Research Article Open Access
Fatty Acids Profile of Microbial Populations in a Mining Reclaimed Region Contaminated with Metals: Relation with Ecological Characteristics and Soil Respiration
| Ramya Narendrula1 and Kabwe K. Nkongolo1,2* | |
| 1Biomolecular Science Program, Laurentian University, Sudbury, Ontario, P3E 2C6, Canada | |
| 2Department of Biology, Laurentian University, Sudbury, Ontario, P3E 2C6, Canada | |
| Corresponding Author : | Kabwe K. Nkongolo Department of Biology, Laurentian University Sudbury, Ontario, P3E 2C6, Canada Tel: 1-705-675-1151 ext-2307 E-mail: knkongolo@laurentian.ca |
| Received November 11, 2014; Accepted January 28, 2015; Published January 30, 2015 | |
| Citation: Narendrula R, Nkongolo KK (2015) Fatty Acids Profile of Microbial Populations in a Mining Reclaimed Region Contaminated with Metals: Relation with Ecological Characteristics and Soil Respiration. J Bioremed Biodeg 6:274. doi:10.4172/2155-6199.1000274 | |
| Copyright: © 2015 Narendrula R, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Mining activities cause drastic disturbances to terrestrial ecosystems affecting the landscape, soil organisms and quality. Ecosystem consists of aboveground and belowground components that interact and influence community and ecosystem level processes and properties. The present study aimed at determining the effects of mining on plant population diversity, genetic variation, soil respiration, and the abundance and composition of soil microbiota in Northern Ontario. Results from population diversity analyses revealed that species diversity and abundance were lower in sites close to smelters. The mean Shannon index value was significantly higher in reference sites compared to eroded/disturbed sites. Tree species richness was 4.7, 5.3, and 7.7 for eroded/disturbed, stable upland and reference sites, respectively. Molecular analysis revealed no differences in genetic variation among plant populations from eroded/disturbed, stable upland and reference sites for the three hardwood species. Soil respiration and PLFA analysis revealed that respiration, total microbial biomass, fungal and bacterial abundances were significantly lower in eroded/disturbed sites compared to reference sites. Overall, microbial community biomass, respiration and fungal abundance significantly increased with higher plant diversities as did soil C and N concentrations. The ratios between fungi and bacteria biomass and among other PLFA measures were extremely low suggesting that the targeted region is still under environmental stress.
| Keywords |
| Plant diversity and abundance; Genetic analysis; Soil respiration; PLFA; Microbial biomass |
| Introduction |
| Surface mining related activities result in severe disturbance of land areas throughout the world. The Greater Sudbury Region (GSR) in Ontario, Canada has a history of logging, mining and smelting of metalliferous ores since late 1800 [1-3]. These activities led to the release of enormous amounts of sulfur ore and released more sulphur dioxide (SO2) into the atmosphere than any other complex in the world resulting in severe contamination and acidification of soils and water at sites approximately 30 km from the smelters in the GSR [1,2]. These factors devastated forest ecosystems by greatly reducing the diversity of plants, animals and microorganisms making the GSR area one of the most ecologically disturbed regions in Canada. Numerous studies have demonstrated the adverse effects of metal contamination on microbial diversity and activities in soil [4]. |
| During the last 30 years, production of nickel (Ni), copper (Cu) and other metals have remained at high levels but through combination of industrial technological developments and legislated controls the industrial SO2 emission has been reduced by 90% [3]. This allowed for a certain degree of recovery to occur, such as improved air quality and natural recovery of damaged ecosystems. Further, the recovery has been achieved through soil liming, seed distribution and reforestation program (Sudbury Regreening/Land Reclamation). Over 12 million trees has been planted in the GSR leading to the increase of soil organic matter and microbial biomass/communities [5,6]. |
