Gnetol, a Resveratrol Derivative Ameliorates Malathion-Induced Neurotoxicity through Modulating Lysosomal Membrane Permeabilization in N2a Cells
Received: 08-Oct-2017 / Accepted Date: 16-Oct-2017 / Published Date: 23-Oct-2017 DOI: 10.4172/2161-0460.1000388
Abstract
Malathion is a highly neurotoxic organophosphate (OP) pesticide which is in wide use. It is known for its high toxicity to insects, which is caused by inhibition of acetylcholinesterase activity, and for being neurotoxic to humans and other mammals. The present study mainly focused on potential effect of 2,3’,5’,6-tetrahydroxy-trans-stilbene (gnetol) on malathion-induced neuronal cell death in N2a mouse neuroblastoma cells. Malathion activated lysosomal cathepsin B release, resulting in defective autophagy and induction of apoptotic cell death. Interestingly, gnetol (5, 10, and 20 μM) protected cells from apoptosis by regulating heart fatty acid binding protein 3 (hFABP3) and vascular endothelial growth factor (VEGF) expression, leading to improvements in the cellular and nuclear morphological changes induced by malathion. Gnetol induced autophagy by reducing lysosomal cathepsin B release as assessed by immunofluorescence staining, which ameliorated apoptotic cell death in N2a cells. Furthermore, the neurite outgrowth and the NGF level were upregulated by gnetol treatment. Taken together, gnetol, a resveratrol derivative, may protect neuronal cells against malathion-mediated apoptosis and potentiate neuritogenesis.
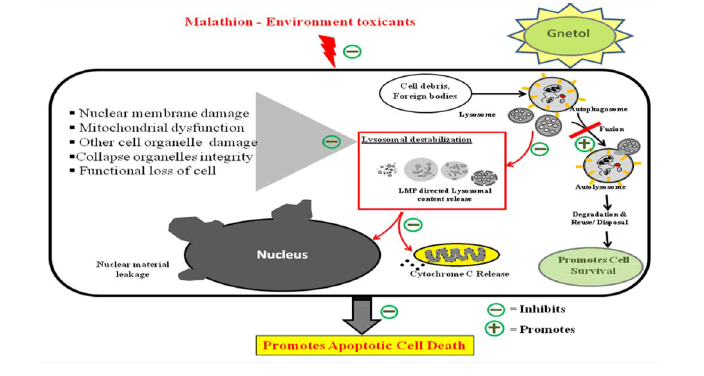
Graphical Abstract
Schematic representation of the proposed role of gnetol on lysosomal destabilization in malathion-induced apoptosis in N2a neuroblastoma cells
Keywords: Malathion; Autophagy; Gnetol; Phytochemicals; Neurodegenerative disease; Lysosomal destabilization
Abbreviations
ND: Neurodegenerative Disease; AD: Alzheimer’s Disease; WHO: World Health Organization; Cas: Caspase; LMP: Lysosomal Membrane Permeabilization; Cyto C: Cytochrome C; hFABP3: Heart Fatty Acid Binding Protein 3; VEGF: Vascular Endothelial Growth Factor; NGF: Nerve Neurotrophic Growth Factor; DMSO: Dimethyl Sulfoxide; CB: Cathepsin B; LAMP-1: Lysosomal Associated Membrane Protein 1; LDH: Lactate Dehydrogenase; PBS: Phosphate- Buffered Saline; ANOVA: Analysis Of Variance; TEM: Transmission Electron Microscopy; OP: Organophosphate; ROS: Reactive Oxygen Species; AChE: Acetylcholinesterase; FATP 1: Fatty Acid Transport 1 Protein; GDNF: Glial Cell-Derived Neurotrophic Factor
Introduction
Neuronal loss is major pathophysiological issue in neurodegenerative diseases such as AD, Parkinson’s disease. Many researchers have focused on finding various therapies and drugs with the potential to regulate neurodegenerative implications, but it still remains as major issue [1,2]. Recently, some reports have found that the prevalence of neurodegenerative disease is associated with regular, prolonged exposure to toxic substances [3,4]. Things we encounter on a daily basis, such as food, chemical preservatives, pest repellents, and health and personal care items, should be investigated for potential harmful effects. Pesticides, especially organophosphates, have been implicated in neurodegenerative disease states by data on agricultural field exposure reported by the World Health Organization (WHO) [5,6]. Clinical samples taken from AD patients contained greater concentrations of pesticides than did samples from control subjects. Malathion, an organophosphate pesticide, is used worldwide to combat household pests, insects, lice, mosquitoes, and agricultural pests that afflict a wide variety of vegetables [7]. Hence, our study was designed to evaluate the effect of natural products against malathion-induced neurotoxicity. Previous studies reported that the administration of antioxidants such as α-tocopherol, selenium, sulforophane (SFN), and curcumin may partially protect against malathion-induced hepatic oxidative stress and injury [8,9]. Therefore, firstly, we aimed to investigate the effects of a number of potential antioxidants, stilbene derivatives, on acute malathion toxicity in neuronal cells. Dose optimization of malathion and the resveratrol derivatives trans-resveratrol, cis-resveratrol, gnetol, isorhapontigenin, tetrahydroxy stilbene, dihydroxy resveratrol, pterostilbene, pinosylvin, and piceatannol were done with an MTT cell proliferation assay. Gnetol, 2,3’,5’,6-tetrahydroxy-trans-stilbene, was identified as one of the most potent molecules and was therefore studied further in an effort to identify its effects and mechanism of action against malathion-induced toxicity.
Previous studies often concentrated on the protective effects of antioxidants against caspase (Cas)-dependent cell death, although some work has also been done on non-caspase-dependent death [10]. With an eye towards the latter, we exposed mouse neuroblastoma cells to malathion and found that it initiated lysosome-mediated cytotoxicity. Similarly, hydroxychloroquine enhances lysosomal membrane permeabilization (LMP) in HeLa and BJAB cells transfected with pcDNA3.1 and human Bcl2 vectors [10,11]. LMP is brought about by exposure of cathepsin to the cytosolic space prior to fusion with autophagosomes [12]. The mechanism of autophagy in a previously generated mouse neuroblastoma model of malathion treatment involved defective expression of the LC3 protein, which facilitated mitochondrial membrane permeabilization and the release of cytochrome C (Cyto C) and triggered apoptosis via alterations in pro/anti-apoptotic protein levels (AKT, Bax, Bcl2, Cas 3). Thus, LMP induction is considered to be a rate-limiting step in cell survival. Also, the AD model had defects in a fatty acid transportation protein (hFABP3) and a vascular growth promoting protein (VEGF).
Hence, the present study was designed to elucidate the potential of gnetol, a resveratrol derivative, against malathion-induced toxicity in N2a cells based on the knowledge that malathion induces LMPmediated apoptotic cell death, mitochondrial dysfunction, DNA damage, and alterations in the expression levels of hFABP3 and VEGF. Furthermore, we measured the neurite outgrowth and the NGF level after treatment of gnetol.
Materials And Methods
Cell culture and treatment
N2a cells were obtained from the Korean Cell Line Bank (Seoul, Korea). N2a cells cultured in high glucose Dulbecco’s modified Eagle medium (DMEM; Thermo Scientific, Seoul, Korea) supplemented with 10% fetal bovine serum (FBS; Atlas Biologicals) and 1% penicillin and streptomycin were kept in an incubator at 37ºC under 5% CO2. All the cells were seeded and incubated overnight before the start of experiments. 5 × 105 cells/ml were plated in 96- or 24-well plates as needed. 2% FBS DMEM was used throughout the experimental period for all treatments.
Reagents and antibodies
Malathion (Sigma-Aldrich, Seelze, Germany) and Gnetol (TCI, Tokyo, Japan) were dissolved in dimethyl sulfoxide (DMSO) for preparation of appropriate stock concentration. Primary antibodies were as follows: caspase-3, AKT, p-AKT (Cell signaling, Danvers, MA, USA), VEGF, Bax, BcL-2, Cathepsin B, LAMP-1 (Santacruz, CA, USA), cytochrome C (BD Bioscience, CA, USA), hFABP3 (Proteintech, Chicago, IL, USA), LC3B (Abcam, Cambridge, MA, USA) and Retinoic acid, α-tubulin (Sigma-Aldrich, St. Louis, MO, USA). All the primary antibodies were diluted and used as per the manufacturer’s instructions.
Cell viability
Cell viability was measured using the MTT (3-(4,5-dimethylthiazol- 2-yl)-2,5-diphenyl tetrazolium bromide) assay, based on the formazan formation. N2a cells were seeded in 96-well plates, incubated overnight, and treated with different concentrations of gnetol (5, 10, and 20 μM). After 1 h of gnetol treatment, malathion (1 mM) was added to the wells and incubated for 8 h. Following incubation, the supernatant was collected and the cells were exposed to 0.5 μg/ml MTT solution for 1 h. The purple-colored formazan was solubilized using DMSO and read at 570 nm to assess the concentration of viable cells. Data are expressed as a percentage of viable cells compared to the control.
