Grafting, Scion and Rootstock Effects on Survival Rate, Vegetative Growth and Fruit Yield of High Tunnel-grown Grafted Pepper (Capsicum annuum L.) Plants
Received: 16-Sep-2017 / Accepted Date: 24-Oct-2017 / Published Date: 27-Oct-2017 DOI: 10.4172/2329-8863.1000312
Abstract
The objective of this study was to develop information useful to pepper (Capsicum annuum L.) rootstock breeders, grafted plant propagators and growers managing commercial plantings in semi-protected settings. To accomplish this, an experiment involving four phenotypically diverse scion cultivars and five rootstocks grafted in all combinations along with self-grafted and non-grafted cultivars as controls was completed twice. The effects of the grafting process, scion cultivars and rootstock genotypes on grafted plant performance were delineated by measures of post-grafting plant survival (%), plant vegetative vigor and by early and total season yield parameters. The grafting process significantly increased vegetative vigor parameters, most notably in 2014 whereas the yield parameters of self-grafted plants exceeded their non-grafted counterparts only in 2015. In both years, scion performance in graft combinations remained characteristic of the cultivar type; long-fruited cultivars ‘Eigman’ and ‘Kurtovszka Kapia’ had lower survival rates, generally exhibited greater vegetative vigor, and amassed greater yields per m2 than blocky-fruited ‘Toronto’ and ‘Zedinca’. In general, graft combinations using commercial pepper rootstocks 52-03 RZ and Tan Tan (No: 12G076) outperformed those composed of standard cultivars or breeding lines used as rootstocks. Scions on these rootstocks exhibited greater vegetative vigor in both years and higher yields per m2 than non-grafted controls in 2014. The superior performance of 52-03 RZ and Tan Tan (No: 12G076) in this study exemplified the advantage of using rootstocks specifically bred for optimum root system performance and compatibility with a variety of scions. However, significant scion × rootstock interactions and seasonal differences in performance suggested, that as new rootstocks are developed or as new graft combinations are introduced, it will be necessary to rigorously test them in multiple environments to insure commercial success.
Keywords: Capsicum; Grafting; Rootstock development; Grafting effects; Rootstock effects; Scion effects; Survival; Vegetative growth; Fruit yield
Introduction
In many countries, cultivation of peppers (Capsicum annuum L.) in open-framed, plastic-covered tunnels is widespread because these systems provide a greater amount and duration of crop protection from unwanted precipitation, wind, and other abiotic conditions, thereby typically leading to greater production per unit area per unit time. However, soil-based stresses, including disease, can spike in these systems, undercutting their value. Regardless, combining superior root system traits with desirable scion characteristics through grafting is an increasingly recognized approach, along with other practices [1].
Historically, vegetable crop grafting was used to alleviate soil-borne diseases [2], but the range of vegetable species grafted has expanded and the reasons for its use have increased over time. Today, grafting is used as a means to confer resistance against low and high temperature stress [3] improve nutrient uptake [4] enhance synthesis of endogenous hormones [5] improve water use efficiency [6] improve alkalinity tolerance [7] raise salt, drought and flooding tolerance [8] reduce the assimilation of agrichemical residues present in soils [9] diminish metal ion toxicity damage [10] extend production-market windows [11] facilitate organic vegetable cultivation [12], control pests [13] confer food security [14] and improve fruit quality [15]. Although the use of grafted plants for vegetable production in protected environments has increased globally over the last decade [16] it is a proven technique for enhancing a crop’s genetic potential that is still considered to be underutilized [17].
The current status of vegetable grafting and its potential for improving production of tomato and other solanaceous crops have been recently reviewed [18]. However, to date, the influence of grafting and rootstocks on grafted pepper survival, vegetative growth, fruiting characteristics and yield has been investigated in few studies and with only a limited number of rootstocks. Moreover in pepper, current expansion of the practice is hampered by the lack of the following: a cadre of well-tested commercially-available rootstocks; information about the interactive nature of rootstock-scion combinations in regards to performance factors; an understanding about how performance factors are interrelated during grafted plant production and cropping; and an awareness of how cropping potential for a given rootstockscion combination may vary when cultured in different production environments.
The objective of this study was to verify the effects of grafting and pepper rootstock-scion combinations on grafted plant survival rate, growth, fruit yield and their components establishing a range of performance expectations for current and future grafted pepper propagators, producers and rootstock developers. To do so, we included a diverse set of rootstock and scion genotypes in order to represent a range of possible production outcomes. Moreover, outlining performance interrelationships among these parameters and assessing possible environmental effects may provide rootstock breeders or grafted plant producers alike with useful information for evaluating germplasm and/or production techniques. The twenty rootstock-scion combinations of grafted pepper plants along with their non-grafted and self-grafted controls were cultured in a semiprotected, plastic-covered environment to mimic current production trends.
Materials and Methods
The study was completed twice (Season 1, July 2013-May 2014; Season 2, July 2014-May 2015) in a plastic house (high tunnel) without environmental controls at the faculty of agriculture-Kafr El-Sheikh University, Egypt. Four pepper scions and seven root treatments were included in both seasons; the four scions were grafted onto five rootstocks (20 treatments) and in addition, each scion was self-grafted (4 treatments) and grown as a non-grafted plant (4 treatments). The experimental units were arranged in a randomized complete block design with three replicates of each treatment.
Plant material and grafting procedures
Diversity in the horticultural characteristics among the five rootstocks and four pepper varieties used for this study are summarized in Table 1. The rootstocks displayed a wide range of disease resistance and included commercial rootstocks [Tan Tan (No: 12G076) and 52-03 RZ], cultivars used as rootstocks (‘Budai csipős’ and ‘Nourdine’) and CCA-4758, a pepper breeding line. The four selected scion cultivars represented consumer-accepted shapes for pepper fruit; ‘Eigman’ and ‘Kurtovszka Kàpia’ both produced elongated fruit (long-fruited), and ‘Toronto’ and ‘Zedinca’ were round or blockyfruited cultivars. All seed stocks were obtained through personal contact or from commercial distributors. Rootstock and scion seedlings to be grafted were cultured in a high tunnel environment; rootstock seeds were sown ten days before sowing scion seeds to ensure stem diameter uniformity at the time of grafting. Pepper seedlings of the same diameter and with two to four true leaves were grafted 35-40 days after sowing using the tube (splice) grafting method. The grafted seedlings were transferred immediately to a healing chamber [19]. All grafted seedlings were held for 7-10 days for further growth development and acclimation.
| Pepper genotype | Country of origin | Seed source | Disease/pest resistance | Fruit characteristics | Plant vigor level | Usage |
|---|---|---|---|---|---|---|
| Rootstocks | ||||||
| CCA- 4758 | Taiwan | AVRDC | Fusarium wilt, Nematodes | Small- Hot elongated | Very high | Breeding line |
| Budai csipős | Hungary | ZKI | Bacterial wilt | Large-Hot elongated | Low | Cultivar used as a rootstock |
| Nourdine | Egypt | Domiatec Group | Phytophthora blight, Nematodes | Medium-Hot elongated | Medium | Cultivar used as a rootstock |
| 52-03 RZ | Egypt | Rijk Zwaan | Phytophthora blight Potato Y virus Tomato mosaic virus Nematodes | Medium-Hot elongated | Medium to high | Commercial rootstock |
| Tan Tan (No: 12G076) | Egypt | Kanza Group | Bacterial wilt Phytophthora blight Nematodes | Medium- Hot elongated | Medium | Commercial rootstock |
| Scions | ||||||
| Toronto | Egypt | Rijk Zwaan | Sweet- Blocky | Medium | Commercial cultivar | |
| Zedinca | Egypt | Rijk Zwaan | Sweet- Blocky | Medium | Commercial cultivar | |
| Kurtovszka Kàpia | Hungary | ZKI | Sweet- Elongated | Medium to high | Commercial cultivar | |
| Eigman | Egypt | Enza Zaden | Sweet- Elongated | Medium to high | Commercial cultivar | |
Table 1: Characteristics of rootstock and scion genotypes employed to illustrate a range of performance in the survival rate, vegetative growth and yield among grafted pepper plants and their self-grafted and non-grafted controls grown under high tunnel culture.