| Soils with high concentrations of metals from natural or anthropogenic activity may pose considerable challenge to exposed biota. For example, Cu, iron (Fe), manganese (Mn) and zinc (Zn) are essential micronutrients required for a wide variety of cellular processes in plants [7,8] However, these same metals can be toxic and inhibit growth of plants and their associated soil microorganisms when present at excessive levels [7]. Soil microbes play significant roles in recycling of plant nutrients, maintenance of soil structure, detoxification of noxious chemicals and the control of plant pests and plant growth [4]. Soil microorganisms mediate key processes that control ecosystem carbon (C) and nitrogen (N) cycling and they potentially represent a mechanistic link between plant diversity and ecosystem function [9]. With the increasing emphasis on sustainable fertility and environment benefits, protection of soil and restoration of soil microbial activity is therefore of high priority and a thorough understanding of ecosystem processes is a critical factor in assuring that soil remains healthy [10]. |
| To date, information on landscape degradation, soil toxicity, acidification, plant metal accumulation, genetic diversity and forest composition in Northern Ontario is readily available but the knowledge and relationship between plant population diversity, soil health and soil quality within the GSR in lacking. The objectives of the present study were 1) to assess plant population diversity and sustainability in mining disturbed areas in Northern Ontario; 2) to determine microbial diversity and abundance in various disturbed and undisturbed sites; and 3) to establish relationships between above ground (plant population diversity) and below ground biodiversity. |
| Materials and Methods |
| Field site |
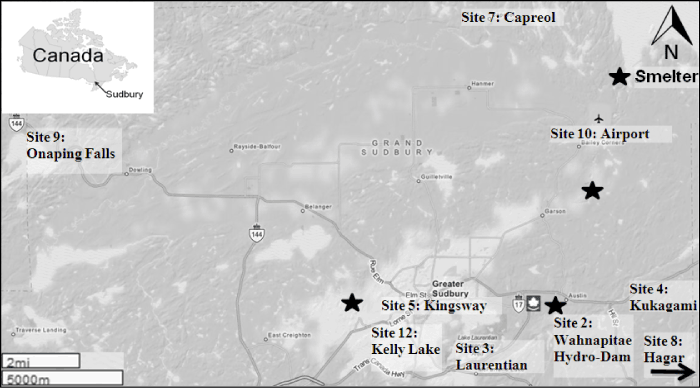
| The study was conducted in a mining region of Greater Sudbury in Northern Ontario, Canada (46°30′ N, 80°00′ W). It lies on the Pre- Cambrian Shield and has a mean elevation of 300 m above sea level and its topography is characterized by mosaic of rock outcrops, glacial till deposits, numerous lakes and narrow valleys resulted during the Wisconsin glaciations [2]. Overall, nine sites were selected from the Northern Ontario and grouped based on distance from the smelter (Figure 1). Group 1: Eroded/Disturbed sites (0-5 km from smelter) included Airport, Wahnapitae Hydro-Dam and Kelly Lake; Group 2: Stable upland sites (5-15 km from smelter) included Kingsway, Kukagami and Laurentian and Group 3: Reference sites (>15 km from smelter) included Onaping Falls, Capreol and Hagar. Sites close to smelters were characterized as sandy/clay soil rich in Cu and Ni. |
| Plant population diversity |
| Each site was sampled along a designated transect with a minimum of three plots. Each plot consisted of 10 m diameter where trees/shrub and ground cover species were documented to assess Shannon-Wiener index, Simpson’s index of diversity, species richness and evenness. Individual trees/shrub species were counted and the percentage cover for each species was estimated. For the ground cover, only the percentage cover for each species was recorded. |
| Molecular analysis |
| Leaf samples from Betula papyrifera (white birch), Quercus rubra (red oak) and Acer rubrum (red maple) were collected from eroded/ disturbed, stable upland and reference sites based on leaf morphology. The locations of the sampling sites are illustrated in Figure 1. In general 10% to 20% of each population was analyzed. For each species, 70 trees representing each targeted population were selected. For each tree, leaf samples were wrapped in aluminum foil, frozen in liquid nitrogen and stored at -20oC until DNA extraction. |
| DNA extraction |
| Genomic DNA from individual samples was extracted from fresh frozen leaf material using method described by Nkongolo [11]. The protocol is a modification of Doyle and Doyle [12] procedure which included the addition of 1% polyvinyl pyrrolidone (PVP) and 0.2% beta mercaptanol to the cetyl trimethylammonium bromide (CTAB) buffer solution, two additional chloroform spins prior to the isopropanol spin and no addition of RNAse. The concentration of the DNA was determined using the flurochrome Hoechst 33258 (busdensimide) flurorescent DNA qunatification kit from Bio-Rad (cat # 170-2480) and the purity was determined by running the samples on a 1% agarose gel. DNA samples were stored at -20oC untill further analysis. |
| ISSR analysis |
| Several primers synthesized by Invirogen were chosen for preliminary amplification with DNA samples from each population. Ten primers were selected based on polymorphism, reproducibility and band resolution (Table 1). PCR amplification was carried out as described by Narendrula and Nkongolo [13] and Tran et al. [14]. All PCR products were separated for analysis on a 2% agarose gel stained with ethidium bromide in 0.5x Tris-Borate-EDTA (TBE) buffer. The gel was run at 3.14 V/cm, documented with the Bio-Rad ChemiDoc XRS system and analyzed for band presence (1) or absence (0) with the Discovery series Quantity One 1D Analysis software. The resulting data matrix of the ISSR phenotype was analyzed using Popgene software (version 1.32) [15] to determine genetic diversity parameters. |