Lactate dehydrogenase (LDH) release assay
Supernatant from the cell culture media was used to assess the LDH level using an LDH assay kit (Roche Diagnostics GMBH, Mannheim, Germany). Cytotoxicity is directly proportional to LDH release into the supernatant. Lactic acid, generated by LDH in the media, was reduced to formazan by iodotetrazolium dye. The experiments were conducted as per the user manual and color was measured at an absorbance of 490 nm. Cytotoxicity data is expressed as percent over control.
Neurite outgrowth potentiation in N2a cells by malathion
Gnetol pre-supplemented Malathion treatment was examined for neurite outgrowth potentiation in N2a cells. N2a cells were seeded at a cell density of 5 × 104 per well in 6-well plates and treated with malathion (0.25, 0.5, and 1 mM) for 8 h, as described above. The automated images were captured using Essen IncuCyte ZOOM v2013B Rev1 (Essence Bioscience Inc., Ann Arbor, MI, USA). Retinoic acid was used as a positive control for neurite outgrowth. The collected images were analyzed to measure neurite length and percent neurite outgrowth was calculated and plotted
Western blotting analysis
At the end of the experiments, the cells were washed with phosphatebuffered saline (PBS) 3x, then harvested using a cell scraper and centrifuged at low rpm to get a pellet. The pellet was lysed using RIPA lysis buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 0.1% sodium dodecyl sulfate and 0.5% deoxycholate, and 1% NP40) comprised of 1 mM phenylmethylsulfonyl fluoride (PMSF), protease inhibitor cocktail, and phosphatase inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA) for 30 min on ice. The cells along with lysis buffer were sonicated on ice and subjected to centrifugation at 12,000 rpm for 20 min at 4°C. The total protein was collected and determined for protein concentration using the Bradford reagent BioRad DC kit (Bio-Rad Laboratories, Seoul, Korea). Protein samples of 25 μg were separated using SDS-PAGE and blotted to nitrocellulose membranes. The membranes were incubated overnight with the primary antibodies at 4°C in a shaker.
Fluorometric analysis of nuclei using hoechst dye
N2a cells (5 × 104) were seeded in 8-well Lab-Tek II chamber slides with covers (Nunc, Roskilde, Denmark) and incubated overnight. The cells were treated with different concentrations of gnetol (0, 5, 10, and 20 μM) for 1 h and then exposed to malathion (1 mM) for 8 h. After treatment, the cells were washed with PBS and fixed with 4% paraformaldehyde for 20 min at room temperature. Then, cells were stained with Hoechst solution for 30 min in the dark at room temperature. DNA damage was measured by staining nuclei with Hoechst dye and observing the cells under a confocal laser scanning microscope (Nikon A1+, Japan); 200 cells were manually counted to determine the concentration of condensed/fragmented nuclei.
Soluble NGF secretory level (ELISA)
To estimate the soluble NGF level in C6 cells co-cultured with media from treated N2a cells, we first treated N2a cells with gnetol followed by malathion exposure, then collected the media. Next, the media was added to C6 cells in 24-well plates and NGF level were measured 24 h later. After the 24 h treatment period, the supernatant of C6 cells was analyzed for NGF level. Quantification of NGF was performed as described in the manufacturer’s instructions for β-NGF ELISA kit (R&D system, Minneapolis, MN). The NGF levels are expressed in pg/ ml and are compared with control cells.
Autophagy detection assay
N2a cells (5 × 104) were seeded in an 8-well Lab-Tek II chamber slide with cover (Nunc, Roskilde, Denmark) and incubated overnight. The cells were treated with different concentrations of gnetol (0, 5, 10, and 20 μM) and malathion (1 mM), as above. Autophagy was detected using the Cyto-ID fluorescent dye for autophagy from the Cyto-ID® Autophagy Detection Kit (Enzo Life Science, Farmingdale, NY 11735). Along with malathion, 500 nM rapamycin+10 μM chloroquine were maintained separately as positive controls. Experiments were carried out according to the product manual. The fluorescence intensity images of the cells were captured by a confocal laser scanning microscope (Nikon A1+, Japan) with the excitation ~488 nm, and emission ~530nm. The induction of autophagy was defined with increase in the fluorescent intensity in the cytosolic space.
Immunocytochemistry analysis of LMP
N2a cells (5 × 104) were seeded in an 8-well Lab-Tek II chamber slide with cover (Nunc, Roskilde, Denmark) and incubated overnight. Cells were treated as described previously. At the end of the experiment cells were washed with PBS (pH 7.4) and fixed with using 4% paraformaldehyde (PFA) for 20 min at room temperature, then by blocking in 1% horse/goat serum and 3% triton X-100 in PBS for 1 h at room temperature. Then cells were inspect for lysosomal associated membrane protein 1 (LAMP1) and cathepsin B (CB) by using anti- LAMP1 (mAb from Santa Cruz Biotechnology) and anti-CB (pAb from Santa Cruz Biotechnology) antibodies, respectively, and incubated overnight in a shaker at 4°C. Upon completion of incubation, the cells were washed 3x with PBS. FITC (Alexa Fluor® 488 conjugate, Life Technologies) and Rhodamine (Alexa Fluor® 594 conjugate, Invitrogen Technologies) secondary antibodies at 1:200 dilution were incubated in the dark at room temperature for 2 h. Finally, the cells were washed 3x with PBS and mounted in slides. The images of stained cells were captured with a confocal laser scanning microscope (Nikon A1+, Japan).
TEM analysis lysosomal structural morphology
To confirm the LMP, lysosomal morphological structural analysis was performed based on the methods reported by Kang. To examine lysosomal activity, N2a cells were seeded in 24 well plate and treated as described above. At the end of the treatment period, cells were immersed in fixative (PBS-buffered 2.5% glutaraldehyde) and incubated overnight at 4°C. Additionally, specimens were dehydrated by drenching in buffered 2% osmium tetroxide for 2 h at 4°C and embedded. Further, 50-60 nm thin sections were taken and stained with uranyl acetate and lead citrate. The lysosomal morphology was examined using Jeol 1400 transmission electron microscopy (Jeol Ltd., Tokyo, Japan). The sample preparation and TEM instrument facility were provided by Korean Basic Science Institute (KBSI, Chungbuk Ochang Center, South Korea).
Statistical analysis
The data are presented as the mean ± standard deviation of atleast three independent experiments. Statistical comparisons were performed between control and treatment groups using Tukey’s post hoc test for multiple comparison of one-way analysis of variance (ANOVA) using GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA, USA). P<0.05 were considered statistically significant.
Results
Malathion-induced cytotoxicity in N2a cells is ameliorated by gnetol
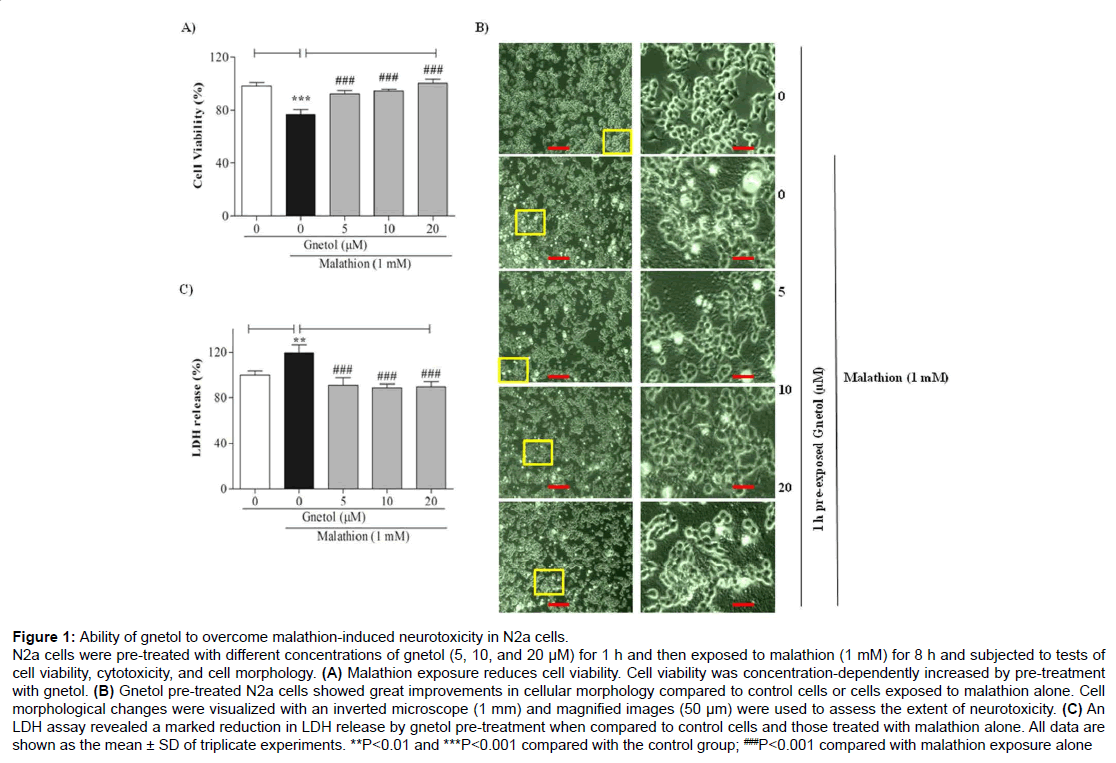
To measure protective effects of gnetol on malathion-induced neurotoxicity, N2a cells were treated with various doses of gnetol (5, 10, and 20 μM) for 1 h and were then exposed to 1 mM malathion for 8 h. Cell viability was assessed with the MTT assay (Figure 1). Malathion treatment alone brought about a decrease in cell viability (Figure 1A). Gnetol treatment dose-dependently protected the cells from malathion-induced neurotoxicity. Cells treated with malathion alone for 8 h were 70-80% viable, whereas cells pre-treated with gnetol (5, 10, and 20 μM) for 1 h showed a drastic increase in cell viability (92.1%, 94.5%, and 100.1%, respectively). Cellular morphology observed under a microscope showed distinct changes in appearance after malathion treatment and dose-dependent improvements with gnetol pre-treatment (Figure 1B). Alterations in cell bodies indicative of toxicity could be seen following malathion treatment; transmission electron microscopy (TEM) images demonstrated the extent of toxicity.
