High Platelet-Lymphocyte Ratio may Predict Poor Therapeutic Response in Breast Cancer Patients Treated with Neoadjuvant Chemotherapy
Received: 10-Dec-2024 / Manuscript No. DPO-24-154639 / Editor assigned: 12-Dec-2024 / PreQC No. DPO-24-154639 (PQ) / Reviewed: 26-Dec-2024 / QC No. DPO-24-154639 / Revised: 02-Jan-2024 / Manuscript No. DPO-24-154639 (R) / Published Date: 09-Jan-2024
Abstract
Abstract Background: To investigate the correlation between Blood Inflammatory Markers (BIMs), including Neutrophil to Lymphocyte Ratio (NLR), Monocyte to Lymphocyte Ratio (MLR) and Platelet to Lymphocyte Ratio (PLR), and the efficacy of Neoadjuvant Chemotherapy (NACT) in BC patients. Besides, the relationship between Tumor Infiltrating Inflammatory cells (TIIs) and BIMs has also been preliminarily studied. Methods: A total of 315 BC patients between January 2018 and September 2022 were recruited. Receiver operating characteristic curve was used to determine the cut-off value of each BIM. Logistic regression analysis was used to evaluate the predictive power of BIMs for pathological Complete Response (pCR). TIIs were assessed by immunohistochemistry and their relationships with BIMs also were analyzed. Results: NLR, MLR, PLR in the pCR group were significantly lower than those in the non-pCR group after NACT (p ≤ 0.05). PLR had the largest Area Under the Curve (AUC) (0.73) compared to NLR (0.57) and MLR (0.67) (p< 0.01). Univariate analysis showed ER, PR, HER2, NLR, MLR, PLR were significantly associated with pCR, but multivariate analysis found only HER2 and PLR were independently predictors for pCR (p<0.01). PLR was positively correlated with the expression of P-selectin in tumor tissue (r: 0.26, p<0.01). Survival analysis showed that NLR, MLR, PLR had no significant correlation with disease prognosis. Conclusion: PLR after NACT could serve as a predictor for pCR and it correlated with the imbalance of the tumor-infiltrating platelet to lymphocyte ratio, which might cause drug resistance.
Keywords: Breast cancer; Neoadjuvant chemotherapy; Platelet to lymphocyte ratio; Pathological complete response
Abbreviations
BC: Breast Cancer; BIMs: Blood Inflammatory Markers; NACT: Neoadjuvant Chemotherapy; TIIs: Infiltrating Inflammatory Cells; ROC: Receiver Operating Characteristic; pCR: pathological Complete Response; DFS: Disease-Free Survival; NLR: Neutrophil to Lymphocyte Ratio; MLR: Monocyte to Lymphocyte Ratio; PLR: Platelet to Lymphocyte Ratio; AUC: Area Under the Curve; TINs: Tumor-Infiltrating Neutrophils; TAMs: Tumor-Associated Macrophages; TILs: Tumor-Infiltrating Lymphocytes; TEPs: Tumor- Educated Platelets; BMI: Body Mass Index; HR: Hormone Receptor; MP: Miller-Payne scoring system; 95% CI: 95% Confidence Interval; OR: Odds Ratio; PBS: Phosphate-Buffered Saline.
Introduction
Neoadjuvant Chemotherapy has become the standard treatment for locally advanced Breast Cancer (BC). It not only can downstage locally advanced disease and make it operable, but also improve the breastconserving surgery rate [1]. However, the response to NACT among patients is variable. Although pCR is the most important curative indicator, it can only be evaluated on resected specimen [2]. So far, there is no convenient biomarker that can reflect the tumor burden of patients during NACT in real time [3]. Some studies have suggested that imaging, positron emission tomography and gene expression profiling can predict the NACT outcome [4-6]. But these methods are costly and not conducive to general clinical implementation. Inflammation has been considered one of the characteristic of cancer [7]. It can promote tumor initiation and progression, whereas escape from immune surveillance, which may favor cancer invasiveness [8]. The main inflammation and immune cell components in the tumor microenvironment include TINs, TAMs, TILs and TEPs that are also considered to have certain proinflammatory activity [9-12]. Some studies have found that TINs, TAMs and TEPs can promote angiogenesis and form an immunosuppressive microenvironment, which lead to tumor progression and drug resistance [13]. While TILs usually inhibit tumor growth and have a positive correlation with chemotherapy efficacy and prognosis of BC patients [14]. Besides, one study has reported that intra-tumoral inflammatory cells to T cells ratio can reflect the inflammatory-immune imbalance in the tumor microenvironment and has the potential to be a predictor of survival in some types of cancer [15]. However, the analysis of inflammation and immune cell components in tumor microenvironment also is not convenient and timely. More importantly, even if TILs are considered to have a good application prospect, they are only limited to specific molecular subtypes of BC. As for TINs, TAMs and TEPs, they have not been applied in clinical practice at present due to the lack of support from large randomized controlled studies. Some researchers believe that since inflammation and immune cells in tumor microenvironment are derived from peripheral blood, then blood cell populations can provide information about the intra-tumoral status [16]. There have been some studies reported that peripheral monocyte count was associated with the density of the TAMs and high absolute monocyte count could predict poor survival in cancer patients. Besides, high platelet counts also were reported to be associated with adverse outcomes in BC [17]. However, they are nonspecific inflammatory indexes and cannot be used as tumor biomarkers. In recent years, novel BIMs, such as NLR, PLR and MLR derived from the count of inflammatory cells in peripheral blood, have been extensively studied. Several studies have found that some BIMs could be used as potential prognostic factors for survival in different types of cancers including BC [18-20]. In the context of NACT, BIMs also have been suggested as potential predictors for pCR but with conflicting results [21,22]. Therefore, the significance of BIMs in BC remains unclear. In addition, whether inflammation cell components in the tumor microenvironment are affected by circulating cells and whether local and circulating inflammation cells have consistent predictive effects on the efficacy of NACT is unclear.