| Soil respiration |
| Soil respiration was assessed as described by Goupil and Nkongolo [16]. For each site, four soil samples (each consisting of 10 sub-samples) were collected from the organic layer (0-5 cm in depth) and placed in a plastic bag. Plant material, stones and residues were removed and resulting soil samples were well mixed. Soil samples were completely dried, labeled and stored prior to analysis. Samples of 40 g of dried soil were weighed into a capillary cup (with perforations at the bottom) after placing a fiber filter disk at the bottom of the cup. These cups were placed inside a glass jar with the use of forceps. To the glass jar, 20 mls of distilled water was added carefully by not spilling on the soil sample. A carbon dioxide (CO2) probe was then gently placed into the glass jar using forceps. The lid of the glass jar was screwed on tightly and the start time was recorded. After 24 hour period, the probe was carefully removed and placed in the Solvita digital reader to determine CO2 concentration. Interpretations of the data were based on Solvita’s guidelines. |
| Phospholipid fatty acid (PLFA) analysis |
| Phospholipid analysis (PLFA) was performed at FAME Lab, Microbial ID. Inc, Newark, Delaware (USA) as described in Buyer and Saaser [17]. Mole percentage of each PLFA was used to indicate the relative abundance of bacteria, actinomycetes and fungi in soil. Total PLFA extracted from soil was used as an index of living microbial biomass [17]. |
| Statistical analyses |
| Data were analyzed using SPSS statistics version 20 for Windows. One-way ANOVA was used to determine significance of differences the groups for plant diversity, microbial biomass (total PLFA), composition (bacterial, actinomycetes and fungal PLFAs) and functions (respiration, total C and N concentrations). Relationship between plant diversity and the above mentioned attributes of the soil microbial community were calculated using Pearson correlation analysis. |
| Results |
| Plant population diversity |
| The proportions of different tree/shrub species and ground cover found in eroded/disturbed, stable upland and reference sites are described in Tables 2 and 3. The sites were mainly composed of hardwoods that accounts for over 90% of tree populations. Only a small percentage of plant population was attributed to conifers. Overall, 16 tree/shrub species were identified in all the sites. Acer rubrum (red maple), Betula papyrifera (white birch), Pinus resinosa (red pine), Populus tremuloides (quaking aspen), Quercus rubra (red oak) and Salix spp. (willow) were found in all the sites. Acer rubrum and Betula papyrifera were the most dominant species. Betula papyrifera trees were more abundant and healthier close to smelter compared to other sites. Opposite trend was observed for Acer rubrum. |
| A large array of vascular plants was recorded in the ground cover but most species occurred only in small quantities and at few sites (Table 3). Vaccinium angustifolium (lowbush blueberry) was found on established mineral soils and is known to be important in the prevention of erosion. Vaccinium angustifolium represented 38.89%, 18.33% and 6.11% in eroded/disturbed, stable upland and reference areas, respectively. This was followed by Deschampsia spp. (hair grass) with 18.33% in the eroded/disturbed and 1.67% in the references sites. Deschampsia spp. is an excellent colonizer and is characteristic of the true barren areas. Aralia nudicaulis (wild sarsaparilla) was specifically found in the reference sites (16.67%) whereas Cladonia rangiferina (reindeer lichen) and Lycopodium digitatum (clubmoss) were found only in eroded/disturbed sites (5.00% and 6.11%, respectively). The reference sites were dominated by Pteridium aquilinum (bracken fern) (46.67%) (Table 3). |
| Vaccinium angustifolium and Deschampsia spp. species were present in all the sites and a high percentage of these species were observed in the barren areas. The location of barren areas is linked to mining activities and it is for this reason that they are common in the vicinity of the smelters. The barren areas are also associated with acidic soil conditions with soil pH below 4. |
| Results of the ecological diversity parameters for each site are described in Table 4. For tree/shrub species, mean Shannon index value was significantly higher (1.13 and 0.97) in stable uplands and reference sites compared to eroded/disturbed sites (0.52). Simpson index followed the opposite trend with the mean value of 0.40 and 0.54 for stable upland and reference sites, and 0.76 for eroded/disturbed sites. Tree species richness was 4.67, 5.33 and 7.67 for eroded/disturbed, stable upland and reference sites, respectively (Table 4). Table 4 depicts the values of other parameters for tree/shrub species as well as for ground cover. Figures 2 represents total number of trees present in eroded/disturbed, stable upland and reference sites. |
