Case Report Open Access
Groove Pancreatitis: A Malignant Masquerade in the Duodenum
Karin Eshagh1, Chris M Reid1,2, Michael Chan1,3, Grace Y Lin1,4, Thomas Savides1,3 and Jason K Sicklick1,2*1University of California San Diego, School of Medicine, La Jolla, CA, USA
2Department of Surgery, Division of Surgical Oncology, Moores UCSD Cancer Center, University of California San Diego, La Jolla, CA, USA
3Department of Medicine, Division of Gastroenterology, University of California San Diego, La Jolla, CA, USA
4Department of Pathology, Division of Surgical Pathology, University of California San Diego, San Diego, CA, USA
- *Corresponding Author:
- Jason K. Sicklick
Division of Surgical Oncology
University of California, 3855 Health Sciences Drive
Room 2313, La Jolla, CA 92093-0987, USA
Tel: 858-822-3967
Fax: 858-228-5153
E-mail: jsicklick@ucsd.edu
Received date: June 27, 2014; Accepted date: September 18, 2014; Published date: September 25, 2014
Citation: Eshagh K, Reid C, Chan M, Lin G, Savides T, et al. (2014) Groove Pancreatitis: A Malignant Masquerade in the Duodenum. J Gastroint Dig Syst 4:217. doi:10.4172/2161-069X.1000217
Copyright: © 2014 Eshagh K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Groove pancreatitis is a rare form of chronic pancreatitis that has been described by several names including para-duodenal wall cyst, pancreatic hamartoma of the duodenum, cystic dystrophy of heterotopic pancreas, and myoadenomatosis. It is characterized microscopically by the presence of a pseudocyst arising from the “groove” between the duodenum, common bile duct, and pancreatic head. It is thought to be caused by functional and/or anatomical minor papilla obstruction from viscous pancreatic secretions. In turn, this leads to impaired pancreatic enzyme outflow, Brunner’s gland proliferation, and resultant pancreatitis. This often appears as a submucosal mass in the region. Thus, the differential diagnosis ranges from benign pathologies such as Brunner’s gland hyperplasia, leiomyoma, and schwannoma to malignant pathologies such as gastrointestinal stromal tumor and ampullary carcinoma. Herein, we present a case of a middle aged male smoker with a remote history of alcohol abuse that presented with one month of epigastric pain associated with bilious emesis and weight loss. Computed tomography (CT) and endoscopic ultrasound (EUS) revealed a submucosal cystic mass that was obstructing the second portion of the duodenum. Cytology demonstrated crowded groups of atypical epithelioid and spindle cells that were suspicious for a neoplasm. The patient subsequently underwent pancreaticoduodenectomy in order to relieve his duodenal obstruction, as well as to establish a definitive tissue diagnosis. Despite the use of multiple imaging modalities including CT and EUS with fine needle aspirates, the final diagnosis of groove pancreatitis was only made following resection. This case now demonstrate that groove pancreatic often mimics submucosal duodenal tumors and malignant periampullary cancers clinically, radiographically, endoscopically, and cytologically.
Abstract
Groove pancreatitis is a rare form of chronic pancreatitis that has been described by several names including para-duodenal wall cyst, pancreatic hamartoma of the duodenum, cystic dystrophy of heterotopic pancreas, and myoadenomatosis. It is characterized microscopically by the presence of a pseudocyst arising from the “groove” between the duodenum, common bile duct, and pancreatic head. It is thought to be caused by functional and/or anatomical minor papilla obstruction from viscous pancreatic secretions. In turn, this leads to impaired pancreatic enzyme outflow, Brunner’s gland proliferation, and resultant pancreatitis. This often appears as a submucosal mass in the region. Thus, the differential diagnosis ranges from benign pathologies such as Brunner’s gland hyperplasia, leiomyoma, and schwannoma to malignant pathologies such as gastrointestinal stromal tumor and ampullary carcinoma. Herein, we present a case of a middle aged male smoker with a remote history of alcohol abuse that presented with one month of epigastric pain associated with bilious emesis and weight loss. Computed tomography (CT) and endoscopic ultrasound (EUS) revealed a submucosal cystic mass that was obstructing the second portion of the duodenum. Cytology demonstrated crowded groups of atypical epithelioid and spindle cells that were suspicious for a neoplasm. The patient subsequently underwent pancreaticoduodenectomy in order to relieve his duodenal obstruction, as well as to establish a definitive tissue diagnosis. Despite the use of multiple imaging modalities including CT and EUS with fine needle aspirates, the final diagnosis of groove pancreatitis was only made following resection. This case now demonstrates that groove pancreatic often mimics submucosal duodenal tumors and malignant periampullary cancers clinically, radiographically, endoscopically, and cytologically.
