Eye Toxoplasmosis – A Case Report
Received: 17-Dec-2018 / Accepted Date: 11-Jan-2019 / Published Date: 25-Jan-2019 DOI: 10.4172/2314-7326.1000285
Abstract
Background: Ocular toxoplasmosis is one of the most frequent etiologies of posterior uveitis. Toxoplasma can be considered an agent of high infectivity and low pathogenicity. The purpose of this study is to report a clinical case of ocular toxoplasmosis.
Material and methods: A case report of a patient, with the accomplishment of diagnostic tests with the confirmation of ocular toxoplasmosis.
Results: Serological tests for toxoplasmosis and hemogram were performed in 1987, with the hypothesis of toxoplasmic retinochoroiditis. In the prenatal care, the result of the serology for toxoplasmosis was negative. In 2010, the examinations of Tonometry, Ocular Biomicroscopy and Eye Fund were performed. In 2011, the examinations Tonometry, Coagulogram, Hemogram, Toxoplasmosis, Simple Bilateral Retinography, Ocular Biomicroscopy, Eye Fund examination showed a sequela of peripheral toxoplasmosis in the left eye, being the first recurrence of ocular toxoplasmosis. In 2016, the second recurrence of ocular toxoplasmosis was verified, with the Ocular Biomicroscopy (BO), Intraocular Pressure (IOP) and Eye Fund tests. In 2017, a simple Retinography examination was performed, concluding the presence of a retinochoroiditis scar in the right eye and two chorioretinal scarring in the left eye. The treatment was performed and after the tests were Visual Acuity, Ocular Biomicroscopy and Computerized Visual Campimetry. The latter allowed diagnosing, in both eyes, global indices within normality.
Conclusion: We report a case of ocular toxoplasmosis with ocular recurrences being performed therapeutic approaches, as well as present examinations performed to accompany the patient, thus achieving a more accurate diagnosis to perform a more effective treatment.Y.
Keywords: Exams; Toxoplasmosis ocular; Uveitis
Introduction
Ocular toxoplasmosis has become the most frequent etiology of posterior uveitis worldwide. The etiological agent Toxoplasma gondii presents a high infection rate in the general population. It is estimated that approximately one-third of the world’s population is infected [1]. Most serological studies refer to normal populations with no clinical signs or symptoms present; Therefore, Toxoplasma gondii can be considered an agent of high infectivity and low pathogenicity. Most cases of ocular toxoplasmosis were believed to be of congenital origin, but evidence leads us to believe that its later acquired form is the most frequent in our environment, and in congenital form, 40% had ocular lesions and 75% of women and 50% of men had unilateral ocular cysts [2].
Toxoplasma gondii, an obligate intracellular parasite found in many animal species worldwide, causes a variety of clinical syndromes in humans. The definitive hosts are the cats and other felines, which are infected, mainly, for being carnivorous [3,4].
Infection is often asymptomatic and most cases of acquired Toxo-plasma infection are subclinical. However, patients infected with congenital and immunodepressed transmission may manifest severe systemic disease. In immunocompetent individuals, toxoplasmosis is manifested mainly by ocular lesions, accounting for up to 85% of infectious uveitis in the posterior segment [5].
Ocular toxoplasmosis usually manifests from the second to the fourth decade of life [6,7]. In most cases, it causes very characteristic ocular lesions, which makes clinical diagnosis possible [8]. Scar lesions are characterized by retinal necrosis, typical of toxoplasmic retinocoriditis. The lesion in macular rosacea is pathognomonic of congenital ocular toxoplasmosis, according to some authors [9].
The active lesion presents a white-yellowish exudate, occasionally gray, with poorly defined limits, which may be multiple or satellite of a preexisting, always in a focal way, which differentiates it from another posterior diffuse uveitis [10].
The symptoms most frequently reported by patients are low visual acuity, appearance of flies or increases of existing ones. Rarely does the patient complain of eye pain, photophobia, and tearing. At the examination, the main signs found are: certain precipitates, corneal edema, presence of flare, cells in the aqueous humor, posterior synechiae, even rubeosis iridis, and vitreitis, which is present with great frequency [11].