After grafting and acclimation were completed, the seedlings were transplanted into the clay loam soil of the plastic house on two-row raised beds at spacing 50 cm between plants (plant density=2 plants/ m2). Standard commercial cultural practices such cultivation, irrigation, fertigation, pruning, pest control and others were followed throughout the experimental period. Grafted seedlings were pruned to three main stems and fruits were harvested at the maturity generally associated with each scion cultivar.
Data recorded
For the 20 rootstock-scion combinations and their self-grafted and non-grafted control counterparts, the variables tested included survival rate following grafting, plant growth parameters and fruit yield components. The survival of grafted plants was rated 15 days after transplanting (DAT) based on the appearance of the scion; plants with completely wilted scions were regarded as dead, otherwise, as living. Grafting survival was expressed as a percentage. Vegetative growth parameters such as plant height (cm), number of leaves, number of branches and leaf area per plant (cm2) were recorded three times at 30, 50 and 70 DAT. Measurements were performed on samples of three plants from each replicate. Plant height was measured from the ground surface to the tip of the plant. Leaf area was determined on five mature acropetal-most leaves per plant using portable area meter model LI-3000A (LI-COR Biosciences, Lincoln, NE, USA). Total chlorophyll was measured by color reflectance using a portable chlorophyll meter (SPAD-502, Konica Minolta Inc., Osaka, JAPAN) at 60 DAT. Commercially-mature fruit was harvested at 7 to 15 day intervals. Yield component data including average fruit weight (g), the number of fruits per plant, and fruit weight per m2 and were compiled for the first 45 days of harvesting (early yield) and again after the seven months in each experimental season (total yield).
Data analysis
Season 1 (2014) and Season 2 (2015) data were analyzed separately. The main and interactive effects of four scion genotypes grafted to five rootstock genotypes along with their self-grafted and non-grafted scion controls (i.e., 28 combinations) were determined using SAS software (version 9.4; SAS Institute, Cary, NC). Multiple comparisons were made with Fisher’s least significance difference test (P ≤ 0.05). The effects of the grafting process were evaluated by contrasting the survival, horticultural performance and yield of self-grafted scion cultivars with that of their non-grafted genotypic counterparts (i.e., four combination pairs). Similarly, the effects of rootstocks on scion survival, growth and yield parameters were considered by comparing rootstock means across scion cultivars with each other and with control plants. With respect to these parameters, scion effects were determined by comparing scion means across all rootstocks and controls. Patterns exemplifying significant interactions [i.e., scion × the grafting process (Sc*Gr) and/or scion × rootstock (Sc*Rs)] were graphed using Sigma Plot (version 12.5; Systat Software, Inc., San Jose, CA). SAS software was also used to determine Pearson correlation coefficients among survival, growth and yield parameters using arrays of root treatment means.
Results And Discussion
Grafting effects
Grafting success and the survival of the grafted plant depends on the cohesion between rootstock and scion (i.e., callus formation, vascular bundle differentiation and connectivity at the graft interface) that insures the balanced development of both scion and rootstock [20] necessary for optimum horticultural performance. Poorly connected grafts limit water and xylem sap transfer at the graft union; low hydraulic conductance causes impaired stomatal activity, defoliation and a loss of shoot vigor (scion growth), yield and quality [21].
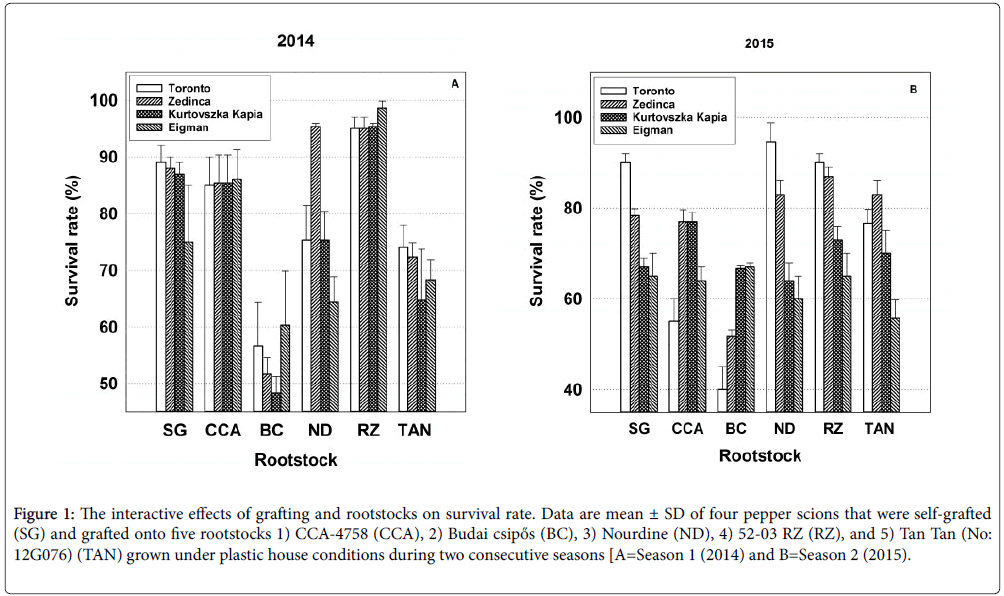
Herein, the effects of the grafting process were evaluated by contrasting the mean survival rate, horticultural performance and yield parameters of self-grafted scion cultivars with that of their nongrafted genotypic counterparts (i.e., four combination pairs, Tables 2 and 3). The grafting process significantly reduced the percentage of self-grafted plants that survived the 15 day healing and hardening-off processes by 15.2% in 2014 and by 24.9% in 2015 in comparison with their non-grafted seedling counterparts (rated at 100% survival). However, in accordance with Sc*Gr significance, self-grafted plant survival percentages significantly differed among cultivars with nearly 90% of self-grafted ‘Toronto’ seedlings surviving in both years, but less than 75% and 65% of self-grafted ‘Eigman’ seedlings surviving in 2014 and 2015, respectively (Figure 1; see SG bar set).
Figure 1: The interactive effects of grafting and rootstocks on survival rate. Data are mean ± SD of four pepper scions that were self-grafted (SG) and grafted onto five rootstocks 1) CCA-4758 (CCA), 2) Budai csipős (BC), 3) Nourdine (ND), 4) 52-03 RZ (RZ), and 5) Tan Tan (No: 12G076) (TAN) grown under plastic house conditions during two consecutive seasons [A=Season 1 (2014) and B=Season 2 (2015).
