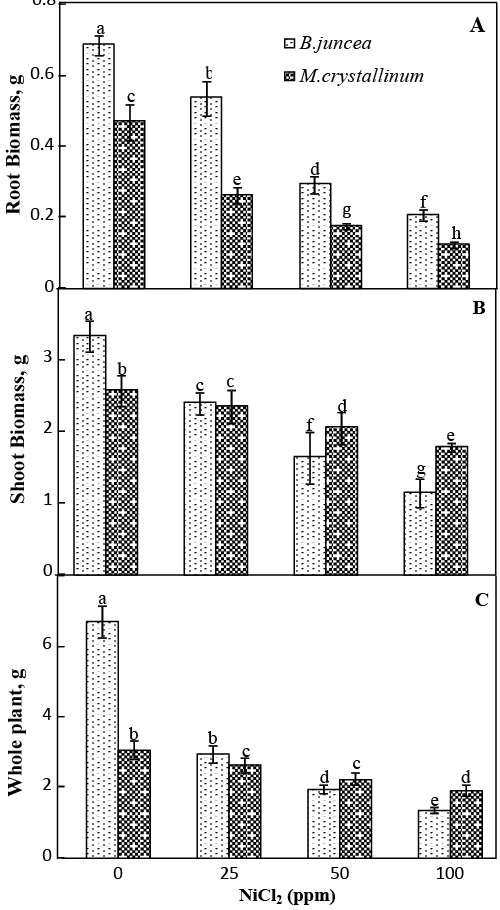
| Results related to the effect of Ni2+ on plant morphology are presented in Figure 1. Ni-exposure of B. juncea plants induced early morphophytotoxicity symptoms as young leaf chlorosis, which was visible 10 days after starting the treatment. Two week later, chlorosis was more severe and necrosis appeared on the oldest leaves with a subsequent falling of these senescing leaves at the highest Ni2+ concentrations. In contrast, such toxicity symptoms were not observed on leaves of Ni-treated M. crystallinum plants, even at the highest Ni2+ concentration. Both root and shoot biomass decreased significantly in both species with increasing Ni2+ concentrations (Figure 2a and 2b). On average, the reductions recorded at 100 μM NiCl2 in shoot biomass was 65% and 30% respectively, for B. juncea and M. crystallinum. For both species, the reduction percentage observed in root biomass reached ca. 60 % as compared to the control at 100 μM NiCl2. As a result, the whole plant biomass production of both species was adversely affected by Ni addition (Figure 2c), with B. juncea more impacted than M. crystallinum at 100 μM NiCl2 (−79% and −37% as compared to the control values respectively). |
| Plant mineral status |
| Significant differences were found in the nutrient uptake and accumulation pattern of Ni-treated plants depending on the element, the species investigated and even the organ (Table 1). With respect to macronutrients, Ca2+, Mg2+ and K+ concentrations decreased significantly with increasing Ni2+ external concentration in M. crystallinum shoots. For this species, root Ca2+ increased significantly, while Mg2+ and K+ remain almost constant. In Ni-treated B. juncea plants, shoot and root concentrations of Ca2+ and Mg2+ were notably higher than those of the control, whereas, K+ remained unchanged. For micro-nutrients, Ni treatment led to a significant increase of Fe2+ and Mn2+ concentrations in B. juncea shoots. However, addition of Ni2+ significantly reduced shoot Fe2+, Zn2+ and Mn2+ concentration in M. crystallinum. In B. juncea roots, a slight increase of micro-nutrients concentrations was noted. In contrast, for M. crystallinum, nickel treatment resulted in a significant decrease of Mo2+ and Zn2+ concentrations. A similar trend was found for root Fe2+ and Mn2+ concentrations, only at low treatment (25μM NiCl2). |
| Ni2+ effect on chlorophyll and carotenoid contents |
| The photosynthetic pigments of Ni-treated B. juncea plants was adversely impacted as reflected by the significant decrease of Chl a, Chl b, and total Chl concentrations (Table 2). For instance, compared to the control, the reductions recorded at 100 μM NiCl2 in Chl a, Chl b and total Chl were 39%, 55%, 44%, respectively. In contrast, for M. crystallinum plants, Ni2+ led to a slight decrease of Chl a, Chl b and total Chl concentrations, excepting in the 100 μM NiCl2 dose, Ni-treated M. crystallinum plants showed a significantly higher Chl concentration as compared to the control (Table 2). For both species, the carotenoid concentration was generally constant following Ni exposure, whereas it decreased significantly in B. juncea at the highest NiCl2 concentration (Table 2). |
| Ni2+ accumulation and translocation |
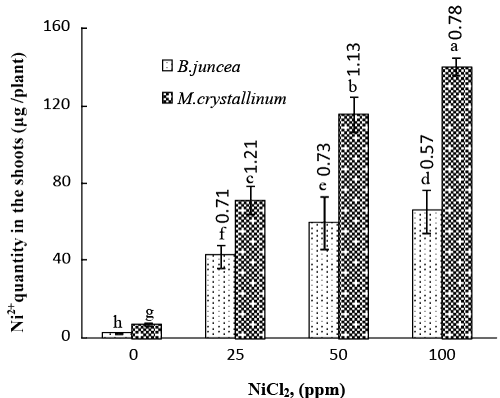
| In treated plants, Ni2+ concentrations increased markedly in both under- and above-ground organs following Ni exposure (Table 3). It is noteworthy that roots of both B. juncea and M. crystallinum accumulated much more Ni2+ than did shoots. M. crystallinum shoot Ni2+ concentrations were significantly higher than B. juncea (for instance 78 μg g−1 DW and 57 μg g−1 DW at 100 μM NiCl2 respectively), the same trend was also observed in roots (for instance 371 μg g−1 DW and 152 μg g−1 DW at 100 μM NiCl2 respectively). The phytoextraction potential of a given species depends not only on metal shoot concentration but also on shoot biomass production. In terms of shoot Ni2+ content (calculated as the product of the shoot metal concentration by its biomass), M. crystallinum translocated more Ni2+ toward shoots as compared to B. juncea irrespective of NiCl2 concentration (Figure 3). For instance, at 100 μM NiCl2, shoot Ni2+ contents were 141 μg plant−1 and 66 μg plant−1 in M. crystallinum and B. juncea, respectively. The higher phytoextraction capacity of M. crystallinum is better highlighted by using the nickel absorption efficiency, which showed higher values for M. crystallinum as compared to B. juncea plants (Table 3). |