The limitations of serological tests are evident, however, in the presence of ophthalmological manifestations considered not suggestive of ocular toxoplasmosis. In addition to a significant percentage of the population, up to 58%, has already been infected by the parasite, presenting positive serologies, the antibody titers do not seem to be related to the activity of retinitis, which makes diagnosis and of therapeutic decisions [12,13]. Thus, the need for the use of tests to rapidly detect and/or exclude the presence of Toxoplasma gondii in the eye confirms the cause of uveitis as well as the exclusion of other infectious agents, which may in some cases, with a similar table, but which require specific treatments and give different prognoses.
Based on clinical and laboratory evidence, the main discussion is about the treatment of uveitis by toxoplasmosis, as well as the evaluation of the interval between the documented infection of systemic toxoplasmosis and the first detection of ocular toxoplasmosis (TO).
The authors present this case to highlight the possibility of ocular toxoplasmosis in an immunocompetent patient, illustrating the severity and irreversibility of the typical retinochoroiditis lesions caused by Toxoplasma gondii and acute phaseopharyngeal alterations.
The purpose of this study is to report a clinical case of ocular toxo-plasmosis. This is a case report of a 33-year-old patient, attended at the IMESP ophthalmology clinic and at the Pediatric Clinic in the city of Pouso Alegre -MG, where diagnostic tests were performed to confirm ocular toxoplasmosis. The work was carried out after approval of the Research Ethics Committee of the mentioned Medical School of Itajuba.
Methods
A case report of a patient, with the accomplishment of diagnostic tests with the confirmation of ocular toxoplasmosis. The present study is presented as a case report, with descriptive characteristics, based on a clinical case of ocular toxoplasmosis in an immunocompetent individual. This study was approved by the Ethics Committee under CAAE nº 95361318.6.0000.5559 opinion nº 2,839,926. To achieve the proposed goal, it was necessary to compile the clinical information, such as symptomatology, case evolution, drug treatment and laboratory tests, such as blood, immunological and imaging tests contained in the patient’s medical record.
The information was analyzed according to the script applied and recorded in the report, considering the annotations relevant to this study. The results and analysis consisted in the characterization of data considered important for such study, considering the theoretical references. The data collected were organized and presented as a table.
Case Report
A.C.D., a 35-year-old, female, Caucasian, from the city of Pouso Alegre, acquired toxoplasmosis in February 1987, when she was 4 years old. She was referred to the pediatrician with complaint of low fever and crying. The complementary tests requested, and their results were: serological reaction for toxoplasmosis, IgG positive (1 for 2000) and negative IgM - indirect immunofluorescence (IFI) method, and blood count with no changes and platelets 310,000/mm3. Based mainly on the clinical and laboratory conditions, the hypothesis of toxoplasmic retinochoroidits was raised and treatment with Sulfamethoxazole 800 mg and Trimethoprim 160 mg (Bactrim F®) was started: 1 oral longterm every 12 hours. Bactrim treatment was performed for 45 days. In the survey of his medical history, on December 15, 1982, during the prenatal care of his mother, the result of the serology of the same for toxoplasmosis was IgG negative and IgM negative as shown in Table 1.
| Year | Exams | Treatment |
|---|---|---|
| 1987 | Laboratory tests: IgG positive (1 for 2000) and negative IgM. | Sulfamethoxazole 800 mg and Trimethoprim 160 mg (Bactrim F®) |
| 2010 | Normal Tonometry and Ocular Biomicroscopy. The Eye Fund exam, being the right eye, normal; left eye, inferior temporal atrophic focus | Ophthalmic follow-up. |
| 2011 | Eyes Fund Exams: peripheral retinochoroiditis - scar lesion of peripheral toxoplasmosis in the left eye and right atrophic superior nasal (NS) right eye. Laboratory tests: IgG> 250.0, and IgM, 0.26. |
Sulfamethoxazole 800 mg and Trimethoprim 160 mg (Bactrim F®) |
| 2016 | Simple Retinography: presence of peripheral degeneration, without holes. Eye Fund Screening: Retinochoroiditis by toxoplasmosis in remission. |
Sulfamethoxazole 800 mg and Trimethoprim 160 mg (Bactrim F®) |
| 2017 | Simple Retinography: retinochoroiditis scar in the right eye and two chorioretinal scars in the left eye. | Sulfamethoxazole 800 mg and Trimethoprim 160 mg (Bactrim F®) and oral corticosteroid. |
Table 1: Exams done.