Keywords
Groove pancreatitis; Cystic dystrophy of heterotopic pancreas; Pancreatic hamartoma of duodenum; Para-duodenal wall cyst; Myoadenomatosis; GIST; gastrointestinal stromal tumor; Brunner’s gland hyperplasia
Introduction
Groove pancreatitis (GP) is a rare form of chronic pancreatitis that has been described by several names including para-duodenal wall cyst, pancreatic hamartoma of the duodenum, cystic dystrophy of heterotopic pancreas, and myoadenomatosis. GP is most common in males aged 40-50-years old with a history of alcoholism and/or smoking that present with abdominal pain, vomiting, and weight loss. Imaging typically reveals duodenal stenosis and cystic lesion(s) near the head of the pancreas [1]. This form of focal, chronic pancreatitis affects the “groove” between the duodenum, common bile duct, and pancreatic head. It is thought to be caused by functional and/or anatomical minor papilla obstruction from viscous pancreatic secretions [2]. Most commonly, this occurs due to alcohol or smoking. In turn, there is impaired pancreatic enzyme outflow, Brunner’s gland proliferation, and resultant pancreatitis [3]. Heterotopic pancreas in the duodenum and peptic ulcer disease are also possible contributing factors. Unlike chronic pancreatitis, there is no known association between groove pancreatitis, autoimmune disease or gallstones [4].
The diagnostic imaging modalities of choice include CT and upper endoscopy with EUS. CT typically reveals a cystic duodenal wall and local inflammation. Endoscopic imaging may reveal inflamed duodenal mucosa with luminal stenosis. On EUS, cysts are found within the groove and duodenal wall [3,5]. Despite the aforementioned findings, making a tissue diagnosis is often difficult due to the heterogeneity of cells within the duodenal wall and para-duodenal cysts.
Materials and Methods
Report of a case
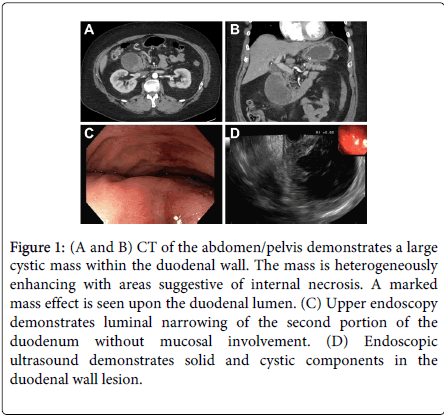
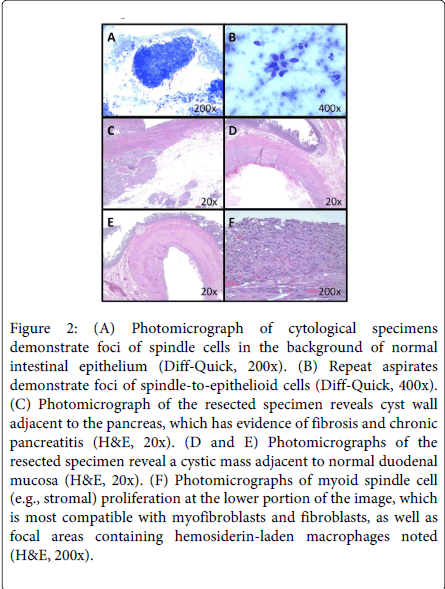
A 50-year old male smoker with schizoaffective disorder, morbid obesity, Type II diabetes mellitus, and a remote history of alcohol abuse presented with one month of epigastric pain associated with bilious emesis and weight loss. Upon workup he had a total bilirubin of 1.5 mg/dL. Computed tomography (CT) revealed a 7.3×5.9-cm cystic mass that was obstructing the second portion of the duodenum (Figure 1A and B). Upon upper endoscopy he was found to have a near obstructing, submucosal duodenal mass (Figure 1C). Endoscopic ultrasound (EUS) examination demonstrated that this lesion had solid and cystic components. Mucosal biopsies revealed benign duodenal mucosa while cytology from fine needle aspirates displayed foci of spindle cells in the background of intestinal epithelium (Figure 2A). Repeat fine needle aspiration showed foci of somewhat spindle-to-epithelioid cells (Figure 2B). Neuroendocrine-like cells were also present. However, these findings were not diagnostic for malignancy