In 2014, the process of grafting significantly increased all vegetative growth parameters at 70 DAT (Table 2); chlorophyll content at 60 DAT was significantly improved by self-grafting in 2015, along with all 70 DAT vegetative parameters except plant height (Table 3). Grafters have previously offered anecdotal reports concerning the horticultural superiority of self-grafted plants, but discussions of this phenomenon in the scientific literature are rare. Aloni et al. [22] reported significant increases in root and shoot fresh weight and in root length of selfgrafted ‘Arava’ muskmelon over their non-grafted counterparts when grown in either salt-stress or salt-free (control) environments. In either the muskmelon experiment of Aloni et al. or in the pepper experiment reported herein, the process of self-grafting likely did not improve the vascular connectivity or xylem and phloem function over that of plants that were left intact.
| Effects | Survival (%) | Vegetative growthz | Yieldy | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Plant height (cm) | No. of branches | No. of leaves | Leaf area (cm2) | Chlorophyll content | Fruit weight (g) | No. fruits per plant | Yield (kg) | ||
| Graftingx and rootstock genotypew | |||||||||
| Non-grafted | 100.0 av | 125.4 cd | 29.9 d | 109.3 e | 741.3 e | 54.3 bc | 131.5 ab | 54.2 c | 11.6 c |
| Self-grafted | 84.8 c | 132.4 b | 38.8 c | 124.8 d | 795.5 d | 54.5 bc | 134.4 ab | 52.5 c | 10.9 c |
| CCA-4758 | 85.4 c | 130.8 bc | 41.5 bc | 125.7 d | 802.5 d | 53.4 cd | 116.5 c | 38.8 d | 6.8 d |
| Budai csipős | 54.3 f | 123.3 d | 41.7 b | 124.7 d | 915.0 a | 51.5 d | 126.8 b | 56.7 bc | 10.7 c |
| Nourdine | 77.6 c | 136.0 b | 40.9 bc | 135.1 c | 869.6 c | 52.7 cd | 140.0 a | 52.4 c | 10.9 c |
| 52-03 RZ | 96.0 b | 145.7 a | 45.8 a | 160.5 a | 915.8 a | 55.8 ab | 131.0 ab | 65.2 ab | 13.9 b |
| Tan Tan (No:12G076) | 69.8 e | 150.0 a | 41.3 bc | 148.8 b | 877.5 b | 56.7 a | 134.9 ab | 67.7 a | 15.7a |
| Scion genotypeu | |||||||||
| Toronto | 82.1 ab | 123.7 c | 40.3 ab | 136.5 a | 899.3 a | 54.2 b | 177.7 a | 26.4 c | 9.3 c |
| Zedinca | 84.0 a | 129.6 b | 39.6 ab | 132.5 b | 870.3 b | 57.2 a | 183.1 a | 25.9 c | 9.5 c |
| Kurtovszka Kàpia | 79.4 bc | 142.6 a | 41.2 a | 123.2 b | 807.3 c | 51.3 c | 86.5 b | 73.1 b | 12.6 b |
| Eigman | 79.0 c | 143.3 a | 38.8 b | 129.4 b | 804.3 c | 53.7 b | 75.6 c | 95.9 a | 14.7 a |
| Interactive effects | |||||||||
| Scion*grafting (Sc*Gr) | 0.0180 | 0.6746 | 0.4193 | 0.3226 | <0.0001 | <0.0001 | 0.0139 | 0.0291 | 0.1458 |
| Scion*rootstock (Sc*Rs) | <0.0001 | 0.0936 | 0.0095 | 0.0003 | <0.0001 | <0.0001 | 0.0386 | 0.0641 | 0.2596 |
Table 2: The effects of the grafting process, rootstock genotype and scion genotype on the survival, vegetative growth and yield of four pepper cultivars when grafted as scions onto five rootstocks, self-grafted, or as a non-grafted control grown under high tunnel conditions during Season 1. zParameters measured 70 DAT. yParameters measured cumulatively from July 2013 to May 2014. xGrafting effects evaluated by contrasting the survival, horticultural performance and yield of self-grafted scion cultivars with that of their non-grafted genotypic counterparts. wRootstockBreeding genotype effects on survival, horticultural performance and yield evaluated by contrasting rootstock means across scion cultivars with each other and with non-grafted control plants. vMain effect means with similar postscripts were not significantly different (p <0.05) according to Fisher’s least significant difference test (SAS version 9.4, SAS Institute, Cary, NC). uScion effects on survival, horticultural performance and yield were determined by contrasting scion means across all rootstocks, including self-grafted and non-grafted controls.
| Effects | Survival (%) | Vegetative growthz | Yieldy | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Plant height (cm) | No. of branches | No. of leaves | Leaf area (cm2) | Chlorophyll content | Fruit weight (g) | No. fruits per plant | Yield (kg) | ||
| Graftingx and rootstock genotypew | |||||||||
| Non-grafted | 100.0 av | 107.6 b | 26.7 b | 109.3 e | 324.3 e | 54.5 d | 134.8 bc | 51.1 b | 11.2 abcd |
| Self-grafted | 75.1 c | 110.9 b | 22.6 c | 123.9 d | 346.5 d | 57.7 b | 138.0 ab | 64.1 a | 12.2 ab |
| CCA-4758 | 68.3 e | 112.9 b | 19.8 d | 128.0 cd | 341.3 d | 55.5 cd | 128.9 c | 55.6 ab | 10.7 bcd |
| Budai csipős | 56.3 f | 113.2 b | 27.7 ab | 131.7 c | 361.6 c | 56.9 bc | 132.5 bc | 48.2 b | 10.3 cd |
| Nourdine | 75.4 c | 113.3 b | 25.7 b | 140.4 b | 387.0 b | 56.2 bcd | 138.5 ab | 47.8 b | 9.8 d |
| 52-03 RZ | 78.8 b | 124.3 a | 29.2 a | 159.5 a | 433.3 a | 58.2 b | 146.5 a | 52.9 b | 12.7 a |
| Tan Tan (No:12G076) | 71.3 d | 126.3 a | 25.8 b | 158.3 a | 438.5 a | 61.5 a | 140.8 ab | 52.5 b | 11.7 abc |
| Scion genotypeu | |||||||||
| Toronto | 78.0 b | 90.0 d | 22.6 c | 131.3 c | 436.2 a | 55.8 b | 184.3 b | 24.4 c | 8.9 b |
| Zedinca | 80.0 a | 97.2 c | 22.0 c | 131.6 c | 391.0 b | 58.8 a | 197.7 a | 24.2 c | 9.5 b |
| Kurtovszka Kàpia | 74.0 c | 140.7 a | 29.9 a | 142.5 a | 355.3 c | 58.7 a | 93.9 c | 68.1 b | 12.7 a |
| Eigman | 68.1 d | 134.1 b | 26.8 b | 138.3 b | 321.9 c | 55.4 b | 72.7 c | 96.1 a | 13.8 a |
| Interactive effects | |||||||||
| Scion*grafting (Sc*Gr) | <0.0001 | 0.3652 | 0.0006 | <0.0001 | <0.0001 | 0.0009 | 0.0331 | <0.0001 | 0.0444 |
| Scion*rootstock (Sc*Rs) | <0.0001 | 0.5703 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Table 3: The effects of the grafting process, rootstock genotype and scion genotype on the survival, vegetative growth and yield of four pepper cultivars when grafted as scions onto five rootstocks, self-grafted, or as a non-grafted control grown under high tunnel conditions during Season 2. zParameters measured 70 DAT. yParameters measured cumulatively from July 2014 to May 2015. xGrafting effects evaluated by contrasting the survival, horticultural performance and yield of self-grafted scion cultivars with that of their non-grafted genotypic counterparts. wRootstock genotype effects on survival, horticultural performance and yield evaluated by contrasting rootstock means across scion cultivars with each other and with non-grafted control plants. vMain effect means with different postscripts were not significantly different (p<0.05) according to Fisher's least significant difference test (SAS version 9.4, SAS Institute, Cary, NC). uScion effects on survival, horticultural performance and yield were determined by contrasting scion means across all rootstocks, including self-grafted and non-grafted controls.
Rather, plants are known to generate ethylene in response to both wounding and salt stress which, in turn, leads to increased oxidative stress and eventually to the activation of inherent plant defense mechanisms that alleviate it. The possible association of self-graft performance superiority with up-regulated plant antioxidant defense mechanisms was supported by melon data showing decreased levels of hydrogen peroxide (a reactive oxygen species) and of malonyl dialdehyde (a marker for membrane fatty acid oxidation) found in selfgrafted plants of either the control or salt-stress treatments. According to Aloni et al. [22] the phenomenon warrants further investigation.