Figure 1: Ability of gnetol to overcome malathion-induced neurotoxicity in N2a cells.
N2a cells were pre-treated with different concentrations of gnetol (5, 10, and 20 μM) for 1 h and then exposed to malathion (1 mM) for 8 h and subjected to tests of cell viability, cytotoxicity, and cell morphology. (A) Malathion exposure reduces cell viability. Cell viability was concentration-dependently increased by pre-treatment with gnetol. (B) Gnetol pre-treated N2a cells showed great improvements in cellular morphology compared to control cells or cells exposed to malathion alone. Cell morphological changes were visualized with an inverted microscope (1 mm) and magnified images (50 μm) were used to assess the extent of neurotoxicity. (C) An LDH assay revealed a marked reduction in LDH release by gnetol pre-treatment when compared to control cells and those treated with malathion alone. All data are shown as the mean ± SD of triplicate experiments. **P
We also assessed LDH release in the N2a cell supernatant. Malathion treatment increased the release of LDH (119.5%) (Figure 1C). In contrast, gnetol (5, 10, 20 μM) pre-treatment attenuated the malathionmediated release of LDH dose-dependently (90.8%, 88.5%, and 89.5%, respectively). These results clearly support that gnetol protects neuroblastoma cells from the neurotoxicity induced by malathion.
Gnetol ameliorates malathion-mediated apoptotic death, mitochondrial dysfunction, and nuclear damage in N2a cells
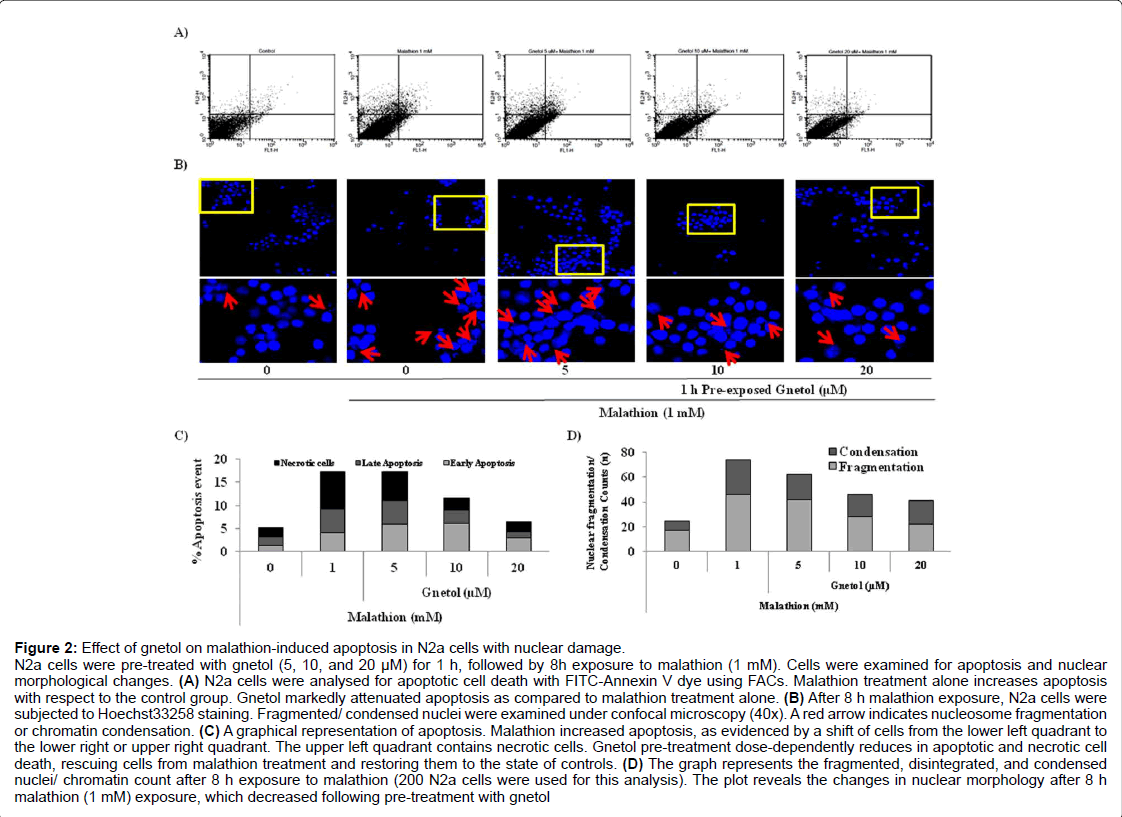
Figure 2: Effect of gnetol on malathion-induced apoptosis in N2a cells with nuclear damage.
N2a cells were pre-treated with gnetol (5, 10, and 20 µM) for 1 h, followed by 8h exposure to malathion (1 mM). Cells were examined for apoptosis and nuclear morphological changes. (A) N2a cells were analysed for apoptotic cell death with FITC-Annexin V dye using FACs. Malathion treatment alone increases apoptosis with respect to the control group. Gnetol markedly attenuated apoptosis as compared to malathion treatment alone. (B) After 8 h malathion exposure, N2a cells were subjected to Hoechst33258 staining. Fragmented/ condensed nuclei were examined under confocal microscopy (40x). A red arrow indicates nucleosome fragmentation or chromatin condensation. (C) A graphical representation of apoptosis. Malathion increased apoptosis, as evidenced by a shift of cells from the lower left quadrant to the lower right or upper right quadrant. The upper left quadrant contains necrotic cells. Gnetol pre-treatment dose-dependently reduces in apoptotic and necrotic cell death, rescuing cells from malathion treatment and restoring them to the state of controls. (D) The graph represents the fragmented, disintegrated, and condensed nuclei/ chromatin count after 8 h exposure to malathion (200 N2a cells were used for this analysis). The plot reveals the changes in nuclear morphology after 8 h malathion (1 mM) exposure, which decreased following pre-treatment with gnetol
Apoptotic cell death is the rate-limiting step in neuronal cell death, so we analyzed the extent of apoptotic cell death using flow cytometry (Figure 2A). Figure 2A revealed that malathion treatment induces cell death, whereas gnetol pre-treatment reduces apoptotic cell death. Using quadrant-stat, the extent of apoptotic cell death was calculated and plotted in a bar graph. Malathion treatment significantly enhanced the number of cells in early and late stages of apoptosis and necrosis. Gnetol pre-treatment dose-dependently diminished apoptotic and necrotic cell death (Figure 2C).
Malathion causes high vulnerability to nuclear damage. We stained cellular nuclei with Hoechst33258 fluorescent dye and visualized the cells under a confocal microscope at the end of our experiments in order to analyze morphological changes in the nucleus (Figure 2B). From the images (40x), it is clear that nuclear material undergoes a high number of morphological changes, including oligonucleosomal fragmentation and nuclear condensation. Gnetol pre-treatment reduced the extent of nuclear damage. We counted the number of cells that had some degree of nuclear fragmentation or disintegrated nuclei (Figure 2D). The results show that malathion causes a high degree of nuclear fragmentation/condensation, and these changes decline after treatment with gnetol dose-dependently (5, 10, and 20 μM).