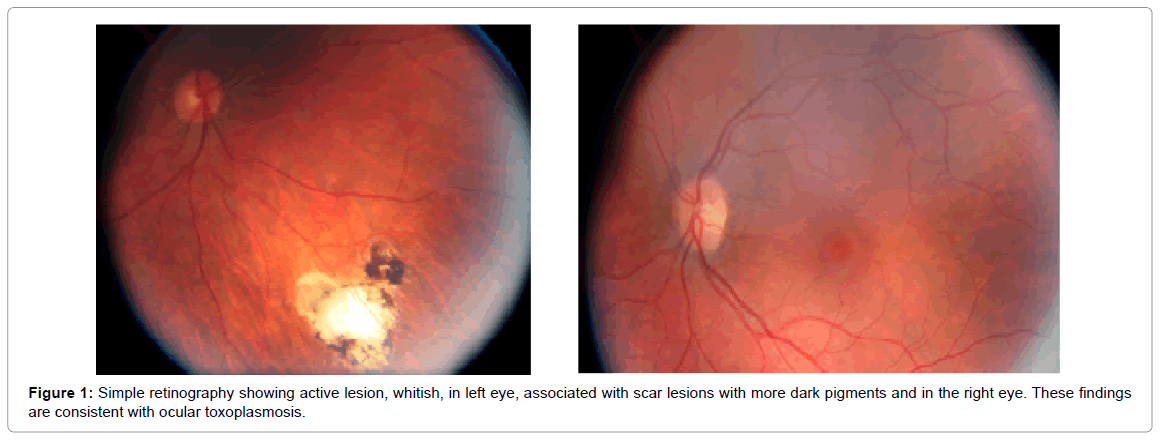
On January 22, 2010, the following tests were performed: Tonometry for left eye, 11 mmHg; for the right eye, 11 mmHg (normal range of intraocular pressure between 11 and 21 mmHg); o Biomicroscopy Ocular for right eye, normal, and for left eye, normal; the Eye Fund exam, being right eye, normal; left eye, inferior temporal atrophic focus [13]. On September 22, 2011, the examinations performed and their results for the right eye were of optic disc with normal size and coloration, with clear margins, without edema, physiological excavation; the vessels presented central emergency, caliber preserved, normal tortuosity, without signs of vasculitis; the inferior pole was without pathological changes, with the macula preserved. For the left eye, the result was an optical disc of normal size and color, with clear margins, without edema, physiological digging; vessels with central emergency, preserved caliber, normal tortuosity, without signs of vasculitis; posterior pole without pathological changes and macula preserved; without pathological changes. The fundoscopy examination revealed characteristic lesions of retinochoroiditis in the periphery of the left eye as shown in Figure 1. The conclusion was a scar lesion of peripheral toxoplasmosis in the left eye as described in Table 1 and Figure 1.
On September 29, 2011 until October 18, 2011, Tonometry presented as a result, for the right eye, 12 mmHg, and for the left eye, 12 mmHg. An assessment of the complaints of blurred right eye was made.
On October 8, 2011, the tests performed were the Coagulogram (coagulation plays a critical protective role in the context of T. gondii infection), [14] with a bleeding time of 1.42 minutes (reference value: up to 4 minutes) ; the time and activity of prothrombin, with international normalized ratio (RNI) of 1.00 (reference value: up to 1.25); prothrombin activity of 99% (reference value: >70%) and prothrombin time of 11.9 seconds; the time course of activated partial thromboblastin presented a patient/control ratio of 1.00 (reference value: up to 1.20), and the hemogram showed results for the Erythrogram: red blood cells, 4.15 (reference value: 3.80 to 5.00 ml/mm3), hemoglobin, 12.8 g/dL (reference value: 12.0 to 16.0 g/dL); hematocrit, 38% (36- 48%); V.C.M., 91.6 fL (reference value: 81 to 100 fL); H.C.M., 30.9 pg (26-36 pg); C.H.C.M .: 33.7 g/dL (reference value: 32 to 35 g/dL); R.D.W., 13.3% (reference value: 11 to 16%). Results for Leukogram: total leukocytes, 4,200/mm³ (reference value: 3,500 to 10,000), blasts, 0 (reference value: 0); promyelocytes, 0 (reference value: 0); Myelocytes, 0 (reference value: 0); metamielocytes, 0 (reference value: 0); rods, 0 (reference value: 0); segmented, 2,142 (reference value: 1500 to 8000); eosinophils, 92 (reference value: up to 500); basophils, 38 (reference value: up to 100); lymphocytes, 1642 (reference value: 800 to 4000); atypical lymphocytes, 0 (reference value: up to 200); monocytes, 286 (reference value: up to 900); platelets, 271,000 (reference value: 130,000 to 450,000/mm3). For the Toxoplasmosis test, the results were: IgG antibodies> 250.0 (reference value: positive: >8.0 IU/mL, undefined 6.5 to 8.0 IU/mL, negative: <6.5 IU/mL). Toxoplasmosis: IgM antibodies, 0.26 (reference value: positive: >3.50 IU/mL, undefined: 3.0 to 3.5 IU/mL, negative: <3.0 IU/mL).