| Molecular analysis |
| Table 1 describes the main characteristics of ISSR primers used in the present study. These primers were selected for the amplification of DNA to determine the genetic variation in the Betula papyrifera, Quercus rubra and Acer rubrum species growing in the disturbed and undisturbed areas. For Acer rubrum and Quercus rubra the polymorphism ranged from 52.01% to 55.56% and 58.82% to 60.78%, respectively (Table 5). The level of polymorphism for Betula papyrifera ranged from 38.43% to 45.07% (Table 5). This indicated that the level of polymorphism analyzed for each population in each species were similar. Thus, data was compiled to compare the overall polymorphism between eroded/disturbed, stable upland and reference sites. No significant differences for the level of polymorphism and Shannon’s information index were observed between the three groups of sites (Table 5). The mean Shannon’s information index was 0.18, 0.22 and 0.23 for Betula papyrifera, Quercus rubra and Acer rubrum, respectively. |
| Soil characterization and respiration |
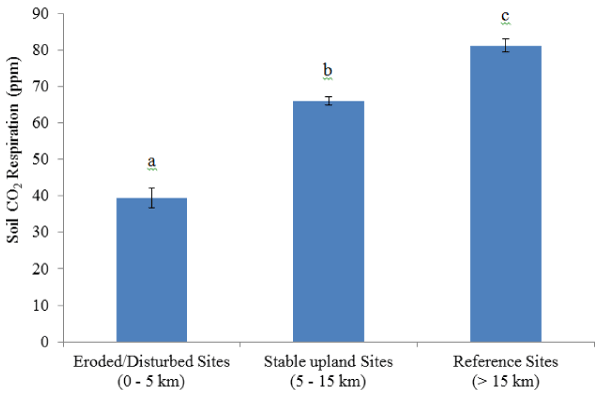
| The pH of the soil ranged from 3.5 to 4.5 in the top organic layer. However, no differences in pH values were observed between eroded/ disturbed, stable upland and reference sites (Table 6). Total organic carbon (C) and nitrogen (N) content differed with soil type, which was lower in eroded/disturbed soil relative to stable upland and reference soil (Table 6). Additionally, the C/N of the total was significantly different for eroded/disturbed sites compared to stable upland and reference areas (Table 6). Net mineralization of N was significantly affected lower in eroded/disturbed soil compared to stable upland and reference soil (Table 6). Similar trend were observed for soil respiration indicating depletion of available organic matter and low biological activity in eroded/disturbed sites compared to stable upland and reference areas (Table 6 and Figure 3). |
| Phospholipid fatty acid (PLFA) analysis |
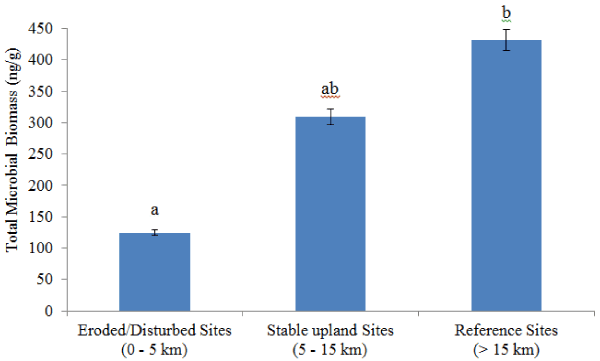
| Results from fatty acid analysis are described in Tables 7 and 8. Fatty acid analysis revealed significantly high microbial biomass in soil samples collected away from the smelters (stable upland and reference sites) compared to eroded/disturbed areas (Figure 4). Significant difference was observed for total fungi and arbuscular mycorrhizal fungi abundance between the three groups (Table 7). Significant difference was noted for eukaryote and actinomycetes between the three groups (Table 7). The analysis also revealed significant differences for gram negative and gram positive bacteria between the groups (Table 7). There were twice more gram negative than gram positive bacteria in all the sites. Overall, there were more bacteria than fungi in all the sites analyzed (Table 7). Total bacteria represent 67.4%, 72.1%, and 71.7% of total microbial biomass for eroded/disturbed, stable upland and reference sites, respectively. These values were only 15.4% (eroded/ disturbed), 13.2% (stable upland), and 14.0% (reference sites) for total fungi biomass. The ratio between fungi and bacteria was also low for all the groups (Table 8). Fungal to bacterial ratio was 0.19 for eroded/ disturbed, 0.15 for stable upland and 0.16 for reference sites. |
| Palmitic acid (16:0) was a common fatty acid in all the samples with the highest percent (13%). In addition to palmitic acid, other common and abundant fatty acids were 14:0 (1.1%), a15:0 (2.6%), i15:0 (7.7%), i16:0 (2.1%), 16:1w7c (7.7%), 16:1w5c (3.4%), 10Me16:0 (4.5%), 17:0w7c (2.9%), 18:0 (2.4%), 18:1w9c (10.7%), 18:2w6c (7.1%) and 19:0w7c (10.7%). These fatty acids were present in all samples and made up about 69.0%, 73.0% and 74.0% of total fatty acid content in the eroded/disturbed, stable upland and reference sites, respectively. The i15:0, a15:0, i17:0 and a17:0 fatty acids commonly used as signature fatty acids for bacteria, 18:2w6c for fungi and 10Me16:0, 10Me17:0, 10Me18:0 for actinomycetes were present in all the samples. Monounsaturated fatty acids were found in the highest amount in all soil samples, followed by saturated and branched chain fatty acids. The ratios between unsaturated and saturated fatty acid were low (Table 8). |
| Correlation between ecological diversity and microbial biomass |