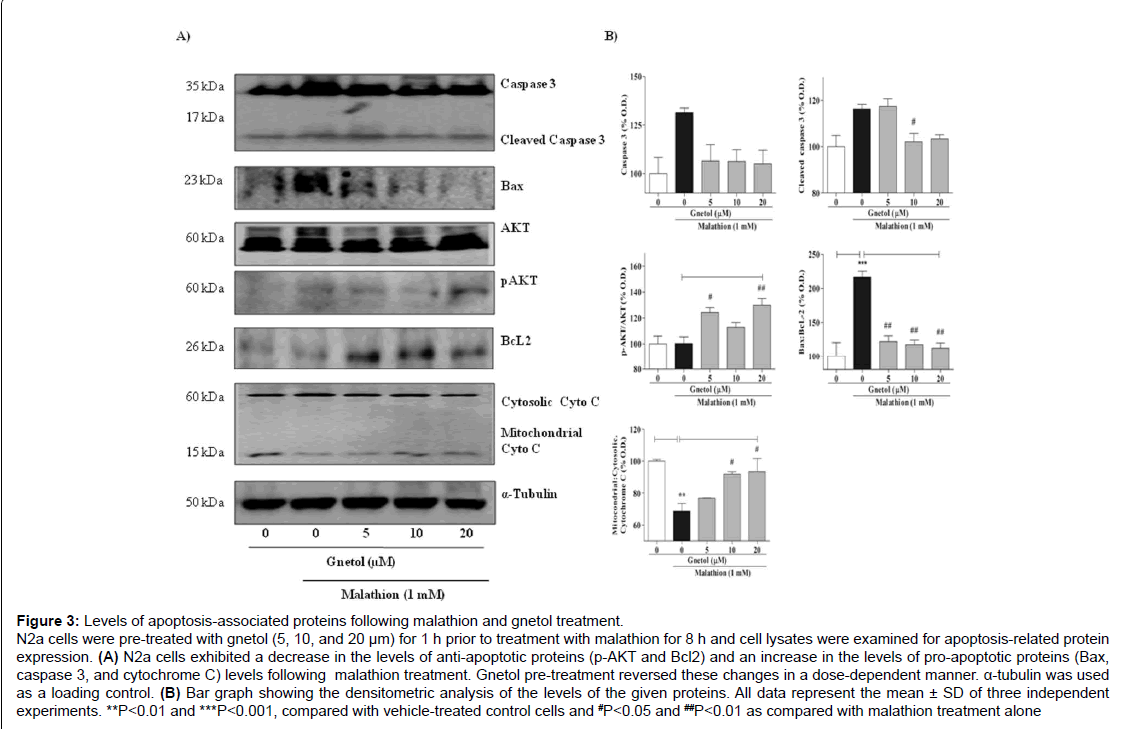
Figure 3: Levels of apoptosis-associated proteins following malathion and gnetol treatment.
N2a cells were pre-treated with gnetol (5, 10, and 20 μm) for 1 h prior to treatment with malathion for 8 h and cell lysates were examined for apoptosis-related protein expression. (A) N2a cells exhibited a decrease in the levels of anti-apoptotic proteins (p-AKT and Bcl2) and an increase in the levels of pro-apoptotic proteins (Bax, caspase 3, and cytochrome C) levels following malathion treatment. Gnetol pre-treatment reversed these changes in a dose-dependent manner. α-tubulin was used as a loading control. (B) Bar graph showing the densitometric analysis of the levels of the given proteins. All data represent the mean ± SD of three independent experiments. **P
To verify apoptotic cell death, we also analyzed the levels of antiapoptotic and pro-apoptotic proteins such as AKT, Bax, BcL2, and cleaved caspase 3 (Figure 3). After malathion treatment, the level of pAKT was slightly increased, to 114.0% of the protein level in the control group, whereas the level of BcL2 was diminished to 94.7% by malathion as compared with vehicle (Figure 3A). Gnetol (5, 10, and 20 μM) pre-treatment increased the expression levels of pAKT and BcL2 proteins dose-dependently ([118.9%, 120.3%, and 148.6%] and [154.7%, 141.5%, and 146.1%], respectively) compared to malathion alone. Concurrently, the level of Bax protein in the malathion group increased to 207.4% compared with the control group, but gnetol (5, 10, and 20 μM) pre-treatment concentration-dependently diminished the Bax expression level (to 190.2%, 168.1%, and 165.9%, respectively, compared to malathion treatment alone).
Apoptosis is associated with mitochondrial dysfunction; release of cytochrome C from the mitochondrial outer membrane to the cytosol is known to initiate apoptosis. Hence, mitochondrial cytochrome C release was analyzed by Western blotting. Following malathion treatment, there is release of cytochrome C from the mitochondria to the cytosol; the mitochondria had 82.3% of the cytochrome C seen in control cells, whereas the cytosol had 120.4% of that in control cells (Figure 3A). Gnetol (5, 10, and 20 μM) pre-treatment produced a gradual increase in the mitochondrial cytochrome C content (81.5%, 93.3%, and 93.6%, respectively, compared to malathion treatment alone) and a slight decrease in the cytosolic cytochrome C content (106.2%, 101.6%, and 97.5%, respectively). To analyze protein expression with Western blotting, α-tubulin was used as a loading control. TEM images of N2a cells also clearly showed that malathion (1 mM) treatment causes serious changes to the nucleus, and weakening of the nuclear membrane causes the leakage of nuclear material into the cytosolic space (Figure 3B). We observed disintegration of cytosolic organelles, which denotes a worsening of cellular morphology, following malathion treatment. Gnetol (20 μM) re-treatment protected cells from malathion-induced toxicity. Also, it preserved the integrity of the cell as a whole, the nucleus, the nuclear membrane, and other organelles, especially the lysosome.
Malathion and gnetol initiate autophagy
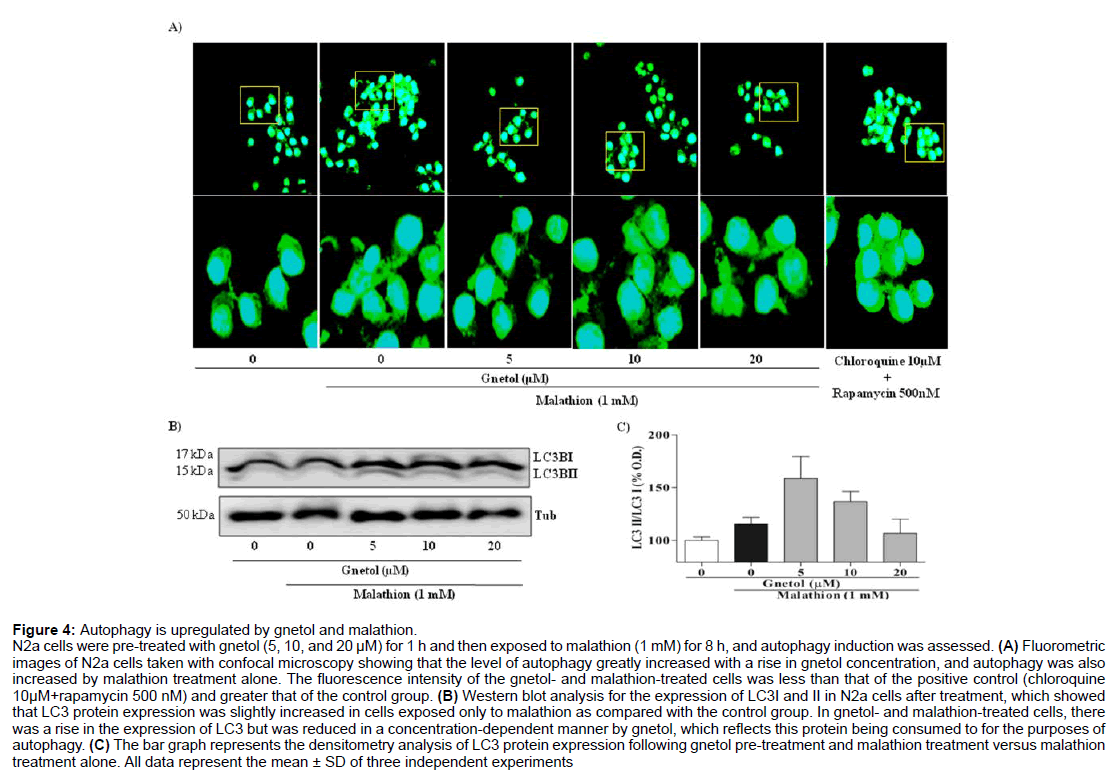
To further characterize the apoptotic cell death mediated by malathion, we focused our attention on the activation of autophagy in N2a cells and its possible neuroprotective role (Figure 4). Using the Cyto-ID autophagy vacuole detecting kit and a confocal microscope, we examined the changes in autophagy brought about by malathion and gnetol treatment (Figure 4A). The results revealed that autophagic flux increased following malathion (1 mM) treatment, as shown by an increase in the autophagosome count, with a high degree of green fluorescence in the cytosolic space as compared with the control group. Gnetol (5, 10, and 20 μM) pre-treatment dose-dependently increased the level of green fluorescence when compared to the malathion-alone group. These results indicated that both malathion and gnetol initiate autophagic flux in N2a cells. The rise in the autophagic flux caused by gnetol and malathion treatment was less than that of the positive control (chloroquine 10 μM+rapamycin 500 nM).