that may be amenable to preoperative chemotherapy. In order to relieve his biliary and duodenal obstructions, as well as to confirm the diagnosis, he subsequently underwent pancreaticoduodenectomy. Histopathological examination revealed a cystic mass adjacent to fibrotic, atrophic pancreas (Figure 2C) and duodenal mucosa (Figure 2D and E). The pseudocystic changes were seen within the duodenal wall (Figure 2D and E). Even after extensive sampling, no definitive epithelial lining was observed in the large cyst. The cyst was denuded, and cases of paraduodenal wall cysts may indeed be completely denuded. Abundant debris was present within the lumen of the cyst (lower portion of Figure 2D). In Figure 2F, there is myoid spindle cell (e.g., stromal) proliferation at the lower portion of the image, which is most compatible with myofibroblasts and fibroblasts, as well as focal areas containing hemosiderin-laden macrophages noted. Brunner's gland hyperplasia was not identified in this particular case. However, it is notable that Brunner's gland hyperplasia is not one of the features of GP listed in the Armed Forces Institute of Pathology (AFIP) criteria. Because the duodenum was quite distorted by the cystic lesion, the minor papilla was not grossly identified, as is classic in GP. However, the lesion does involve, at least partially, the aspect of the duodenum adjacent to the pancreas (Figure 2C), consistent with GP. Immunohistochemical stains for KIT, DOG-1, a-smooth muscle actin, chromogranin A and pan-cytokeratin were negative. Having utilized combined clinical, radiographic, endoscopic, cytological, pathological, and immunohistochemical findings, a final diagnosis of GP was made in our patient.
Figure 1: (A and B) CT of the abdomen/pelvis demonstrates a large cystic mass within the duodenal wall. The mass is heterogeneously enhancing with areas suggestive of internal necrosis. A marked mass effect is seen upon the duodenal lumen. (C) Upper endoscopy demonstrates luminal narrowing of the second portion of the duodenum without mucosal involvement. (D) Endoscopic ultrasound demonstrates solid and cystic components in the duodenal wall lesion.
Figure 2: (A) Photomicrograph of cytological specimens demonstrate foci of spindle cells in the background of normal intestinal epithelium (Diff-Quick, 200x). (B) Repeat aspirates demonstrate foci of spindle-to-epithelioid cells (Diff-Quick, 400x). (C) Photomicrograph of the resected specimen reveals cyst wall adjacent to the pancreas, which has evidence of fibrosis and chronic pancreatitis (H&E, 20x). (D and E) Photomicrographs of the resected specimen reveal a cystic mass adjacent to normal duodenal mucosa (H&E, 20x). (F) Photomicrographs of myoid spindle cell (e.g., stromal) proliferation at the lower portion of the image, which is most compatible with myofibroblasts and fibroblasts, as well as focal areas containing hemosiderin-laden macrophages noted (H&E, 200x).