| No associations among soil respiration data, total soil microbial biomass, and aboveground diversity indices were observed. Total PLFA, an estimate of microbial community biomass showed a strong positive relationship with soil respiration. Strong positive relationship was also observed between tree species diversity and number of plants (Table 9). An examination of correlation between soil respiration and tree species richness displayed a moderate positive correlation of 0.54 (Table 9). Moreover, total PLFA showed no relationship with plant species richness and total number of trees. Total PLFA showed strong positive relationship with total bacteria (1.00) and fungi (0.97) biomass. Strong relationship (0.96) was observed between total bacteria and fungi biomass. Additionally, a strong relationship (0.97) was also observed between total carbon (C) and nitrogen (N) content. |
| Discussion |
| Previous studies on metal analysis of the targeted sites revealed that nickel (Ni) and copper (Cu) continue to be the main contaminants in sites near the smelters in the GSR exceeding the Ontario Ministry of Environment (OMOE) guidelines [18,19]. The levels of total aluminum (Al), iron (Fe) and magnesium (Mg) concentrations were significantly higher in sites closer to smelters compared to the reference sites [19]. Narendrula et al. [3] reported a high concentration of arsenic (As) in sites close to smelters. The proportion of bioavailable metals compared to total metals was found to be very small [19]. The highest percentage of bioavailable element was observed for phosphorus (P) [19]. |
| Plant population diversity |
| Diversity index is a quantitative measure that reflects how many species are present in a dataset and simultaneously takes into account how evenly the individuals are distributed in targeted areas [20]. The value of a diversity index increases when the number of species and evenness increase [21]. Two measures of diversity were calculated: 1) Shannon-Wiener (H’) and Simpson index (D). Shannon-Wiener index is mostly used to determine the complexity of a community, typical values ranging between 1 and 4 [22]. Shannon-Wiener index increases as both the richness and the evenness of the community increase [21]. We observed that diversity and evenness in the sites away from the smelter are higher than in the sites closer to the smelter. The stable upland and reference sites not only have a greater number of species present, but the individuals in the community are distributed more equitably among these species. |
| Simpson’s index is based on the probability, that two individuals randomly selected from a sample will belong to the same species [23,24]. Simpson’s index is a measure of dominance, so as D increases, diversity decreases. The values range between 0 and 1, where 0 represents infinite diversity and 1, no diversity [23]. Species diversity in eroded/disturbed sites was significantly higher compared to stable upland and reference sites indicating the effect of mining and pollution on diversity. Both Shannon-Wiener and Simpson’s index are more robust than species richness [25]. Ma [26] reported that diversity and evenness can be related (positively or negatively) whereas, evenness and diversity indices are not consistently regulated by richness. No differences were observed between sites for species richness. However, diversity and evenness were higher in stable upland and reference sites compared to eroded/disturbed areas. These population health indices have been used in other studies to evaluate recovery after disturbances caused in an area [14,27,28]. |
| Molecular analysis |
| Changes in environmental conditions rapidly shift allele frequencies in populations of species with relatively short generation times [29]. Environmental changes are predicted to decrease population size which can results in overall decrease in the level of genetic variation [29,30]. Genetic markers have been used to monitor whether environmental changes influence species at the level of DNA [29]. Information from genetic analysis can be used to identify the nature of the environmental threats experienced by various organisms and to determine the ecosystem stability and health [29,31,32]. |