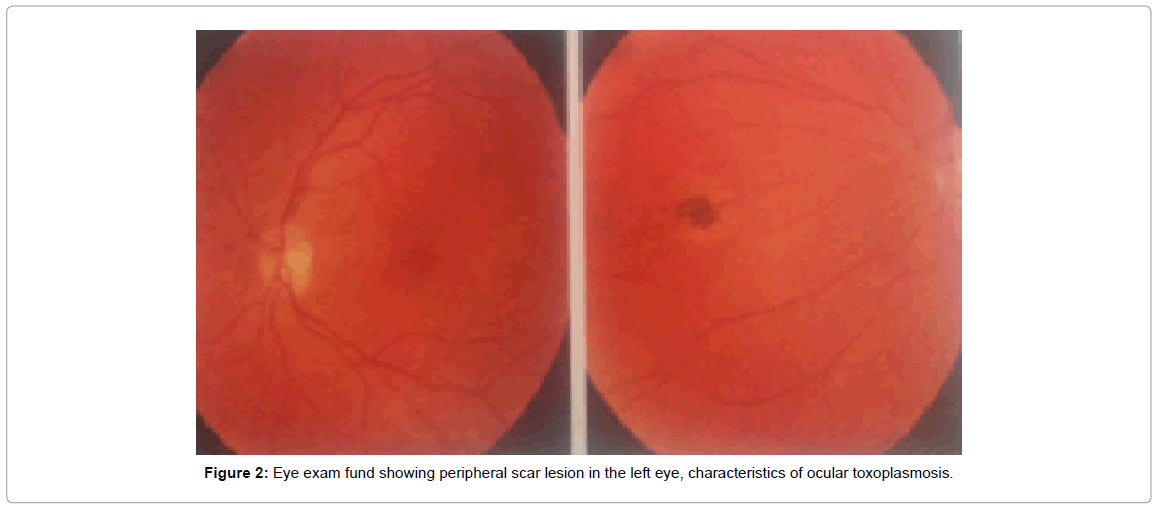
On October 28, 2011, the Bilateral Simple Retinal Examination presented the following results: both eyes presented optic disc with clear borders and normal staining; 0.3 mm digging in the right eye and 0.2 dd in the left eye; vessels with caliber and path without alterations; retina applied 360 degrees at the periphery, with scarring on the mean inferior temporal periphery of the right and nasal eye of the left eye; macula with brightness and shape without changes. Other exams and their results: Ocular Biomicroscopy, being normal right eye; normal left eye; Eye Fund examination, being right eye with superior nasal atrophic focus (NS); left eye, with atrophic NS focus as shown in Figure 2. The guideline was to use Bactrim F® as described in Table 1 and Figure 2.
On December 20, 2016, the second recurrence of ocular toxoplasmosis was observed, and the patient was using Bactrim F® (D34). The follow-up was done with the Ocular Biomicroscopy examinations, which presented results without alterations; o Intraocular Pressure, 11 mmHg; the Eye Fund examination of the right eye resulted in the presence of a chorioretinal scar in the lower nasal periphery, with a pigmented focus, a papilla with a physiological excavation with normal vessels and a preserved macula; the Eye Fund examination of the left eye presented papilla with sharp edges and normal coloration, physiological excavation, with normal vessels, macula and foveal brightness preserved. The presence of peripheral degeneration without holes was observed. The impression was of retinochoroiditis by toxoplasmosis in remission. The patient-oriented approach was to maintain the treatment for up to 45 days. Simple Retinography and Retinal Mapping were also requested.