| Discussion |
| Phytoextraction, the establishment of plants to extract heavy metals from contaminated sites is particularly challenging due to the high toxicity of these pollutants which commonly hamper plant growth. The identification of Ni-accumulator plant species represents the major prerequest for further rehabilitation of Ni contaminated soils. Recently, it has been reported that halophytes species would be candidate for this purpose compared to glycophytes [25,30,31]. For example, [32] have shown that Messembryanthemum crystallinum is more tolerant to Cu stress than Arabidopsis thaliana. Similarly, [12] clearly showed that Chenopodium botrys an annual halophyte may remove up to 180 g Cd ha-1, which is 6 times more than Cd removal by the hyperaccumulator Noccaea caerulescens. |
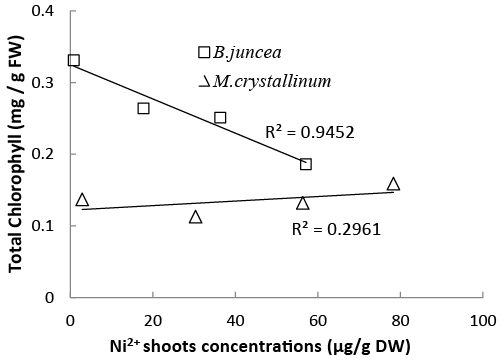
| In the present study, the two tested species showed a different pattern in response to the addition of Ni2+ in the soil. Results showed that the halophyte species M. crystallinum was more tolerant to Ni2+ than the glycophyte B. juncea (Figure 1). Indeed, nickel-induced chlorosis and foliar necrosis were visible only in B. juncea plants, whereas for M. crystallinum seedlings, such toxicity symptoms were not observed even at a shoot tissue concentration higher than 78 μg g−1 dry mass. In both species, Ni negatively affected the plant growth (Figure 1). Biomass productivity of shoots and roots were significantly reduced in response to Ni stress, with root being more impacted than shoot (Figure 2a and 2b). The analysis of total chlorophyll concentrations in apical leaves (Table 2) confirmed that B. juncea was more sensitive to nickel than M. crystallinum. The severe Ni-induced leaf chlorosis observed in B. juncea was associated with lower pigment concentrations in leaves as previously reported in Ni-treated Hordeum vulgare and Triticum aestivum seedlings [33,34]. The abovementioned Ni-related impact on the plant phenotype and/or biomass production may result from direct (toxicity of Ni2+ accumulated in tissues) and/or indirect factors, including the alteration of mineral nutrition, the impairment of the photosynthetic process and the disturbance or imbalance of the plant water status. One of the likely mechanisms that can explain the superiority of the halophyte to maintain its growth potential and to tolerate heavy metals could be, at least partly, linked to the maintenance of an adequate nutrients uptake [31]. Yet, this is not the case in M. crystallinum. In our experiment, Ni-stress adversely affected macro-nutrient (Ca2+, Mg2+ and K+) accumulation in the shoots, and micronutrient (Fe2+, Zn2+ and Mn2+) in the roots of this species. It is known that, heavy metals, including nickel, may compete with essentials nutrients absorption and translocation, which adversely affects the plant mineral status, and even lead to nutrient deficiencies [35,36]. Generally, the effect of toxic heavy metals on nutrients uptake depends at least on two major mechanisms that play a pivotal role in generating metal toxicity. First, the competition for the common binding sites due to the comparable ion radii [37]. For example, Ni has a similar character to Mg, Ca, Fe, and Zn [9]. Second, the decline in nutrient uptake may also result from the metal-induced metabolic impairment that affects the constitution and enzyme activities of cell membranes [38]. Photosynthetic pigments may be used as indicators of metal stress damage [39] and may predict subsequent events at the organism level [40]. In M. crystallinum, the total chlorophyll and carotenoid concentrations were slightly affected in the 0–50 ppm NiCl2 range before significantly increasing at the highest dose likely as a result of rudimentary effect of Ni on Chl and Car biosynthesis concomitant to important reduction of leaf area under 100 ppm Ni leading to the increase of Chl concentration in leaves of these plants (Table 2). The absence of a strong correlation between the shoot chlorophyll concentration and the shoot Ni2+ concentration in M. crystallinum (R2= 0.29) as compared to B. juncea (R2= 0.94) (Figure 4) suggests that nickel is very likely efficiently sequestered in the aboveground organs of the former halophyte species, thus providing a powerful protection of the photosynthetic machinery and hence tolerate better Ni in their