| The availability of a variety of DNA markers, such as restriction fragment length polymorphism (RFLP), amplified fragment length polymorphism (AFLP), random amplified polymorphic DNA (RAPD), simple sequence repeat (SSR) and intersimple sequence repeat (ISSR) has enabled researchers to investigate genetic diversity among various plant species across natural populations [31]. ISSR marker has been used in plant population analysis as they effectively detect low levels of genetic variation [33]. They also may have potential for analyzing biogeographic patterns among populations of a single plant species [31]. ISSR analysis developed by Zietkiewicz et al. [34], uses the SSR motif as the single primer in PCR amplifications [31,33]. ISSR amplification does not require prior knowledge of flanking sequences and has wide applications for all organisms, regardless of the availability of information about their genome sequence [34]. ISSR has proven to be a simple and reliable marker system with highly reproducible results and abundant polymorphism [35]. This is most likely due to the longer lengths of the primers which permit the use of higher annealing temperatures which in turn, reduces non-specific binding and results in higher stringency [35,36]. |
| In the present study, genetic variation and genetic structure of Betula papyrifera, Quercus rubra and Acer rubrum populations was analyzed using ISSR markers. No significant differences in polymorphisms were observed between populations from eroded/ disturbed, stable upland and reference sites. Analysis showed that the average level of polymorphic loci was 41.72%, 53.59% and 59.83% for Betula papyrifera, Quercus rubra and Acer rubrum, respectively. The moderate level of genetic diversity suggests that these hardwood populations are sustainable in the mid-term. Genetic analysis of conifer species growing in the targeted region revealed low levels of genetic variability in Pinus resinosa, moderate in Pinus banksiana (jack pine) and very high in Picea glauca (white spruce) and Picea mariana (black spruce) [3,19]. Analysis of Deschampsia cespitosa showed that the level of genetic variation was significantly reduced due to accumulation of metals [37]. Plants possess homeostatic cellular mechanism to regulate metal concentrations in cells to minimize damage from the exposure to nonessential metals ions [38,39]. These mechanisms control uptake, accumulation and detoxification of these metals. The relative low concentrations of bioavailable metals in Sudbury soils may not cause harm to woody plants since their phytoavailability was negligible [3]. |
| Soil characterization and respiration |
| Soil characterizations have been used to predict the likelihood of flooding and drought [40]. It can also help to determine the types of vegetation, soil moisture and temperature [40]. The soil type and composition play an important role for metal retention [40,41]. In general, coarse grained soils exhibit lower tendency for metal adsorption than fine grained soils [41]. Soil pH is the most important parameter influencing soil-metal chemistry and it is commonly measured in water and/or in 0.01M calcium chloride (CaCl2). Soil pH measured with CaCl2 is usually preferred as it is less affected by soil electrolyte concentration and provides a more consistent measurement [42]. Studies have shown that metal adsorption is small at low pH values and adsorption increases at intermediate pH from zero to near complete adsorption over a relatively small pH range [42,43]. In the present study, pH values were low (> 4.5) in the GSR which is consistent with data reported for coarser textured soils with coniferous vegetation on the Canadian Shield [2]. Low soil pH can affect the availability of nutrients to plants which is often observed in barren lands of the GSR. Low pH is also known to be detrimental to plant growth because of imbalances in the nutrient levels. For example, aluminum (Al) and manganese (Mn) may be available in toxic concentrations whereas phosphate is poorly available. |
| Analyzing the total carbon (C), nitrogen (N) and C/N ratio provides an insight into the carbon and nitrogen supply to soil micro flora and plants. Both nitrogen and carbon are important nutrients for plants and soil microorganisms. Total C and N concentrations were significantly lower in sites closer to the smelters compared to reference sites. Availability of C substrate is shown to be a factor influencing rate of soil respiration [44]. Soil respiration measures total CO2 production in intact soils resulting from the respiration of soil organisms, roots, mycorrhizae and to some extent by chemical oxidation of carbon-containing materials [45]. This activity is sustained by organic matter input to the soil from aboveground and from the roots [45]. Soil respiration rates are critical in the assessment of soil health as it indicates the complete extent of biological activity of living microorganisms available in the soil [16,44,45]. CO2emissions from soils exceed all other terrestrial-atmospheric carbon exchanges [44]. Raich and Potter [46] reported that almost 10% of the atmosphere’s CO2 passes through soils each year which is more than 10 times the CO2 released from fossil fuel combustion. Soil respiration rate shift fundamentally among plant biome, suggesting that vegetation type impacts the rate of respiration [47]. In the present study, respiration rates increased as we moved away from the smelters and similar trend was observed for the number of trees. Various studies that have shown side-by-side comparisons of different plant communities demonstrate differences in soil respiration rates [44,47]. These findings indicate that plant species and number of trees is an important determinant of soil respiration rate and therefore changes in vegetation have the potential to modify the responses of soils to environmental change. |