On January 19, 2017, the evaluation of the Simple Retinography examination (indirect ophthalmoscopy) presented papilla with 0.406 H x 0.367 V digging, with sharp edges and normal staining; vessels with preserved caliber and tortuosity, preserved macula and foveal bright-ness, presence of retinochoroiditis scar on lower nasal periphery, with lesion in remission. The left eye had a papilla with 0.488 Hx 0.308 V digging, with clear borders and normal color, vessels with preserved tortuosity and caliber, preserved macula and foveal brightness, presence of two retinochoroiditis scars on average nasal periphery and extreme nasal periphery without focus of active injury. Printing was of retinochoroiditis in remission in the right eye. Retinography examination showed papillae with sharp edges, pinkish color and physiological CD (diameters), vessels with central emergency, retina applied with 2: 3 arteriovenous relation and path without alterations, macula preserved, presence of scar in the lower nasal peripheral region. For the left eye, papilla with sharp edges, pinkish coloration and physiological C-D relation, vessels with central emergency, retina applied in 2: 3 arteriovenous relation and path without alterations, macula preserved, presence of two chorioretinal scars in the peripheral nasal region. The conclusion was the presence of a retinochoroiditis scar in the right eye and two scars lesions in the left eye. It was suggested to correlate with other clinical exams and to perform routine ophthalmologic follow-up. The indicated course was follow-up in thirty days. The evaluation was of second relapse (the first one was in 2011). Treatment with sulfadiazine and pyrimethamine was initiated with side effects for one week, Bactrim F® for 35 days and used oral corticosteroids. He did not use eye drops. After a week after stopping her medications, the patient complained that her vision had blurred a bit. The impression was of Visual Acuity (AV) without correction, of the right eye, 20-20 p, cloudy, and of the left eye, 20-20 p. Ocular Biomicroscopy showed absence of anterior chamber reaction. The following results were found in the right eye: lower nasal scar - NSINF, satellite focus in resolution without signs of vitreitis. In the left eye: Upper Lattice and nasal scar. On May 17, 2017, Computerized Visual Campimetry was performed, which allowed to diagnose, in both eyes, global indices within the normal range. The conclusion we reached was that the poor defects that appear at some points might be just fluctuations and possibly have no value. They are observed in many normal visual fields.
Discussion
Toxoplasmosis infection in humans is often subclinical, presenting asymptomatic or with nonspecific symptoms, not determining any ocular involvement. The clinical presentation is quite heterogeneous. Some patients have episodes of minimal inflammation, while others have multiple recurrences of severe uveitis, leading to loss of vision. The evolution to retinocoroiditis makes the toxoplasmic infection clinically evident, being the most common cause of posterior uveitis, and may present severe sequelae, including complete loss of vision [15].
Retinochoroiditis rarely occurs as a manifestation of acute infection, although it is found at higher rates in immunocompromised patients. It is more common that it is manifestation of the congenital disease or the reactivation of the chronic infection [16]. The typical retinocoriditis lesion would be necrotizing focal retinocoriditis, accompanied by a vitreous reaction, and may be associated with the healed satellite lesion, indicative of recurrent attack. Retinochoroiditis due to toxoplasmosis may be of congenital or postnatal origin because of acute infection or reactivation [17].
Some individuals do not develop ocular disease, even if they have extensive or multiple retinocoriditis scars, while others have frequent recurrences, even without being associated with an immunosuppressive disease. At each reactivation of toxoplasmic uveitis, a further cycle of infection and inflammation occurs, and the initial lesion may affect the macular region and, the closer to the fovea, the worse the visual prognosis, once tissue damage will not be regenerated [17].
The diagnosis of toxoplasmosis is a sum of findings that proves an eye infection that is suspected to be of toxoplasmic origin. We also excluded other differential diagnoses of posterior uveitis. Diagnosis is often based on serological results, and this is the first method used to evaluate whether the patient has infection with the parasite. Four types of immunoglobulins, IgM, IgG, IgA and IgE, can be identified in the serum of patients infected with T. gondii. Each immunoglobulin has temporal characteristics that will help to determine if the infection is acute or chronic, despite finding situations in ocular toxoplasmosis that doubt will persist. Along with the serology, complementary examinations are used in the area of Ophthalmology [17].