Discussion
The challenge of diagnosing groove pancreatitis preoperatively lies in the ambiguity of imaging findings and cytological variability. Cytology may demonstrate spindle cells, lipid-loaded macrophages, and/or debris [6]. In fact, depending upon the area sampled, features may be suggestive of benign pathology such as Brunner’s gland hyperplasia (BGH) or submucosal tumors (SMTs), such as gastrointestinal stromal tumor (GIST), leiomyoma, and schwannoma. GIST most often has spindle cell histology, but may also have epithelioid or mixed features. Markers typically include KIT (CD117) and a-smooth muscle actin. A major distinguishing factor between GIST and other SMTs is that the latter are almost uniformly KIT-negative. However, about one-in-twenty GISTs are also KIT-negative. Thus, the Discovered on GIST-1 (DOG-1) marker can aid in distinguishing these SMTs.
The presence of Brunner’s glands on biopsies can lead to misdiagnosing BGH. This can present in a similar fashion to GP because there are excessive Brunner’s glands. However, BGH is thought to be caused by hyperactivity of the exocrine modulating factors including the vagus nerve and intestinal mucous membrane factor [7], as well as gastric acid hypersecretion and decreased pancreatic enzyme secretion [2]. Endoscopically, BGH presents with nodular, rather than cystic, lesions in the duodenal submucosa [8]. While Brunner’s gland hyperplasia is often asymptomatic, no consensus exists regarding resection (i.e., pancreaticoduodenectomy), except when patients develop severe symptomatology, such as abdominal pain or hemorrhage.
In cases of GP secondary to alcohol, treatment is conservative management; nil per os, analgesia, parenteral nutrition, and alcohol cessation. In patients with GP secondary to anatomic abnormalities or those refractory to conservative management, pancreaticoduodenectomy is recommended with approximately 75% of patients having complete symptom relief postoperatively [3].
Conclusion
In summary, GP is a rare disorder that often mimics submucosal duodenal tumors and malignant periampullary tumors that present with obstructive jaundice. Surgical therapy remains the gold standard for treatment of this disease when symptoms and obstruction are present. Since resection, our patient is tolerating a diet, and his weight is stable.
References
- Latham J, Sanjay P, Watt DG, Walsh SV, Tait IS (2013) Groove pancreatitis: a case series and review of the literature. Scott Med J 58: e28-31.
- Becker V, Mischke U (1991) Groove pancreatitis. Int J Pancreatol 10: 173-182.
- Casetti L, Bassi C, Salvia R, Butturini G, Graziani R, et al. (2009) "Paraduodenal" pancreatitis: results of surgery on 58 consecutives patients from a single institution. World J Surg 33: 2664-2669.
- Levenick JM, Gordon SR, Sutton JE, Suriawinata A, Gardner TB (2009) A comprehensive, case-based review of groove pancreatitis. Pancreas 38: e169-175.
- Tezuka K, Makino T, Hirai I, Kimura W (2010) Groove pancreatitis. Dig Surg 27: 149-152.
- Chute DJ, Stelow EB (2012) Fine-needle aspiration features of paraduodenal pancreatitis (groove pancreatitis): a report of three cases. DiagnCytopathol 40: 1116-1121.
- Franzin G, Musola R, Ghidini O, Manfrini C, Fratton A (1985) Nodular hyperplasia of Brunner's glands. GastrointestEndosc 31: 374-378.
- Loo CK, Hui PK, Fung TT (1999) Gastrointestinal: Brunner's gland hyperplasia. J GastroenterolHepatol 14: 1137.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 15773
- [From(publication date):
August-2014 - Sep 02, 2025] - Breakdown by view type
- HTML page views : 11064
- PDF downloads : 4709