| Phospholipid fatty acid (PLFA) analysis |
| Significant attention has been focused on the improvements of plant communities in the GSR which have occurred as a result of the land reclamation programs and reduced industrial emissions during the past four decades [11]. Relatively little is known about the corresponding changes in soil microbial populations. Studies have reported that the biological health of soil ecosystem can be used as indicator of ecosystem health [48]. Numerous studies have demonstrated the adverse effects of mining and metals on soil microbial biomass and activity [11,16,49]. |
| In the present study, PLFA analysis was used to analyze soil microbial community responses to mining activities. PLFA profiles are based on the fact that phospholipids are found in the membrane of living cells and bacteria contain a relatively constant proportion of their biomass as phospholipids [48,49]. Microorganisms have unique signature PLFA profiles which can be used to examine community structures [48]. PLFA data indicated decrease in total microbial biomass, AM fungi, total fungi, eukaryote, actinomycetes, gram positive and gram negative bacteria in eroded/disturbed sites compared to stable upland and reference sites. This was expected as various studies have reported differences in response of microorganisms to different environmental conditions, soil types and vegetation [49,50]. Forest soil treated with different metals has shown a change in PLFA composition [49,51]. The interpretation of the changes in patterns of PLFA in soils in terms of changes in specific taxonomic group is difficult since the same PLFA maker may exist in the membranes of organisms belonging to different taxonomic groups. However, certain trends can be pointed out in terms of certain PLFAs belonging to certain groups of bacteria or fungi. Several PLFAs exhibited different trends in different soils. |
| PLFAs 15:0 and 17:0 which have been considered to be of predominantly of bacterial origin showed different concentrations in different sites. A low level was observed in eroded/disturbed sites, while a higher concentration was found in reference sites. This suggests that these PLFAs were affected by the mining activities. It is well established that certain branched, monounsaturated and cyclo fatty acids present in environmental samples are from bacterial population [52]. High percentage of branched fatty acids (a15:0, i15:0, a17:0 and i17:0) were found in stable upland and reference samples than eroded/disturbed samples. Branched PLFA markers have been reported as biomarker for bacteria, anaerobic bacteria and sulfate-reducing bacteria [47,52]. A predominance of gram negative over gram positive bacteria was observed in all sites. Studies have reported an increase in abundance of gram negative bacterial PLFA with simultaneous decrease in gram positive bacterial PLFA to different stress conditions [48,49]. Kaur et al. [48] reported that under stress conditions, survival of gram negative bacteria could be attributed to the presence of cyclo fatty acids in their membrane and the outer lipopolysaccharide layer. |
| It is generally agreed that fungi are less sensitive to metal pollution than bacteria [16,53]. In the present study, PLFA 18:2w6 regarded as a reliable indicator of fungal biomass revealed lower concentrations in areas closer to the smelter. This decrease could be attributed to a high concentration of Cu as this metal is known to be toxic to fungi. In the reference sites, high 18:2w6 concentrations was obtained as the concentrations of Cu found in the area was very low. PLFA 18:2w6 is also found in plant residues and thus the portion of this PLFA that was derived from fungi might therefore be masked by the amount of 18:2w6 derived from plant material in reference sites [49]. This decrease in abundance of 18:2w6 could also be due to a decline in ectomycorrhizal fungi because of damage to the fine roots of trees due to metal pollution, pH and low soil organic matter [48]. Studies by Kaur et al. [48], Hackl et al. [50] reported the effects of pH, soil organic matter, metals and other stress factors on fungal:bacteria ratio. This ratio has been used as a potential tool for discriminating disturbed areas from the undisturbed/reference sites. |