The acute phase of systemic toxoplasmosis is characterized by the presence of anti-T. gondii IgM antibodies in serum; increased titers of anti-T. gondii IgG antibodies four times in serum; or seroconversion. The determination of anti-T. gondii IgA antibodies is an additional test, especially in neonates [17].
It is indicated the accomplishment of the early intrauterine or neonatal diagnosis, important for institution of treatment and reduction of risks of morbidities and sequels. The outpatient follow-up of children with suspected and confirmed diagnosis of congenital toxoplasmosis is mandatory and includes physical examination by the general pediatrician and infectologist, monthly, neurologist and ophthalmologist every six months, and complementary exams such as serological tests, complete blood count, CSF study, ultrasonography and cranial tomography. The maternal serology for IgG and IgM antibodies and the serological screening for T. gondii in the neonate are of great importance, mainly to detect cases in which maternal infection and transmission occurred very late in pregnancy [18].
For treatment, the combination of prednisone, pyrimethamine and sulfonamides is the preferred therapy of the parasite experts, and other combinations of drugs, such as azithromycin and atovaquone, are used. Systematic reviews, however, indicate that none of them was superior in reducing the time of active disease or the rate of recurrence in immuno-competent patients [17]. The response to antibiotic therapy combined or not with corticosteroids varies widely among patient [15].
In ocular toxoplasmosis, there is usually no correlation between antitoxoplasma antibody levels and patient symptomatology. It is common the occurrence of titles less than 1/1024 and the IgM screening is, in most cases, negative. Serological tests are of little help in the diagnosis of ocular toxoplasmosis. In cases where the differential diagnosis with toxoplasmosis is required, determination of antibody titer in the aqueous humor may be enlightening. Comparing serum immunoglobulin levels with those found in the aqueous humor, it is possible to define whether there is intraocular antibody production, ie active ocular toxoplasmosis [19].
A poor understanding of the pathophysiology of ocular toxoplasmosis is mirrored by the inability to unequivocally confirm a clinical diagnosis based on laboratory tests. Although the clinical manifestations of the disease are usually highly characteristic, atypical manifestations are not uncommon, and these are not always recognized as specific of ocular toxoplasmosis even by experienced ophthalmologists [20].
Although the diagnosis of ocular toxoplasmosis can be added by the results of serological tests, these are not in themselves conclusive. Ocular toxoplasmosis always registers positive for Toxoplasma-specific IgG; but so, too, do infected individuals who manifest no signs of ocular involvement. The detection of Toxoplasma-specific IgG is of low diagnostic value [21,22]. Toxoplasma-specific IgM can be detected in the serum, which may be indicative of a recently acquired infection.
However, in cases of acute infection equivocal or positive results are not of diagnostic value. If the serological data confirm the existence of a recently acquired infection, then the alternative of a reactivated latent condition can be excluded. The absence of specific antibodies affords strong evidence against a toxoplasmic origin of the ocular disease. The parasite itself has been detected in the peripheral blood both of patients with ocular toxoplasmosis and of control individuals [23]. Then specific antibodies or of the parasite in peripheral blood is not confirmative of ocular involvement.
Conclusion
Ocular toxoplasmosis is a serious public health problem, understanding its clinical manifestations in immunocompetent individuals becomes a useful tool in the medical field. We report a case of ocular toxoplasmosis with ocular recurrences being performed therapeutic approaches. The therapeutic response was favorable in the case, with the improvement of the clinical manifestations. We also present in this study, various examinations performed to accompany the patient, thus achieving a more accurate diagnosis to perform a more effective treatment.
References
- Foster CS, Vitale A (2002) Diagnosis and treatment of uveitis. Jaypee Brothers Medical Publishers, New Delhi, India.
- Pereira MF, Silva DAO, Ferro EAV, Mineo JR (1999) Acquired and Congenital Ocular Toxoplasmosis Experimentally Induced in Calomys callosus (Rodentia, Cricetidae). Mem Inst Oswaldo Cruz 94: 103-114.