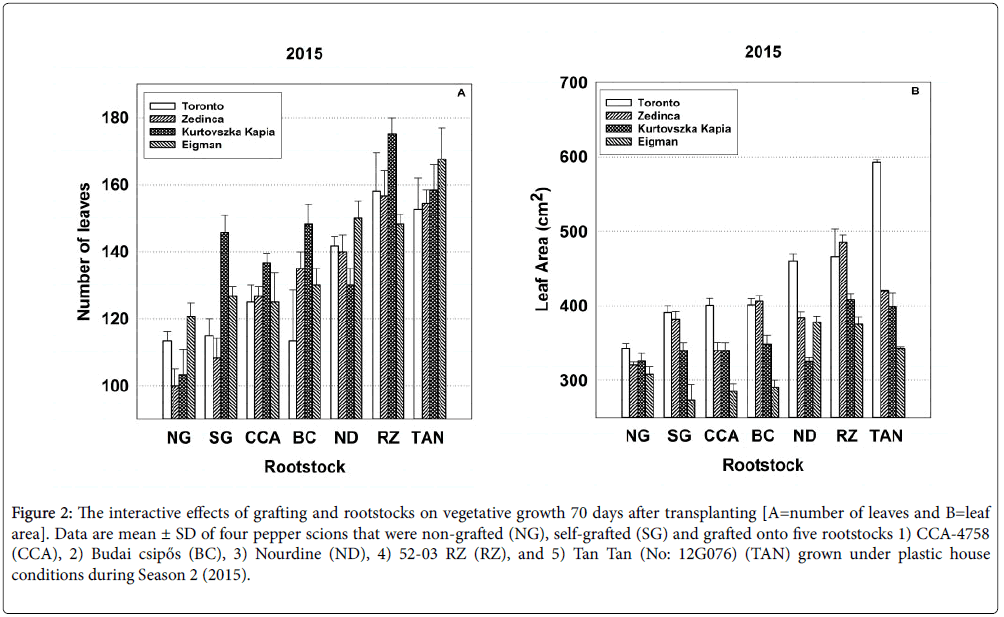
The grafting process affected scion cultivars differently with respect to leaf area per cm2 at 70 DAT and chlorophyll content at 60 DAT in both years, to the number of leaves present at the 70 day sampling period and to chlorophyll content in 2015. Patterns in the significant Sc*Gr interactions are exemplified in Figure 2 displaying the Sc*Gr for number of leaves and leaf area per cm2 for the 70 day sampling period in 2015, respectively (additional Sc*Gr interactions are depicted in Figures S1 through S4 in Supplementary Information). The significant interaction regarding number of leaves (Figure 2) likely results from differences in the behavior of the self-grafted (see the SG bar set) ‘Kurtovszka Kàpia’ in comparison to its non-grafted counterpart (see the NG bar set) whereas the significant interaction regarding leaf area.
Figure 2: The interactive effects of grafting and rootstocks on vegetative growth 70 days after transplanting [A=number of leaves and B=leaf area]. Data are mean ± SD of four pepper scions that were non-grafted (NG), self-grafted (SG) and grafted onto five rootstocks 1) CCA-4758 (CCA), 2) Budai csipős (BC), 3) Nourdine (ND), 4) 52-03 RZ (RZ), and 5) Tan Tan (No: 12G076) (TAN) grown under plastic house conditions during Season 2 (2015).
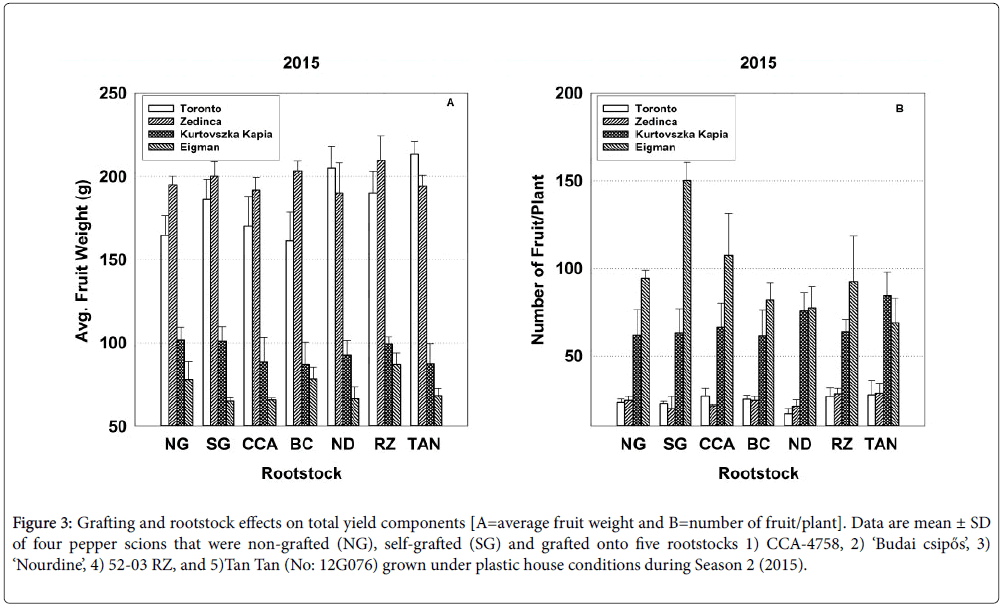
In 2014, grafting did not significantly affect total (seasonal) yield parameters (Table 2), whereas in 2015, self-grafted cultivars produced a statistically significant 25% increase in the number of fruit per plant over their non-grafted counterparts (Table 3). The behavior of ‘Eigman’ likely accounts for much of the increase in this parameter; during Season 2, self-grafted ‘Eigman’ produced smaller fruit than its nongrafted counterpart, but approximately 60% more of them (Figure 3; see bar sets for NG and SG).
Figure 3: Grafting and rootstock effects on total yield components [A=average fruit weight and B=number of fruit/plant]. Data are mean ± SD of four pepper scions that were non-grafted (NG), selfgrafted (SG) and grafted onto five rootstocks 1) CCA-4758, 2) ‘Budai csipős’, 3) ‘Nourdine’, 4) 52-03 RZ, and 5)Tan Tan (No: 12G076) grown under plastic house conditions during Season 2 (2015).
Other significant Sc*Gr interactions either for total yield parameters or those depicting early yield behavior are depicted in Figures S5 through S7).
Scion and rootstock effects
The growth and development, fruiting behaviour and yield of a grafted plant is inherently a function of scion genotype, but rootstock influences may substantially alter these characteristics by controlling the uptake and translocation of water and minerals by fostering the synthesis and translocation of plant hormones, enzymes mRNA and secondary products, and by affecting the availability of these and other substances for photosynthesis and other important metabolic processes [23,24]. The interaction of high-yielding scion genotypes with carefully-selected, complementary rootstocks may improve plant vigor and overall crop performance as well as enhance fruiting behavior and quality, especially when the crop is cultured under sub-optimal environmental conditions [8]. Superior rootstock-scion combinations have also been shown to activate inherent antioxidant defense enzymes, lower levels of lipid peroxidation and up- regulate stress response genes in grafted plants grown under drought stress conditions [25].
The survival of newly grafted plants may also be influenced by scion and rootstock genotypes, but according to Johkan et al. [26], information specific to grafted peppers is quite rare. The Johkan research group found graft success to be associated with rootstock and scion stem diameter parity and the age of seedlings undergoing grafting, but also with factors that may be associated with genotype such as differential callus formation and vascular bundle differentiation as affected by levels of oxidative stress and differential defoliation as a function of hydraulic conductance and stomatal activity [26,27].