Figure 4: Autophagy is upregulated by gnetol and malathion.
N2a cells were pre-treated with gnetol (5, 10, and 20 µM) for 1 h and then exposed to malathion (1 mM) for 8 h, and autophagy induction was assessed. (A) Fluorometric images of N2a cells taken with confocal microscopy showing that the level of autophagy greatly increased with a rise in gnetol concentration, and autophagy was also increased by malathion treatment alone. The fluorescence intensity of the gnetol- and malathion-treated cells was less than that of the positive control (chloroquine 10µM+rapamycin 500 nM) and greater that of the control group. (B) Western blot analysis for the expression of LC3I and II in N2a cells after treatment, which showed that LC3 protein expression was slightly increased in cells exposed only to malathion as compared with the control group. In gnetol- and malathion-treated cells, there was a rise in the expression of LC3 but was reduced in a concentration-dependent manner by gnetol, which reflects this protein being consumed to for the purposes of autophagy. (C) The bar graph represents the densitometry analysis of LC3 protein expression following gnetol pre-treatment and malathion treatment versus malathion treatment alone. All data represent the mean ± SD of three independent experiments.
To verify the increase in autophagy, we proceeded to monitor LC3 protein expression by Western blotting (Figure 4B). Malathion exposure, with or without gnetol pre-treatment, caused an alteration in the LC3 protein expression level. The LC3 protein expression bands were quantified and plotted in a graph (Figure 4C), which showed no major alterations in the expression levels of LC3 or consistent increase in LC3 lipidation, which is a step in the autophagic process. From Figures 4A-4C, it is clear that there is an increase in the autophagic activity in N2a cells following malathion treatment.
Gnetol rescues the LMP phenotype
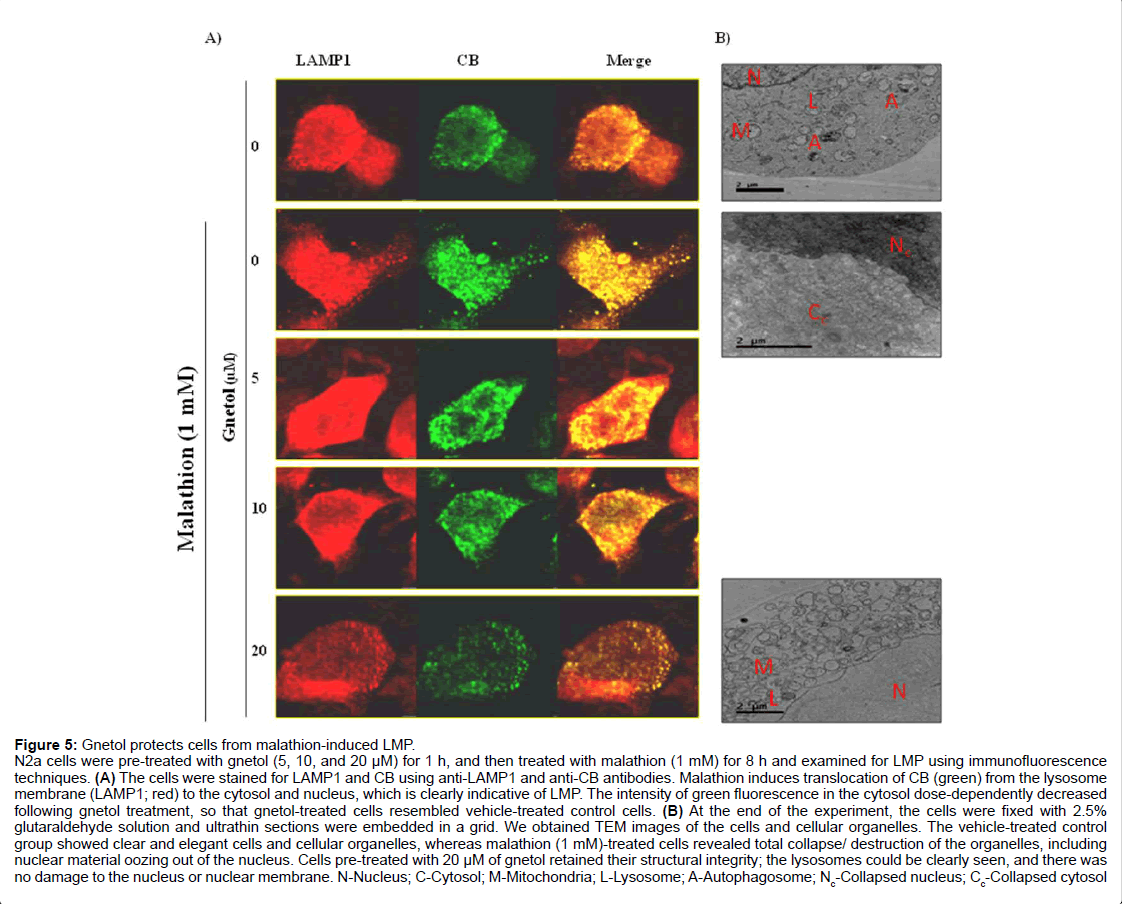
The above findings, which indicate that mitochondrial dysfunction, autophagy, and apoptotic cell death are involved in the toxic effects of malathion, and that gnetol can protect cells from malathioninduced changes, required further explanation (Figure 5A). Hence, we performed lysosomal membrane permeabilization (LMP) assays using immunofluorescence techniques (Figure 5A). The data show that malathion treatment increases release of the lysosomal proteolytic enzyme cathepsin B (CB) from the lysosomal compartment to the cytosol and mediates apoptotic cell death in N2a cells. Gnetol pretreated cells were examined for LMP. The release of CB by malathion was prevented by gnetol (5, 10, and 20 μM) dose-dependently.
Figure 5: Gnetol protects cells from malathion-induced LMP.
N2a cells were pre-treated with gnetol (5, 10, and 20 µM) for 1 h, and then treated with malathion (1 mM) for 8 h and examined for LMP using immunofluorescence techniques. (A) The cells were stained for LAMP1 and CB using anti-LAMP1 and anti-CB antibodies. Malathion induces translocation of CB (green) from the lysosome membrane (LAMP1; red) to the cytosol and nucleus, which is clearly indicative of LMP. The intensity of green fluorescence in the cytosol dose-dependently decreased following gnetol treatment, so that gnetol-treated cells resembled vehicle-treated control cells. (B) At the end of the experiment, the cells were fixed with 2.5% glutaraldehyde solution and ultrathin sections were embedded in a grid. We obtained TEM images of the cells and cellular organelles. The vehicle-treated control group showed clear and elegant cells and cellular organelles, whereas malathion (1 mM)-treated cells revealed total collapse/ destruction of the organelles, including nuclear material oozing out of the nucleus. Cells pre-treated with 20 µM of gnetol retained their structural integrity; the lysosomes could be clearly seen, and there was no damage to the nucleus or nuclear membrane. N-Nucleus; C-Cytosol; M-Mitochondria; L-Lysosome; A-Autophagosome; Nc-Collapsed nucleus; Cc-Collapsed cytosol
To confirm that LMP is affected by malathion, cells were fixed with glutaraldehyde, sliced into ultrathin sections, and examined by TEM imaging (Figure 5B). The malathion (1 mM) treatment group underwent massive collapse of the nucleus and cytosolic organelles, concomitant with breakage in the nuclear membrane, as compared with the control group. Control cells and the organelles in them were comprised of organized structures; the nucleus, mitochondria, lysosome, and autophagosome could clearly be seen, and were separate from the cytosolic space. Gnetol pre-treatment protected the organelles from damage; the nucleus, nuclear membrane, lysosome, mitochondria, and cytosolic space could clearly be seen in gnetol pre-treated cells when compared with malathion-treated cells.