- Bregano RM, Mori FMRL, Navarro IT (2010) Toxoplasmosis acquired in pregnancy and congenital: surveillance in health, diagnosis, treatment and pipelines 1-5.
- Langoni H (2006) Occupational diseases in poultry. In: Andreatti Filho RL. Avian Health and Diseases. São Paulo 52-60.
- Talabani H, Mergey T, Yera H, Delair E, Brézin AP, et al. (2010) Factors of occurrence of ocular toxoplasmosis: a review. Parasite.
- Friedmann CT, Knox DL (1969) Variations in recurrent active toxoplasmic retinochoroiditis. Arch Ophthalmol 81: 481-493.
- Bosch Driessen LE, Berendschot TT, Ongkosuwito JV, Rothova A (2002) Ocular toxoplasmosis: clinical features and prognosis of 154 patients. Ophthalmol 109: 869-878.
- Machado RAF, Bortolli JP, Bassanezi F (2016) Prevalence of chorioretinal scarring on angiographic examinations. Rev bras oftalmol 75.
- Melamed J, Souza CEL, Caramoni CRA (1988) Toxoplasmosis: Ocular manifestations. AMRIGS 32: 163-169.
- Soares P, Hammoud RR, Slavo N (2006) Case Report. Revicience. 8: 10-12.
- Rothova A, Knapen F, Baarsma GS, Kruit PJ, Kijlstra A, et al. (1986) Serology in ocular toxoplasmosis. Br J Ophthalmol 70: 615-622.
- Kanski JJ, Bowling B (2011) Clinical ophtalmology: A systematic approach. 7th ed. Eselvier Saunders 429-433.
- Johnson LL, Berggren KN, Szaba FM, Chen W, Smiley ST (2003) Fibrin-mediated protection against infection-stimulated immunopathology. J Exp Med 197: 801-806.
- Cordeiro CA, Moreira PR, Dutra WO, Young L, Campos WR, et al. (2010) Imunologia da retinocoroidite toxoplásmica. Arq Bras Oftalmol 73: 548-551.
- Castro VM (2012) Active presumed toxoplasmic retinocoroiditis: Clinical parameters and evaluation of the immunophenotypic expression of CD14 + monocytes. Belo Horizonte: UFMG.
- Oréfice F, Cunha Filho R, Barboza AL, Oréfice JL, Calucci D (2010) Toxoplasmose ocular adquirida. Toxoplasmose ocular pós-natal. Rev Bras Oftalmol 69: 184-207.
- Carvalho AGMA, Lima JS, Lima MSPR, Mota CAX (2014) Diagnóstico laboratorial da toxoplasmose congênita. Rev. Ciênc. Saúde Nova Esperança 12: 88-95.
- Affra NA, Seidman CJ, Weiss LM (2013) Toxoplasmose ocular: Controvérsias na Prevenção Primária e Secundária. J Neuroinfect Dis 4: 235689.
- Stanford MR, Ver SE, Jones LV, Gilbert RE (2003) Antibióticos para a retinocoroidite toxoplasmática: uma revisão sistemática baseada em evidências. Oftalmologia 110: 926-931.
- Holliman RE, Stevens PJ, Duffy KT, Johnson JD (1991) Investigação sorológica da toxoplasmose ocular. Br J Ophthalmol 75: 353-355.
- Chapman DJ, Ashburn D, Ogston SA, Ho-Yen DO (1999) A relação entre a toxoplasmose ocular e os nÃveis de anticorpos especÃficos para o toxoplasma. Epidemiol Infect 122: 299-303.
- Bou G, Figueroa, MS, Marti-Belda P, Velez RL, Guerrero A (1999) Valor da PCR para detecção de Toxoplasma gondii em amostras de sangue e humor aquoso de pacientes imunocompetentes com toxoplasmose ocular. J Clin Microbiol 37: 3465-3468.
Citation: Almanca ACD, Jardim SP, Silva BME (2019) Eye Toxoplasmosis – A Case Report. J Neuroinfect Dis 10: 285. DOI: 10.4172/2314-7326.1000285
Copyright: © 2019 Almanca ACD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4798
- [From(publication date): 0-2019 - Oct 09, 2025]
- Breakdown by view type
- HTML page views: 3923
- PDF downloads: 875