In our study, survival rate differences among scions (main effects) were significant. Across all seven root treatments, the blocky scion cultivars ‘Toronto’ and ‘Zedinca’ had higher survival rates in both years than did the long-fruited cultivars ‘Eigman’ and ‘Kurtovszka Kàpia’ (Tables 2 and 3) with significance patterns clearest in Season 2. Although survival rates for blocky-fruited scion cultivars exceeded those of long-fruited cultivars, the height of long-fruited cultivars at 70 DAT was consistently and significantly higher than that of their blocky counterparts in both years, suggesting a strong genotypic component to this trait (Tables 2 and 3). In 2015, long-fruited cultivars also had a greater number of branches and leaves. In contrast, leaf areas per cm2 were significantly and consistently lower for long-fruited cultivars than for blocky-fruited cultivars; the combined leaf data suggests that the shorter, blocky-fruited cultivars had fewer but larger leaves and the taller, long-fruited cultivars had many leaves, but smaller ones. Scion genotype also significantly impacted all seasonal yield parameters in both years of the study (Tables 2 and 3). ‘Eigman’ and ‘Kurtovszka Kàpia’, the two long-fruited cultivars performed similarly producing the highest number of fruits per plant and the highest fruit yields per m2 whereas their blocky-fruited counterparts ‘Toronto’ and ‘Zedinca’ produced fruit that was twice as heavy but only about one-fourth as numerous per plant. Blocky cultivars inherently produce larger fruit with high sink capacities that limit additional flowering and fruit set and may reduce the number of fruits that can be matured on a single plant [28].
The main effects associated with root treatments were also significant in our study (Tables 2 and 3). Across all four scions, use of the 52-03 RZ commercial rootstock resulted in greater grafted seedling survival percentages in both years than those resulting from self-grafts. However, grafts made with Tan Tan (No:12G076) commercial rootstock had a lower seedling survival rate than their counterpart and performed similarly to Nourdine (a scion cultivar used as a rootstock). Overall, when scions were grafted to the cultivar Budai csipős, significantly greater seedling losses were experienced during the healing and hardening-off processes than with any other root treatment. Root treatments also strongly and significantly influenced all vegetative growth parameters measured at 70 DAT in both years (Tables 2 and 3). Scions grafted to commercial rootstocks Tan Tan (No: 12G076) and 52-03 RZ generally outperformed all others in both years. In 2014, non-grafted control plants were often the least vigorous with respect to all growth parameters. In 2015, patterns of significance among root treatments were less distinct. When used as rootstocks, CCA-4758 (a breeding line) and ‘Budai csipős’ had variable effects on scion growth parameters compared to the performance of self-grafted or non-grafted controls. There were also significant differences in seasonal yield and yield components among the five rootstocks in the study when compared to their non- grafted control plants (Tables 2 and 3) but definitive patterns of superiority were not evident across both seasons. In Season 1, CCA-4758 generally underperformed as a rootstock whereas grafting onto either 52-03 RZ or Tan Tan (No: 12G076) rootstocks improved scion yield over those of the non-grafted controls representing the only yield advantage associated with grafted plants in this study.
Scion-rootstock interactions
The survival of newly grafted transplant stock is predicated on the specific compatibility of the rootstock and scion combination as it affects levels of oxidative stress, cohesion, callus formation, vascular differentiation and connectivity, hydraulic conduction, nutrient uptake, stomatal behavior, and scion leaf retention [20-22,26,27]. Likewise, superior rootstock-scion combinations can enhance vegetative growth, increase yield and improve fruit quality in grafted plants under production in greenhouse, high-tunnel or field environments through increased tolerance to biotic and abiotic stresses [3,7-10,13,29-31] improved nutrient and water uptake [4,6,23,24] enhanced photosynthetic capacity, metabolic activity and transport [5,8,23,24] greater leaf area [30] efficient remediation of oxidative stress [25] season extension [11] increases in the number and weight of marketable fruit [29,30,32-34], and improvements in the dry matter, protein and mineral content of fruits [34,35]. Grafted plants composed of inappropriately paired rootstocks and scions lack these advantages and some studies have reported neutral or detrimental effects of specific graft combinations on vegetative growth [29-31], and yield [33].
Significance patterns among scion and rootstock means for percentage survival were distinct and consistent between seasons (Tables 2 and 3). However, the efficacy of specific scion-rootstock combinations to produce viable plants was highly variable within and among years (Figure 1). The significant Sc*Rs interaction within Season 1 might best be exemplified by the consistent behavior of the four scions on the CCA-4758 and 52-03 RZ rootstocks (Figure 1; CCA and RZ bar sets) as opposed to that of the less predictable survival of scions grafted to ‘Budai csipős’ and ‘Nourdine’ (Figure 1; BC and ND bar sets). Survival in Season 2 was characterized by a general lack of consistency for specific scions across rootstocks (Figure 1). For example, ‘Toronto’ exhibited a nearly 95% survival rate when grafted to ‘Nourdine’, but when grafted to ‘Budai csipős’, fewer than half of the grafted plants survived. The success of specific graft combinations also fluctuated between seasons as can exemplified by the inconsistent behavior of the four scions grafted to ‘Budai csipős’ and ‘Nourdine’ (Figure 1; BC and ND bar sets).
These significant Sc*Rs interactions are a reminder that the survival rate of grafted pepper seedlings may be influenced as much by the environment and external factors associated with the grafting process as with the genetic characteristics of the germplasm used.
Significant rootstock-scion interactions (Sc*Rs) were obtained for all growth parameters except plant height in both the 2014 and 2015 seasons (Tables 2 and 3). Again the performance of specific rootstockscion combinations was highly variable. Figure 2 depicting significant Sc*Rs interactions for number of leaves and leaf area at 70 DAT in Season 2 illustrates the types of inconsistent patterns observed for other parameters in all sampling periods (see Supplementary Figures S1 through S4). ‘Kurtovszka Kàpia’ produced a substantially greater number of leaves than other scions when self-grafted or grafted to CCA-4758 ‘Budai csipős’ and 52-03 RZ, but relatively fewer leaves when grafted to ‘Nourdine’ or Tan Tan (No: 12G076) (Figure 2, all bar sets). In 2015, Toronto produced an extensive leaf area in all graft combinations (Figure 2 all bar sets), especially when grafted to Tan Tan (No: 12G076). The leaf area associated with ‘Zedinca’, its blocky counterpart was similar to that of ‘Toronto’ when self-grafted or grafted to ‘Budai csipős’ and 52-03 RZ, significantly reduced when grafted to CCA-4758, ‘Budai csipős’ and Tan Tan (No: 12G076).