Gnetol enhances neuritogenesis, neuronal protein marker level in N2a cells, and NGF secretion in C6 cells exposed to malathion
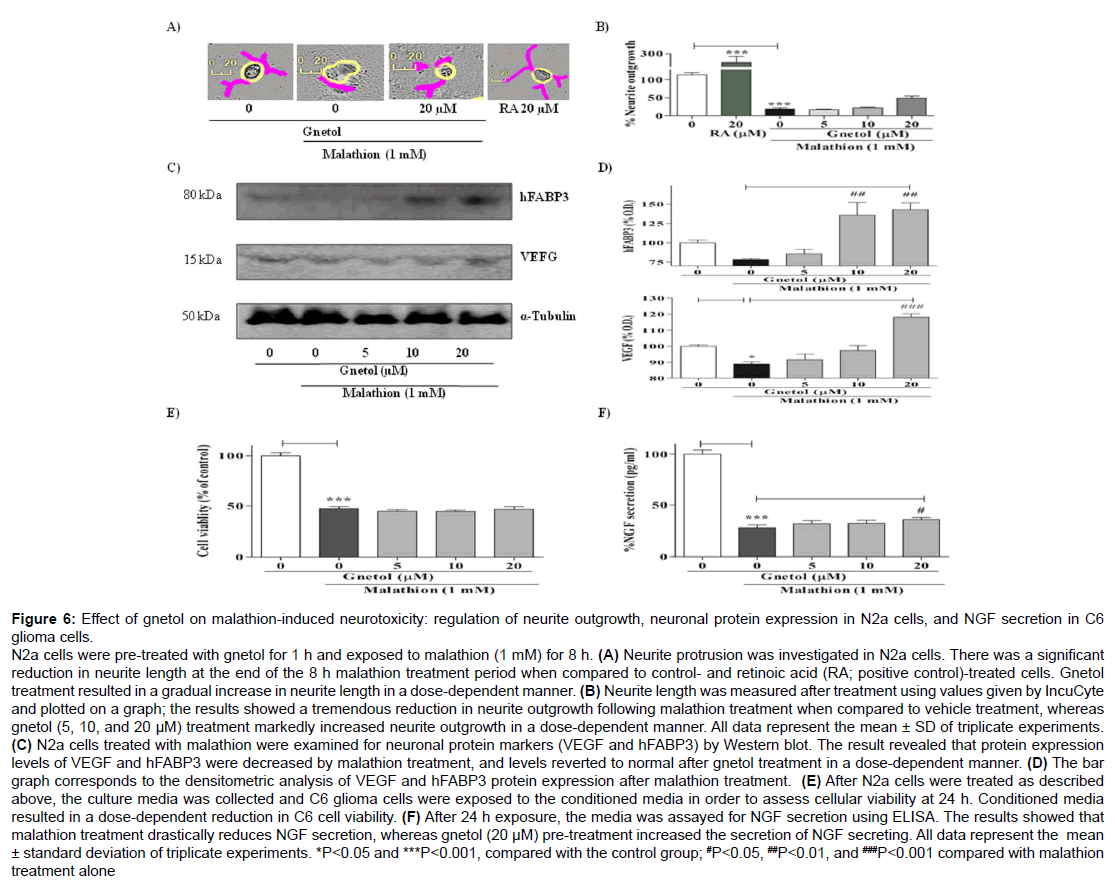
Figure 6: Effect of gnetol on malathion-induced neurotoxicity: regulation of neurite outgrowth, neuronal protein expression in N2a cells, and NGF secretion in C6 glioma cells.
N2a cells were pre-treated with gnetol for 1 h and exposed to malathion (1 mM) for 8 h. (A) Neurite protrusion was investigated in N2a cells. There was a significant reduction in neurite length at the end of the 8 h malathion treatment period when compared to control- and retinoic acid (RA; positive control)-treated cells. Gnetol treatment resulted in a gradual increase in neurite length in a dose-dependent manner. (B) Neurite length was measured after treatment using values given by IncuCyte and plotted on a graph; the results showed a tremendous reduction in neurite outgrowth following malathion treatment when compared to vehicle treatment, whereas gnetol (5, 10, and 20 μM) treatment markedly increased neurite outgrowth in a dose-dependent manner. All data represent the mean ± SD of triplicate experiments. (C) N2a cells treated with malathion were examined for neuronal protein markers (VEGF and hFABP3) by Western blot. The result revealed that protein expression levels of VEGF and hFABP3 were decreased by malathion treatment, and levels reverted to normal after gnetol treatment in a dose-dependent manner. (D) The bar graph corresponds to the densitometric analysis of VEGF and hFABP3 protein expression after malathion treatment. (E) After N2a cells were treated as described above, the culture media was collected and C6 glioma cells were exposed to the conditioned media in order to assess cellular viability at 24 h. Conditioned media resulted in a dose-dependent reduction in C6 cell viability. (F) After 24 h exposure, the media was assayed for NGF secretion using ELISA. The results showed that malathion treatment drastically reduces NGF secretion, whereas gnetol (20 μM) pre-treatment increased the secretion of NGF secreting. All data represent the mean ± standard deviation of triplicate experiments. *P
Malathion-induced neurotoxicity has an impact on neuronal transmission (Figure 6). To identify the effect of malathion on neuritogenesis, N2a cells were treated with gnetol and malathion as above and observed for neurite outgrowth (Figure 6A). The results showed that malathion strongly reduces neurite outgrowth in N2a cells, by comparison with control (untreated) cells. Gnetol (5, 10, and 20 μM) pre-treatment for 1 h ameliorated the effect of malathion on neurite outgrowth. These experiment were verified using retinoic acid as a positive control. The images were used to graph neurite length (Figure 6B). The graph shows that malathion significantly reduces neurite length, whereas gnetol pre-treatment tends to increase neurite length.
To examine the effect of gnetol in the malathion-induced neurodegenerative disease model, we examined the functional properties of neuronal cells, such as memory, energy supply, neurotransmission, differentiation, multiplication, waste disposal, and so on. We measured the levels of hFABP3 and VEGF proteins, which are associated with fatty acid transportation for energy generation and neuronal growth and maintenance, respectively. Malathion (1 mM) treatment led to a stark reduction in hFABP3 and VEGF levels (Figure 6C). Gnetol treatment markedly reversed the changes in expression of VEGF and hFABP3. The expression levels of VEGF and hFABP3 were statistically analyzed in order to quantify the significance of the differences between the different groups, and those results are plotted in a graph (Figure 6D).
Many reports have found that neurotrophic factors are necessary for the induction of neuritogenesis in N2a cells. So, we assessed the level of NGF secreted from C6 glioma cells. The supernatant from N2a cells treated with malathion with or without gnetol was collected, and C6 cells were incubated with that supernatant for 24 h, at which point we examined cell viability (Figure 6E) and the concentration of soluble NGF (Figure 6F). Malathion treatment significantly reduced C6 cell viability when compared to vehicle treatment. The gnetol pre-treated group did not show any morphological changes and appeared healthy. Malathion caused a marked decrease in NGF secretion when compared with the controls, whereas gnetol caused a slight increase in NGF secretion. Neuronal cells were proceeding towards a neurodegenerative disease condition, and this effect was controlled by pre-treatment with gnetol; thus, gnetol is considered to be a potential candidate for the prevention or treatment of neurodegenerative disease.
Taken together, these results indicate that gnetol ameliorates malathion-induced neurotoxicity by increasing the cell ability and neurite outgrowth, working against apoptosis, preventing mitochondrial dysfunction, and preventing DNA condensation/fragmentation. In addition, gnetol enhances autophagy by its effects on LMP.
Discussion
Previous reported gnetol as potent butyrylcholinesterase inhibitor and traditionally Gnetum sp. were used as highly valued nutritious leave among tribal peoples of Centre and West Africa [13,14]. The present study aimed, first to investigate the protective effect of gnetol against malathion-induced neurotoxicity. Also, our objectives were to establish a model of neurodegenerative disease using N2a cells and show that gnetol supplementation is protective, along with elucidate its underlying molecular mechanisms. Malathion, an organophosphate pesticide, is used and available for agricultural and household purposes, for example, controlling pests and lice. Most organophosphate pesticides promote irreversible cholinesterase inhibition, which strongly affects cholinergic system of the brain and caused neurotoxicity with depression, convulsion, paralysis, coma, bradycardia, hypotension, nausea, vomiting, lachrymation, ataxia, etc. [15-17]. Malathion exposure reduces the viability of N2a cells, but pre-treatment with gnetol increases cell survival (Figure 1A). Likewise, terbufos, an organophosphate pesticide, inhibits mouse testicular cell growth and proliferation by induction of reactive oxygen species (ROS) and alterations in the cell cycle, pro-apoptotic proteins, and mitochondrial membrane potential [18]. The morphology of cells also greatly improved after pre-supplemented with gnetol as compared with malathion treatment alone (Figure 1B). Previously, researchers found that subcutaneous exposure of young adult rats to chlorpyrifos promotes substantial nigral dopaminergic neuronal deathmediated development of neurodegeneration, and also led to intense accumulation of microglia- and astrocyte-related neuroinflammation [19]. Similar to gnetol, pentoxifylline protects rat brains from malathioninduced oxidative stress by enhancing antioxidative enzyme levels and antioxidants, and also by functioning as an immunomodulator [20].