| Overall, the microbial communities in all the sites from the GSR were dominated by bacteria, mostly gram negative indicating that the region is still under severe environmental stress. Similar results have been found by various studies performed in metals contaminated soils [49,54]. |
| Relationship between aboveground and belowground diversity |
| Soil sustains life aboveground, therefore, a better understanding of the interactions between aboveground and belowground biodiversity is required to anticipate the potential results of biodiversity change for the maintenance of ecosystem and environment properties [55]. The clear changes in microbial population structure and diversity in soil samples may have significant implication for plant growth, development, vegetation succession and other critical functions [56]. Our results show no correlations between the aboveground and belowground diversity which can be attributed to several factors. First, species or groups could be responding to different or same abiotic constrains but on different temporal or spatial scales [57,58]. Second, species could be linked biologically via interactions that decrease diversity in other component [57]. Studies reported that some below ground species may be less influenced by the aboveground diversity as they are modulated by top-down controls within belowground food webs rather than from the bottom-up controls (ex: aboveground vegetation) [57,58]. Various studies have observed that not all groups within either component necessarily follow the same diversity trends even when they are closely linked ecologically [59]. Third, diversity in one domain could depend on the composition rather than the diversity of organisms in other domain. Hooper et al. [58] and Hooper and Vitousek [59] found no relationship between aboveground and belowground components. They concluded that soil biodiversity is more likely to be related to the traits of the dominant plant species present than to the diversity of the plant community itself. Fourth, genetic variation, evolutionary relationships, species richness, composition and resource quality and quantity can play a major role in defining diversity in a community [57,58]. These factors may not influence all aboveground and belowground components in a similar manner, particularly across gross differences in size and dispersal capability. However, there is evidence that aboveground and belowground components are functionally linked as microorganisms facilitate mineralization of soil organic matter for plant use and the later provides appropriate environment for microbial growth [60]. The results suggest that although general soil characteristics may be most important in determining the dominant bacterial populations in soil, microbial communities are plant driven to a far greater extent. Although we are starting to recognize patterns of microbial diversity in soil and the role of plants in shaping the distribution of microbial populations, identification of species of microorganisms that are present in soil ecosystems is not yet established. Therefore, metagenomic analysis of soil sample has been initiated to establish the types of fungi and bacteria present in each site and to determine the level of soil microbial diversity. |
| Conclusion |
| In the present study, various plant diversity indices were measured to assess the effects of mining on areas close to a smelter. Results revealed that sites closer to smelters have decreased plant population diversity and abundance. Molecular analysis of hardwood species revealed no differences in genetic variation among plant populations from eroded/disturbed, stable upland and reference sites. Soil respiration and microbial biomass were also decreased in disturbed sites. Gram negative bacteria were the main component of soil microbiome present in all sites at high levels. The ratios between fungi and bacteria were extremely low suggesting that the targeted region is still under environmental stress. The present study showed a positive relationship between above ground plant abundance and below ground microorganism abundance. Metagenomics analysis is being performed to determine individual bacteria and fungi species present in each site. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 | Table 5 |
| Table 6 | Table 7 | Table 8 | Table 9 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 14628
- [From(publication date):
March-2015 - Aug 16, 2025] - Breakdown by view type
- HTML page views : 10016
- PDF downloads : 4612