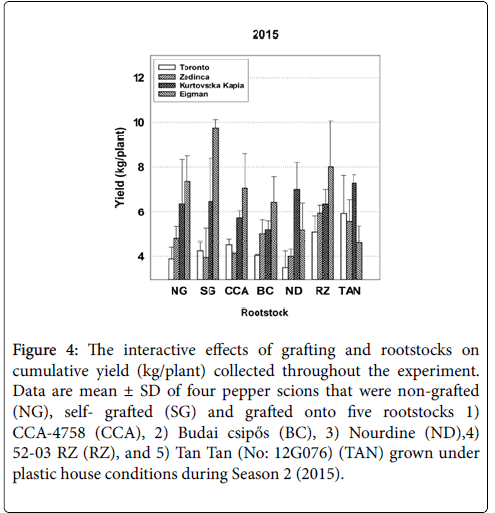
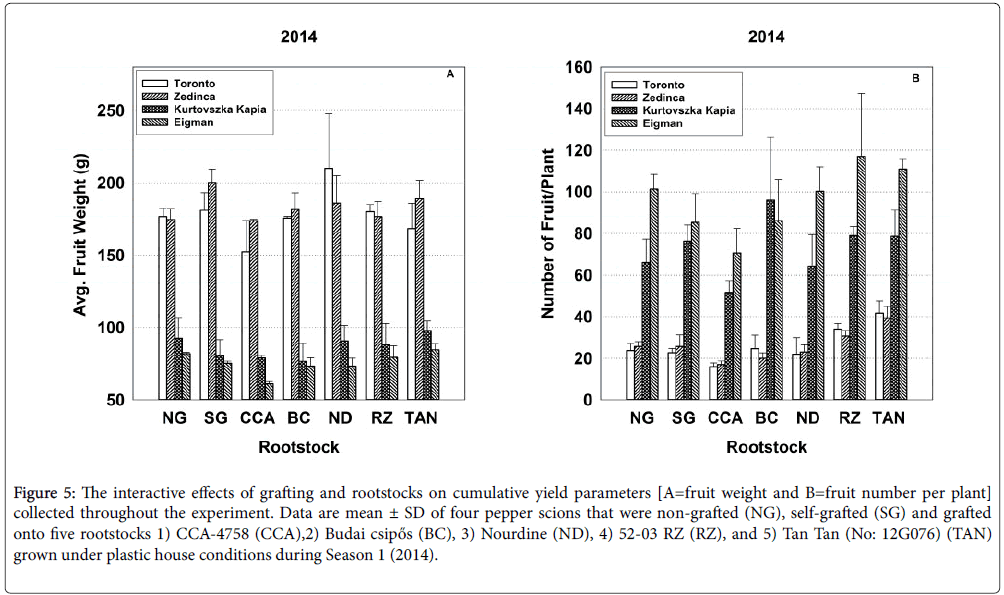
In 2015, strong interactive effects of rootstock-scion combinations (Sc*Rs) were evident for all seasonal yield parameters (Table 3). For example, with respect to average fruit weight over the entire season, ‘Zedinca’ a blocky-fruited cultivar, performed well when grafted to all rootstocks whereas its counterpart ‘Toronto’ outperformed ‘Zedinca’ when grafted to Tan Tan (No: 12G076) but underperformed its partner only when grafted to ‘Budai csipős’ (Figure 3, all bar sets). Among long-fruited scions, fruit weights were higher in ‘Kurtovszka Kàpia’ than those of ‘Eigman’, but the disparity in fruit weights was highly dependent on the rootstock to which they were grafted. Regardless of root treatment, the number of fruit produced on the blocky-fruited cultivars was similar (Figure 3, all bar sets). The behavior of longfruited cultivars was more highly dependent on root treatment with ‘Eigman’ significantly out-performing ‘Kurtovszka Kapia’ when selfgrafted or grafted to either CCA-4758 or 52-03 RZ. The Sc*Rs pattern variability associated with fruit numbers of the two blocky-fruited cultivars and those found for the two long-fruited cultivars was reflected in the Sc*Rs patterns displayed for cumulative yield per plant (Figure 4).
Figure 4: The interactive effects of grafting and rootstocks on cumulative yield (kg/plant) collected throughout the experiment. Data are mean ± SD of four pepper scions that were non-grafted (NG), self- grafted (SG) and grafted onto five rootstocks 1) CCA-4758 (CCA), 2) Budai csipős (BC), 3) Nourdine (ND),4) 52-03 RZ (RZ), and 5) Tan Tan (No: 12G076) (TAN) grown under plastic house conditions during Season 2 (2015).
In our study, scion genotype strongly influenced several important performance factors of grafted pepper transplants grown under high tunnel conditions, but significant rootstock effects for survival, growth and yield were also evident denoting that root genotypes differ in their ability to form effective graft unions and/or to supply water, nutrients and other needed metabolites to the developing aerial tissues. Moreover, significant Sc*Rs interactions indicated that, in many cases, the effects of scions and rootstocks were not additive. Rather, the level of synergy achieved in optimizing grafted plant production factors was combination-specific, pointing to the need to test new combinations for compatibility and performance in order to speed the adoption of superior combinations. Furthermore, inconsistencies in Sc*Rs interaction patterns between years (e.g., Figures 1, 3 and 5, see other examples in Supplementary Information) demonstrate that environmental factors can alter combination-specific responses.
Figure 5: The interactive effects of grafting and rootstocks on cumulative yield parameters [A=fruit weight and B=fruit number per plant] collected throughout the experiment. Data are mean ± SD of four pepper scions that were non-grafted (NG), self-grafted (SG) and grafted onto five rootstocks 1) CCA-4758 (CCA),2) Budai csipős (BC), 3) Nourdine (ND), 4) 52-03 RZ (RZ), and 5) Tan Tan (No: 12G076) (TAN) grown under plastic house conditions during Season 1 (2014).
Season differences regarding grafted plant survival, vegetative vigor and yield parameters
Years had a noticeable effect on plant performance patterns in this study (Tables 2 and 3). For instance, survival rate of self-grafts was reduced by nearly 10% over their non-grafted controls and mean survival rates of scions grafted to CCA-4758 and 52-03 RZ both diminished by approximately 17% in Season 2. Mean scion survival was also curtailed by as much as 10% in 2015.
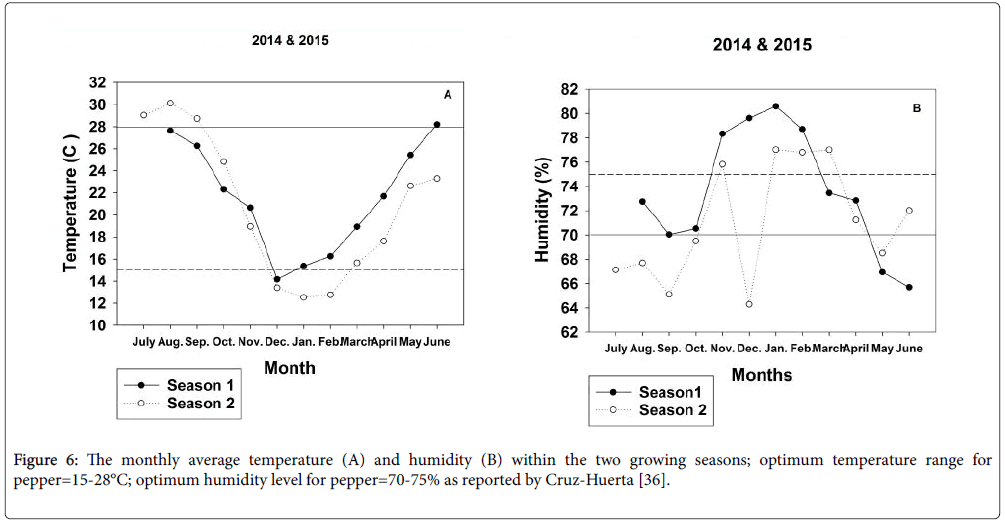
Survival rate of grafted vegetable plants is known to be affected not only by the genotype of the rootstock and scion, by the grafting technique but also by conditions within the healing and acclimatization environment. Ultimately, survival percentages hinge upon how effectively vital compounds can move from the root system to the shoot system to achieve balanced development within the growing plant [20,26,27]. Since seedling growth, the grafting process and the healing and hardening-off periods were conducted in a high tunnel lacking climatic controls, differences in ambient conditions among years may account for a portion of the variable behaviour of grafted plant survival especially during the hardening-off process when newly grafted planting stock was removed from the healing tents. Cruz-Huerta [36] reported ideal growing conditions for pepper to range from 15-28°C and from 70-75% relative humidity (RH). In the early months of 2015, temperatures in the high tunnel exceeded the ideal range and RH was dryer than optimal (Figure 6).
Rootstock-scion combinations as well as self-grafted and nongrafted controls varied substantively in performance for survival rate and measures of vegetative vigor between Season 1 and Season 2. As with the hardening-off process, ambient conditions within the high tunnel environment during the 30-70 day period when measurements were taken most closely matched ideal conditions for pepper production during 2014 (Figure 6). Under the more ideal conditions, plants generally grew taller and were substantially more heavily branched. In addition, leaf areas per cm2 were more than double those of their counterparts grown under stressful conditions. Also, the stressful environment in the latter year of study tended to reduce differences in plant vigor among root treatments but delineate differences among cultivar types more effectively in comparison with the data recorded in Season 1 (Tables 2 and 3).