Recent reports state that dermal exposure to chlorpyrifos and cypermethrin causes CNS toxicity associated with a defect in acetylcholinesterase (AChE) signaling along with histological changes in nissel granules [21]. Similarly, combined exposure of human neuroblastoma cells to pesticides induces apoptosis by modulating the levels of cleaved cas 3, BcL-2, and BcL-xL proteins through FAS/ TNF signaling pathways [22]. We confirmed that 8 hr malathion exposure induces apoptotic cell death in N2a cells with the activation of the pro-apoptotic proteins caspase 3 (Figures 3A and 3B) and bax, mitochondrial cyto C release, and suppression of the anti-apoptotic proteins AKT and BcL-2, and simultaneously activating autophagy. From (Figures 2A and 2C), it is obvious that malathion promotes apoptosis. But, pre-supplement with gnetol ameliorates malathioninduced cell apoptosis in a concentration-dependent manner. Also, as revealed by Hoechst33258 nuclear staining (Figures 2B and 2D), gnetol protects mouse neuroblastoma cells from malathion-induced nuclear fragmentation/condensation. Hypoxia/ischemia or head trauma, excitotoxicity, and glutamate administration induce apoptotic degeneration of the hypothalamus and hypothalamic neurons as observed with DNA fragmentation [23]. Recently, Raszewski et al. reported that chlorpyrifos and cypermethrin induced apoptosis in human neuroblastoma SH‐SY5Y cells [22]. Reports from other research groups found that dieldrin treatment in rat mesencephalic cells induces oxidative stress-mediated apoptotic cell death by modifying caspase 3 activity, mitochondrial cyto C release, and promoting DNA damage. Rottlerin, a protein kinase C inhibitor, blocks the dieldrin-induced DNA fragmentation/condensation in mesencephalic neurodegenerative model [24]. Boron protects the brain, liver, and kidneys from the histological changes in a dose-dependent manner by subsidizing malathion-induced oxidative stress, and enhancing the regenerative effect [25].
Interestingly, our finding suggests that 1 h pre-treatment with gnetol protects N2a cells from malathion-induced apoptotic cell death via activation of autophagy (Figure 4A). Also, elaiophylin, a novel autophagic inhibitor, promotes autophagosome accumulation-mediated blockade by inducing lysosomal destabilization in ovarian cancer cells [26]. Autophagic flux was reassessed by analyzing the expression level of LC3 protein, which revealed no major alteration in LC3 expression from the control (Figure 4B), even in the gnetol-treated group. Hence, we examined LMP after treatment with malathion and gnetol. Gnetol pre-treatment significantly decreased LMP in a dose-dependent manner than malathion treatment alone (Figures 5A and 5B).
Recently, factors such as heart-type fatty acid binding protein (hFABP) and vascular endothelial growth factor can be a one of important biomarkers in hallmark of AD and other dementias [27]. Amyloid β and phosphorylated tau have been studied to elucidate the mechanism of action of neurodegeneration. Some compounds used in traditional Chinese medicine (Huang-Lian-Jie-Du-Decoction) induce neurogenesis, probably by upregulating expression of VEGF [28]. VEGF is a therapeutic target in diabetic macular edema; sustained neutralization of VEGF in diabetic mice induces retinal degeneration with leakage of albumin from the vascular endothelium [29]. Longchain fatty acid transport 1 (FATP 1) is expressed specifically in adipose tissue, heart muscle, skeletal muscle, and the brain and plays a vital role in the transport of fatty acids across plasma membranes. Defects in FATP 1 are associated with ageing and degenerative disease [30]. Therefore, we examined the protein expression levels of VEGF and heart fatty acid binding protein 3 (hFABP3) following treatment with malathion and gnetol. Our results showed that gnetol pre-treated, malathion-treated cells had increased expression of hFABP3 and VEGF protein, when compared to either control or malathion-treated cells (Figure 6).
Gnetol potential activates AMP-activated protein kinase (AMPK) and ERK directed vascular flexibility in rat spontaneous hypertensive heart failure model compared with other Stilbenoid derivates [31,32]. Resveratrol, a potential activator of SIRT1 inhibits aging disease like AD by mimic caloric restriction in animal models [33]. In diabetic cardiomyopathy and hypertrophy adult rat, resveratrol ameliorates apoptosis, mitochondrial biogenesis, hypertrophy and cardiomyocytes functions by SIRT1 mediated activation of nuclear respiratory factor 1 (NRF-1), NRF-2, AMPK, estrogen-related receptor-α (ERR-α), and mitochondrial transcription factor A (TFAM) [34,35].
Spicatoside A from Liriope platyphylla enhances neurite outgrowth in PC12 cells and also potentiates the release of trophic factors that are beneficial in neurodegenerative diseases [36]. Neurotrophic factors were activated via PI3K-dependent pathways or other intracellular signalling pathways and promoted neuronal survival and neurite outgrowth [37,38]. We examined neurite outgrowth of N2a cells after treatment of gnetol. Gnetol increased the degree of neurite outgrowth and length of neurites (Figure 6A and 6B), which is vital for neuronal cells when transmitting neuronal impulses through network tethering. Glial cell-derived neurotrophic factor (GDNF), NGF, and brainderived neurotrophic factor (BDNF) infusion or application of small molecule agonists, inducers, or modulators are potentially therapeutic in neurodegenerative disease states [37]. Moreover, C6 cells incubated with the conditioned media from N2a cells displayed a significant reduction in cellular viability (Figure 6E). Simultaneously, we analyzed the soluble NGF secretion level in C6 cells treated with supernatant from N2a cells. Malathion treatment alone significantly decreased the NGF level, whereas conditioned media from cells that had been pre-treated with gnetol led to an increase in NGF secretion from C6 cells (Figure 6F). A lot of studies already reported that resveratrol, a representative dietary polyphenol acts to provide neuroprotection in many types of neurodegenerative diseases models [39]. In regard to our research, Kumar et al. reported that trans-resveratrol (RV) protected neuronal cell toxicity exposed to organophosphate pesticide-monocrotophos (MCP) [40]. But, till now, there is no report on protection of resveratrol and its derivative against malathion-induced neurotoxicity. Interestingly, among resveratrol derivatives, gnetol (2,3’,5’,6-tetrahydroxy-transstilbene) had the most effectiveness in malathion-treated neurotoxicity.
Menopause associated with estrogen deficient mediated oxidative stress leads to increase in health issues as osteoporosis, cardiovascular disease, neurodegenerative diseases, and cancer. Estrogen replacement therapy recommended beneficial treatment for the welfare of women health [41]. Clinical trial of postmenopausal women with resveratrol, and soy isoflavone showed resveratrol improved cognitive function by boosting cerebral vasodilator responsiveness [42]. Resveratrol and other stilbenes resembles phytoestrogen which modulates estrogen receptor mediated transcription exerts neuroprotective and antiinflammatory effects in multiple sclerosis and other neurodegenerative disease condition [43].
Gnetol is a potent molecule that we herein showed had a beneficial effect on LMP-mediated apoptotic cell death in neuronal cells and also induced autophagy. Additionally, gnetol enhances cell survival by increasing the levels of antiapoptotic proteins, neuronal function, and neuritogenesis in N2a cells with inhibition of pro-apoptotic proteins and nuclear morphological changes. Taken together, our results suggest that gnetol may be a potential candidate for the neurodegerative disease involving exposure to the organophosphate pesticide malathion. These findings call for in vivo study of gnetol in a neurodegenerative disease model, with assessment of its behavioral and cognitive effects. Further studies are needed to unshed darkness of the gnetol molecular mechanism in sirtuin gene family and estrogen receptor signalling mediated neuroprotective effect in neurodegenerative and neuroprotective role.
Chemical Compounds Studied in this Article
Malathion: PubChem CID: 4004; Gnetol: PubChem CID: 45382232; Retinoic acid: PubChem CID: 444795.
Acknowledgement
This work was supported by the KIST Institutional Program(Project No. 2E27513-17-131).
References
- Sarris J, Panossian A, Schweitzer I, Stough C, Scholey A (2011) Herbal medicine for depression, anxiety and insomnia: a review of psychopharmacology and clinical evidence. Eur Neuropsychopharmacol 21: 841-860
- Folch J, Petrov D, Ettcheto M, Abad S, Sánchez-López E, et al. (2016) Current research therapeutic strategies for alzheimer’s disease treatment. Neural Plasticity
- Beamer PI, Canales RA, Ferguson AC, Leckie JO, Bradman A (2012) Relative pesticide and exposure route contribution to aggregate and cumulative dose in young farmworker children. Int J Environ Res Public Health 9: 73-96
- Peris-Sampedro F, Salazar JG, Cabré M, Reverte I, Domingo JL, et al. (2014) Impaired retention in AßPP Swedish mice six months after oral exposure to chlorpyrifos. Food and ChemToxi 72: 289-294.