leaves. According to literature, there are at several mechanisms that could govern metal tolerance in halophytes when compared to glycophytes. Thus, efficient sequestration was considered an important process allowing the resistance to heavy metals in tissues by its complexation with ligands and/or by their exclusion from metabolically active cytoplasm by moving them into inactive compartments, mostly vacuoles and cell walls [30,41]. It is also worth mentioning that Ni2+ tolerance is also strongly coupled with an effective protection against oxidative stress by the induction dynamic ROSscavenging system [42,43]. Some of these processes might be involved in the rudimentary toxicity signs of Ni expressed by the halophyte M. crystallinum. |
| Regarding Ni2+ accumulation, our results indicate that both species were able to absorb Ni2+ and to translocate it toward their shoots, but the Ni2+ was mainly accumulated in roots (Figure 3). In the shoots, Ni2+ concentrations were significantly lower as compared to those measured in previous work, when the two tested species are cultivated in a Ni enriched nutrient solution [10]. This suggests that Ni2+ is less bioavailable in soil than in hydroponic medium. Heavy metals in soils are intimately associated with different soil components and their mobility and availability in rooting medium is determined mainly by the way metals are bound to these soil components. Numerous edaphic factors such as, pH, soil texture, and organic matter content can affect the heavy metals bioavailability in soils and then the metal accumulation by plants [44]. The soil used in this study is a sandy-loam soil with a limited Ni2+ binding capacity (i.e. low organic matter and clay content). Generally, Ni2+ becomes more totally available under acidic conditions [45]. Elevated pH concomitant with high organic matter content increases the sorption of Ni2+ by soils and thus reduces its bioavailability [46]. Bioavailability of nickel was not assessed after artificial contamination in our substrate. As a consequence, despite a low pH and a low amount of organic matter, it cannot be excluded that a portion of the added Ni became unavailable for a direct absorption by the plant. |
| When cultivated on Ni-contaminated soils, most of Ni2+ taken up was accumulated in roots. Several species adopt this strategy and accumulate toxic metals in the roots such as Thlaspi arvense, Zea mays [47], Hordeum vulgare [48] and Rubus ulmifolius [49]. In plants, it was suggested that nickel could be transported in association with citrate and malate [50,51] as a nickel-peptide complex or as a nickel– histidine complex [52]. Furthermore, nickel may also be sequestered in the cation exchange sites of the walls of xylem parenchyma cells and immobilization in the vacuoles of roots [50]. However, this behavior is not suitable in plants used for phytoextraction of metals. |
| Beside concentrations, a total amount of metals accumulated in the shoots is considered as the most important parameter to evaluate the potential of phytoextraction in plants. M. crystallinum accumulated much more nickel in the shoots as compared with the glycophyte B. juncea (Figure 3). In addition, examining the Bioconcentration Factor (BCF), which is a common index used to estimate plant ability to pump heavy metals from the substrate and to compare species for phytoextraction potentials, revealed that in soil. M. crystallinum showed a higher aptitude to bioaccumulate Ni2+ than B. juncea (Figure 3). For example, BCF values were 0.78 and 0.57 respectively in M. crystallinum and B. juncea exposed to 100 ppm NiCl2. |
| Conclusion |
| Our finding indicated that, on Ni2+-polluted soils, the halophyte species M. crystallinum is more tolerant to nickel than B. juncea and that such a tolerance is associated with a high potential of Ni2+ accumulation in aboveground materials. BCF and the amounts of extracted Ni2+ values indicated that M. crystallinum is more efficient to extract nickel from contaminated soil than B. juncea. Regarding the phytoextraction potential, owing to its capability to accumulate a moderate amounts Ni2+ in the shoots, the halophyte M. crystallinum could be therefore more suitable for metal extraction from moderately polluted sites. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 11092
- [From(publication date):
March-2016 - Aug 29, 2025] - Breakdown by view type
- HTML page views : 10096
- PDF downloads : 996