Figure 6: The monthly average temperature (A) and humidity (B) within the two growing seasons; optimum temperature range for pepper=15-28°C; optimum humidity level for pepper=70-75% as reported by Cruz-Huerta [35].
With respect to yield, stress conditions early in the cropping cycle or in mid-winter months did not appear to influence yield m2 to any great extent; on average differences in early and total yield were reduced only 5.7% and 2.1% respectively from Season 1 to Season 2. However, based on average performance across scions, individual rootstocks varied widely in their response to stress with the early and total yield per m2 of the commercial rootstock Tan Tan (No. 12G076) being reduced by 30.8% and 34.3%, respectively and the under-performing breeding line CCA-4758 actually increasing yield per m2 during the same periods by a similar amount (30.0% and 35.9%, respectively). Nevertheless, even with its substantial yield increase in Season 2, the average fruit yield m2 on plants grafted to CCA-4758 was lowest among all grafted plants.
Phenotypic stability of potential rootstocks and scions when used in graft combinations
Whether inherent scion and rootstock phenotypic characteristics are stable when used in grafts will be an important concern as commercial rootstocks are developed or as new graft combinations are considered. For this study, we purposefully chose germplasm with a diverse set of characteristics (Table 1) in part, to test whether or not the phenotype of the rootstock or scion cultivar would be predictive of performance when used in graft combinations. Performance patterns were most evident among scions that differed in fruit shape; blockyfruited cultivars exhibited higher survival rates, developed shorter plants with fewer branches, larger but fewer leaves, and produced fewer but larger fruit than their long-fruited counterparts. It is possible, but unlikely, that genes controlling graft survival, growth and productivity are closely associated with those that control fruit shape. It is more plausible that performance differences between the two groups result from inherent differences in plant vigor of the scion cultivars. Similarly, scions grafted to the compact cultivar ‘Budai csipős’ used as a rootstock survived the grafting process poorly and performed only adequately with respect to growth or yield, whereas scions grafted to the vegetatively-vigorous rootstock 52-03 RZ had the greatest seedling survival rate, developed plants that were among the tallest most heavily branched with the most extensive leaf areas per cm2 and among those that produced the highest yield per m2 in both stress and non-stress environments. However, a portion of the superiority of 5-03 RZ likely results from the concentration of genes conferring grafting superiority during the commercial rootstock breeding and selection process. Grafted plants using the commercial rootstock Tan Tan (No. 12G076) exhibited similar superior characteristics with respect to vegetative growth and yield even though the Tan Tan (No. 12G076) plant itself is only moderately vigorous.
Finally, the superior vitality of the breeding line CCA-4758 failed to consistently enhance growth or yield capacity in grafted plants using it as a rootstock. Admittedly, the germplasm pool used in our study (i.e., 5 rootstocks and 4 scions) is very limited; the horticultural performance pattern differences associated with blocky-fruited vs. long-fruited cultivars or between commercial rootstocks vs. scion cultivars or breeding lines used as rootstocks may not endure as additional germplasm is tested. In summary, advanced pepper germplasm undergoing rootstock development or cultivars with superior horticultural traits will likely contain genetically stable traits that will be evident across a number of graft combinations, but our data is replete with Sc*Rs interactions and environmental influences suggesting that each combination may exhibit unique performance characteristics that will influence its eventual commerciality.
Performance factor interrelationships
In this study, most vegetative growth parameters (except leaf chlorophyll content) were measured at 30, 50 and 70 DAT and yield parameters were measured cumulatively for the first 45 days of harvest and over the entire season. Scion and root treatment means and the significance of Sc*Gr and Sc*Rs interactions not depicted in the text can be found in Supplementary Information Tables S1 through S6.
Across root treatment means, correlation coefficients between survival percentages and growth and yield parameters regardless of when they were measured were weak (ranging from r=-0.362 to r=0.267). The lack of strong relationships among grafted seedling survival percentage and later horticultural performance suggests that surviving plants had developed strong and well-functioning graft unions.
Stronger relationships were uncovered between vegetative growth parameters and early or total yield per m2. The growth/yield performance superiority of scions grafted to the commercial rootstocks or to rootstocks bearing long-fruited cultivars as scions has been discussed above. Relationships between root treatment mean arrays for individual vegetative growth parameters (e.g., plant height at 50 DAT) and early or total yield m2 were highly variable; correlation coefficients (r) ranged from 0.125 to 0.708 during Season 1 and from 0.017 to 0.717 during Season 2. During Season 1, moderate relationships were more consistently uncovered between plant height and yield (r=0.561 to r=0.708), between number of leaves and yield (r=0.518 to r=0.660), and between SPAD readings and yield (r=0.680 to r=0.706).
Relationships between growth characteristics and yield were generally weaker and less consistent during the Season 2, with the strongest being between early and total yield vs plant height at 50 DAT (r=0.608 and r=0.717, respectively). In Season 2, plants experienced temperatures above and relative humidity levels below optimal levels for pepper growth during the early portion of the season when growth parameter measurements were taken (Figure 6) curtailing the development of both root and shoot systems.
However, plants may have been able to compensate for this early abiotic stress as environmental conditions improved prior to the initiation of fruit harvest.
Relationships among repeated measures for both vegetative vigor and yield parameters are also of interest from an experimental standpoint. For example, values for fruit weight per m2 after 45 days of harvest were highly predictive of seasonal yields. Correlations between root treatment means for early yield m2 and total yield m2 were strong (r=0.994 and r=0.983 for Seasons 1 and 2, respectively), suggesting that in future studies, representative yield data for prolonged production could be amassed using a shorter experimental time frame. During Season 1, values recorded at 30, 50 and 70 DAT for plant height and number of leaves (parameters that most closely related with yield per m2) were consistent among the sampling periods with correlation coefficients (r) ranging from 0.914 to 0.985 and from 0.891 to 0988, respectively. In Season 2, similarities among sampling periods were also strongest for plant height and number of leaves, but the relationships were not as robust as in the previous season (e.g., correlation coefficients for values at 30 DAT vs. those at 70 DAT were r=0.763 and r=0.553, respectively). In general, our data suggests that fewer growth measurements collected at fewer points in the cropping cycle may be necessary to capture horticultural performance that influences grafted pepper yield potential, especially in non-stressful growing environments.
Conclusion
The use of grafted vegetable plants has been shown to increase the production of semi-protected systems [18] and to be economically viable for growers in developed countries, even considering the added cost of grafted seedlings [37]. Still, a greater selection of rootstocks adapted to a variety of environmental conditions and that specifically complement the array of desirable commercial pepper cultivars is needed to facilitate the wider and more effective use of grafted pepper plants. Moreover, because documentation is currently inadequate, grafted plant propagators and growers will need additional information about how the horticultural performance of specific rootstock scion pairs may vary when cultured using their production systems and in their environment. Herein, we demonstrated that graft combinations with the two commercial rootstocks routinely surpassed those of other rootstocks or control plants in growth rate and fruiting capacity in both stressful and non-stressful high tunnel environments, confirming the inherent advantages of stocks that have been selected specifically for root system superiority. However, significant Sc*Rs interactions for survival, growth and yield, even among scions grafted to commercial rootstocks attest to the need for thoroughly testing new rootstocks as they are being developed under a variety of environmental circumstances in order to ensure the commercial success of combinations for both propagators and producers.
References
- Radhouani A, El Bekkay M, Ferchichi A (2008) The actual situation of the geothermic sector in the South of Tunisia. In International Symposium of Biotechnology. Sfax-Tunisia.
- Louws FJ, Rivard CL, Kubota C (2010) Grafting fruiting vegetables to manage soilborne pathogens, foliar pathogens, arthropods and weeds. Sci Hort 127: 127-146.
- Venema JH, Dijk BE, Bax JM, Van Hasselt PR, Elzenga JTM (2008) Grafting tomato (Solanum lycopersicum) onto the rootstock of a high altitude accession of Solanum habrochaites improves suboptimal-temperature tolerance. Environ Exp Bot 63: 359-367.