- Kamel F, Hoppin JA (2004) Association of pesticide exposure with neurologic dysfunction and disease. Environ Health Perspect 112: 950-958
- Tanner CM, Goldman SM, Ross GW, Grate SJ (2014) The disease intersection of susceptibility and exposure: chemical exposures and neurodegenerative disease risk. Alzheimer's & Dementia 10: S213-S25
- Edwards D (2006) Reregistration eligibility decision for malathion. US environmental protection agency-prevention, pesticides and toxic substances EPA 9:738
- AM Aboul-Soud M, M. Al-Othman A, E. El-Desoky G, A. Al-Othman Z, Yusuf K, Ahmad J, et al. (2011) Hepatoprotective effects of vitamin E/selenium against malathion-induced injuries on the antioxidant status and apoptosis-related gene expression in rats. J Toxicol Sci 36: 285-296.
- Alp H1, Aytekin I, Hatipoglu NK, Alp A, Ogun M (2012) Effects of sulforophane and curcumin on oxidative stress created by acute malathion toxicity in rats. Eur Rev Med Pharmacol Sci 16 Suppl 3: 144-148.
- Boya P, Gonzalez-Polo R-A, Poncet D, Andreau K, Vieira HL, et al. (2003) Mitochondrial membrane permeabilization is a critical step of lysosome-initiated apoptosis induced by hydroxychloroquine. Oncogene 22: 3927-3936
- Boya P, Kroemer G (2008) Lysosomal membrane permeabilization in cell death. Oncogene 27: 6434-6451.
- Repnik U, Hafner ÄŒesen M, Turk B (2014) Lysosomal membrane permeabilization in cell death: concepts and challenges. Mitochondrion 19 Pt A: 49-57.
- Sermboonpaisarn T, Sawasdee P (2012) Potent and selective butyrylcholinesterase inhibitors from Ficus foveolata. Fitoterapia 83: 780-784.
- Shiembo PN (1997) The sustainability of eru (Gnetum africanum and Gnetum buchholzianum): over-exploited non-wood forest product from the forests of Central Africa
- Marrs TT, Ballantyne B (2004) Pesticide toxicology and international regulation: John Wiley & Sons.
- Edward D (2006) Reregistration eligibility decision for malathion. US environmental protection agency, prevention, pesticides and toxic substances EPA 738-R-06-030, July 2006
- Venkatesan R, Park YU, Ji E, Yeo E-J, Kim SY (2017) Malathion increases apoptotic cell death by inducing lysosomal membrane permeabilization in N2a neuroblastoma cells: a model for neurodegeneration in Alzheimer’s disease. Cell Death Discovery 3: 17007
- Hung JH, Chen CY, Omar HA (2016) Reactive oxygen species mediate Terbufos-induced apoptosis in mouse testicular cell lines via the modulation of cell cycle and pro-apoptotic proteins. Environ Toxicol 31: 1888-1898.
- Zhang J, Dai H, Deng Y, Tian J, Zhang C, et al. (2015) Neonatal chlorpyrifos exposure induces loss of dopaminergic neurons in young adult rats. Toxicology 336: 17-25.
- Ranjbar A, Ghahremani MH, Sharifzadeh M, Golestani A, Ghazi-Khansari M, et al. (2010) Protection by pentoxifylline of malathion-induced toxic stress and mitochondrial damage in rat brain. Hum Exp Toxicol 29: 851-864
- Latuszynska J, Luty S, Raszewski G, Przebirowska D, Tokarska-Rodak M (2003) Neurotoxic effect of dermally applied chlorpyrifos and cypermethrin. Reversibility of changes. Ann Agric Environ Med 10: 197-201.
- Raszewski G, Lemieszek MK, Lukawski K, Juszczak M, Rzeski W (2015) Chlorpyrifos and Cypermethrin Induce Apoptosis in Human Neuroblastoma Cell Line SH-SY5Y. Basic Clin Pharmacol Toxicol 116: 158-167
- Ishimaru MJ, Ikonomidou C, Tenkova TI, Der TC, Dikranian K, et al. (1999) Distinguishing excitotoxic from apoptotic neurodegeneration in the developing rat brain. J Comp Neurol 408: 461-476
- Kanthasamy AG, Kitazawa M, Yang Y, Anantharam V, Kanthasamy A (2008) Environmental neurotoxin dieldrin induces apoptosis via caspase-3-dependent proteolytic activation of protein kinase C delta (PKCdelta): Implications for neurodegeneration in Parkinson's disease. Mol Brain 1: 12
- Coban FK, Ince S, Kucukkurt I, Demirel HH, Hazman O (2015) Boron attenuates malathion-induced oxidative stress and acetylcholinesterase inhibition in rats. Drug Chem Toxicol 38: 391-399
- Zhao X, Fang Y, Yang Y, Qin Y, Wu P, et al. (2015) Elaiophylin, a novel autophagy inhibitor, exerts antitumor activity as a single agent in ovarian cancer cells. Autophagy 11: 1849-1863
- Guo LH, Alexopoulos P, Perneczky R (2013) Heart-type fatty acid binding protein and vascular endothelial growth factor: cerebrospinal fluid biomarker candidates for Alzheimer's disease. Eur Arch Psychiatry Clin Neurosci 263: 553-560.
- Zou H, Long J, Zhang Q, Zhao H, Bian B, et al. (2016) Induced cortical neurogenesis after focal cerebral ischemia--Three active components from Huang-Lian-Jie-Du Decoction. J Ethnopharmacol 178: 115-124.
- Hombrebueno JR, Ali IH, Xu H, Chen M (2015) Sustained intraocular VEGF neutralization results in retinal neurodegeneration in the Ins2Akita diabetic mouse. Sci Rep: 5
- Vincenzetti S, Nasuti C, Fedeli D, Ricciutelli M, Pucciarelli S, et al. (2016) Proteomic analysis for early neurodegenerative biomarker detection in an animal model. Biochimie 121: 79-86
- Lee DI, Acosta C, Anderson CM, Anderson HD (2017) Peripheral and cerebral resistance arteries in the spontaneously hypertensive heart failure rat: effects of stilbenoid polyphenols. Molecules 22: 380
- Akinwumi BC, Raj P, Lee DI, Acosta C, Yu L, et al. (2017) Disparate effects of stilbenoid polyphenols on hypertrophic cardiomyocytes in vitro vs. In the spontaneously hypertensive heart failure rat. Molecules 22: 204
- Sawda C, Moussa C, Turner RS (2017) Resveratrol for Alzheimer's disease. Ann N Y Acad Sci 1403: 142-149.
- Ma S, Feng J, Zhang R, Chen J (2017) SIRT1 activation by resveratrol alleviates cardiac dysfunction via mitochondrial regulation in diabetic cardiomyopathy mice. Oxid Med Cell Longev 2017: 4602715
- Thandapilly SJ, Louis XL, Yang T, Stringer DM, Yu L, et al. (2011) Resveratrol prevents norepinephrine induced hypertrophy in adult rat cardiomyocytes, by activating NO-AMPK pathway. Eur J Pharmacol 668: 217-224.
- Hur J, Lee P, Moon E, Kang I, Kim SH (2009) Neurite outgrowth induced by spicatoside A, a steroidal saponin, via the tyrosine kinase A receptor pathway. Eur J Pharmacol 620: 9-15.
- Allen SJ, Watson JJ, Shoemark DK, Barua NU, Patel NK (2013) GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol Ther 138: 155-175.
- Segal RA, Greenberg ME (1996) Intracellular signaling pathways activated by neurotrophic factors. Annu Rev Neurosci 19: 463-489.
- Tellone E, Galtieri A, Russo A, Giardina B, Ficarra S (2015) Resveratrol: a focus on several neurodegenerative diseases. Oxidative medicine and cellular longevity.
- Kumar V, Tripathi VK, Singh AK, Lohani M, Kuddus M (2013) Trans-resveratrol restores the damages induced by organophosphate pesticide-monocrotophos in neuronal cells. Toxicol Int 20:48
- Bhavnani BR (2003) Estrogens and menopause: pharmacology of conjugated equine estrogens and their potential role in the prevention of neurodegenerative diseases such as Alzheimer's. J Steroid Biochem Mol Biol 85: 473-482
- Thaung Zaw JJ, Howe PRC, Wong RHX (2017) Does phytoestrogen supplementation improve cognition in humans? A systematic review. Ann N Y Acad Sci 1403: 150-163
- Wei Z, Wang M, Hong M, Diao S, Liu A, et al. (2016) Icariin exerts estrogen-like activity in ameliorating EAE via mediating estrogen receptor ß, modulating HPA function and glucocorticoid receptor expression. Am J Transl Res 8: 1910-1918
Citation: Venkatesan R, Park YU, Lee TH, Kim SY (2017) Gnetol, a Resveratrol Derivative Ameliorates Malathion-Induced Neurotoxicity through Modulating Lysosomal Membrane Permeabilization in N2a Cells. J Alzheimers Dis Parkinsonism 7: 388. DOI: 10.4172/2161-0460.1000388
Copyright: © 2017 Venkatesan R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 7982
- [From(publication date): 0-2017 - Nov 23, 2025]
- Breakdown by view type
- HTML page views: 6958
- PDF downloads: 1024