- Colla G, Suárez CMC, Cardarelli M, Rouphael Y (2010) Improving nitrogen use efficiency in melon by grafting. HortSci 45: 559-565.
- Dong H, Niu Y, Li W, Zhang D (2008) Effects of cotton rootstock on endogenous cytokinins and abscisic acid in xylem sap and leaves in relation to leaf senescence. J Exp Bot 59: 1295-1304.
- Rouphael Y, Cardarelli M, Colla G, Rea E (2008) Yield, mineral composition, water relations, and water use efficiency of grafted mini-watermelon plants under deficit irrigation. Hort Sci 43: 730-736.
- Colla G, Rouphael Y, Cardarelli M, Salerno A, Rea E (2010) The effectiveness of grafting to improve alkalinity tolerance in watermelon. Environ Exp Bo 68: 283-291.
- Schwarz D, Rouphael Y, Colla G, Venema JH (2010) Grafting as a tool to improve tolerance of vegetables to abiotic stresses: thermal stress, water stress and organic pollutants. Sci Hort 127: 162-171.
- Otani T, Seike N (2007) Rootstock control of fruit dieldrin concentration in grafted cucumber (Cucumis sativus). J Pest Sci 32: 235-242.
- Savvas D, Papastavrou D, Ntatsi G, Ropokis A, Hartmann H, et al. (2009) Interactive effects of grafting and manganese supply on growth, yield and nutrient uptake by tomato. Hort Sci 44: 1978-1982.
- Lee JM, Kubota C, Tsao SJ, Bie Z, Echevarria PH, et al. (2010) Current status of vegetable grafting: Diffusion, grafting techniques, automation. Sci Hort 127: 93-105.
- Venema JH, Elzenga JTM, Bouwmeester HJ (2011) Selection and breeding of robust rootstocks as a tool to improve nutrient-use efficiency and abiotic stress tolerance in tomato. Acta Hort 915: 109-115.
- Doñas Uclés F, Jiménez Luna MDM, Góngora Corral JA, Pérez Madrid D, Verde Fernández D, et al. (2014) Influence of three rootstocks on yield and commercial quality of ‘Italian sweet’ pepper. Ciênc Agrotec Lavras 38: 538-545.
- Albacete A, MartÃnez Andújar C, MartÃnez Pérez A, Thompson AJ, Dodd IC, et al. (2015) Unraveling rootstock X scion interactions to improve food security. J Exp Bot 66: 2211-2226.
- Davis AR, Perkins Veazie P, Hassell R, Levi A, King SR, et al. (2008) Grafting effects on vegetable quality. Hort Sci 43: 1670-1672.
- Rivero RM, Ruiz JM, Romero L (2003) Role of grafting in horticultural plants under stress conditions. J Food Agri Environ 1: 70-74.
- Kleinhenz MD, Francis DM, Young M, Aldrich T (2009) Rootstock effects on yield of grafted ‘Celebrity’ tomato in Ohio. The Ohio State University, Wooster, Ohio.
- Keatinge JDH, Lin LJ, Ebert AW, Chen WY, Hughes JD, et al. (2014) Overcoming biotic and  abiotic stresses in the Solanaceae through grafting: Current status and future perspectives. Biol Agric Hort 30: 272-287.
- Bumgarner NR, Kleinhenz MD (2016) Grafting Guide: Apictorial guide to the cleft and splice graft methods for tomatoes and pepper. Ohio State University Extension, Bulletin 950, USA.
- Ogata T, Kabashima Y, Shiozaki S, Horiuchi S (2005) Regeneration of the vascular bundles at the graft interface in auto- and heterografts with juvenile nucellar seedlings of satsuma mandarin, yuzu and trifoliate orange. J Jpn Soc Hort Sci 74: 214-220.
- Oda M, Maruyama M, Mori G (2005) Water transfer at graft union of tomato plants grafted onto Solanum rootstocks. J Jpn Soc Hort Sci 74: 458-463.
- Aloni B, Karni L, Deventurero G, Cohen R, Katzir N, et al. (2011) The use of plant grafting and plant growth regulators for enhancing abiotic stress tolerance in vegetable transplants. Acta Hort 898: 255-263.
- Flores FB, Sanches Bel P, Estan MT, Martinez Rodriguez MM, Moyano E, et al. (2010) The effectiveness of grafting to improve tomato fruit quality. Sci Hort 125: 211-217.
- Lee JM, Oda M (2010) Grafting of herbaceous vegetable and ornamental crops. Hort Rev 28: 61-124.
- Liu J, Li J, Su X, Xia Z (2014) Grafting improves drought tolerance by regulating antioxidant enzyme activities and stress-responsive gene expression in tobacco. Env Exp Bot 107: 173-179.
- Johkan M, Oda M, Mori G (2008) Ascorbic acid promotes graft-take in sweet pepper plants (Capsicum annuum L.). Sci Hort 116: 343-347.
- Johkan M, Mitukuri K, Yamasaki S, Mori G, Oda M (2009) Causes of defoliation and low survival rate of grafted sweet pepper plants. Sci Hort 119: 103-107.
- Wubs AM, Ma Y, Hemerik L, Heuvelink E (2009) Fruit set and yield patterns in six Capsicum cultivars. Hort Sci 44: 1296-1301.
- Del Amor FM, Lopez Marin J, Gonzalez A (2008) Effect of photo selective sheet and grafting technique on growth, yield, and mineral composition of sweet pepper plants. J Plant Nutr 31: 1108-1120.
- López MarÃn J, González A, Pérez Alfocea F, Egea Gilabert C, Fernández JA (2013) Grafting is an efficient alternative to shading screens to alleviate thermal stress in greenhouse-grown sweet pepper. Sci Hort 149: 39-46.
- Kokalis Burelle N, Bausher MG, Rosskopf EN (2009) Greenhouse evaluation of Capsicum rootstocks for management of Meloidogyne incognita on grafted bell pepper. Nematropica 39: 121.
- Colla G, Rouphael Y, Cardarelli M, Temperini O, Rea E, et al. (2006) Influence of grafting on yield and fruit quality of pepper (Capsicum annuum L.) grown under greenhouse conditions. Acta Hort 782: 359-364.
- Doñas Uclés F, Pérez Madrid D, Amate Llobregat C, RodrÃguez GarcÃa EM, Camacho Ferre F (2015) Production of pepper cultivar ‘Palermo’ grafted onto Serrano de Morelos 2, Jalapeno, and three commercial rootstocks. Hort Sci 50: 1018-1022.
- Jang Y, Moon JH, Lee JW, Lee SG, Kim SY, et al. (2013) Effects of different rootstocks on fruit quality of grafted pepper (Capsicum annuum L.). Korean J Hort Sci Tech 31: 687-699.
- Sánchez Torres P, Raigón MD, Gammoudi N, Gisbert C (2016) Effects of grafting combinations on the nutritional composition of pepper fruit. Fruits 71: 249-256.
- Cruz Huerta N (2010) Temperature effects on ovary swelling in sweet pepper: Physiology and anatomy. PhD thesis, The University of Florida.
- Rysin O, Rivard C, Louws FJ (2015) Is vegetable grafting economically viable in the United States: Evidence from four different tomato production systems. Acta Hort 1086: 79-86.
Citation: Soltan MM, ElAidy FA, Scheerens JC, Kleinhenz MD (2017) Grafting, Scion and Rootstock Effects on Survival Rate, Vegetative Growth and Fruit Yield of High Tunnel-grown Grafted Pepper (Capsicum annuum L.) Plants. Adv Crop Sci Tech 5: 312. DOI: 10.4172/2329-8863.1000312
Copyright: © 2017 Soltan MM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 9882
- [From(publication date): 0-2017 - Dec 09, 2025]
- Breakdown by view type
- HTML page views: 8688
- PDF downloads: 1194