In this retrospective study, we intended to explore whether and which BIM could be used as a predictor for the efficacy of NACT. Besides, the corresponding cellular components in the tumor microenvironment also were evaluated to study the relationship with circulating cells and their value in predicting the efficacy of NACT.
Materials and Methods
Participants
This is a single-center retrospective study and the objects included clinically diagnosed BC patients who received NACT followed by surgery at our hospital between 1st January, 2018 and 30th September, 2022.
Inclusion criteria included: 1) Age>18 years old; 2) Invasive BC confirmed by biopsy; 3) At least immunohistochemical results for ER, PR, HER2 and Ki67 were available; 4) All patients should meet the NACT criteria according to established guidelines; 5) Radical surgery for each patient performed at Nanjing Drum Tower Hospital after NACT [23].
Exclusion criteria included: 1) Patients with clinical stage IV or inflammatory breast cancer or history of previous malignant tumor; 2) Clinicopathological data were insufficient; 3) Patients who did not meet the NACT criteria or terminated chemotherapy prematurely; 4) Patients who did not undergo radical surgery at Nanjing Drum Tower Hospital after NACT.
Data collection
Medical electronic records and pathology reports of patients were reviewed. The recorded information included: Age, BMI, menstrual status, clinical stage before chemotherapy (tumor size, axillary lymph node status, distant metastasis or not), pathological results of biopsy and radical surgical specimens, NACT regimens, imaging assessment results during NACT and surgery methods. HR, HER2 and Ki67 index were characterized on biopsy and radical surgical specimens in the central laboratory of our hospital. HER2 was determined according to ASCO/CAP guidelines [24]. HR+ was defined if more than 1% of tumor cells were positive for ER/PR immunohistochemical staining. The absolute counts of neutrophils, monocytes, platelets and lymphocytes in peripheral blood of patients before the first NACT and after the last chemotherapy were recorded in an excel sheet. And then they were used to calculate NLR, MLR and PLR.
Immunohistochemistry
Paraffin sections were deparaffinized at 65℃ for 1 hour and treated in a pressure boiler with EDTA for 10 min. The sections were placed in 3% hydrogen peroxide solution and incubated at room temperature for 10 min to block endogenous peroxidase. Each section was washed 3 times with Phosphate-Buffered Saline (PBS), sealed with Protein Block Serum-free for 20 min and then incubated with primary rabbit monoclonal antibody (Abcam, Cambridge, USA) overnight at 4°C. After that, the sections were incubated with secondary antibody (Dako, California, USA) for 30 min, washed with PBS for 3 times. Tissues were stained for 5 min with fresh DAB solution and then counterstained with Mayer's hematoxylin and mounted in Permount (Fisher Scientific, New Jersey, USA). Samples were evaluated by one independent observer using an optical microscope. Only cancer cells with a distinct brown staining of the nucleus were considered positive.
Outcomes
The primary outcome measured in this study was pCR. The pCR was defined by ypT0/Tis, N0, M0. Secondary outcome was DFS and OS. DFS was defined as the time from radical surgery after NACT until any distant or local recurrence and OS was defined as the period from the date of diagnosis until the date of death assessed by Response Evaluation Criteria in Solid Tumors 1.1 (RECIST 1.1) [25].
Statistical analysis
The statistical analysis was done using SPSS 20 (IBM Corp., Armonk, NY). Continuous variables were expressed as "mean ± standard error" and categorical variables were expressed as percentage (%). Chi-square test or Fisher's exact test was used to compare the categorical parameters between the two groups. T-test were used to compare the normally distributed continuous variables. Correlation analysis of categorical parameters was done by Pearson χ² test. The optimal cut-off value of each index was determined using ROC curve analysis. Kaplan-Meier method was used to plot survival curve and logrank test was used to compare survival difference. A p-value<0.05 was considered statistically significant.
Ethical approval
This observational study was approved by the ethical and scientific committee of Nanjing Drum Tower Hospital. All procedures performed in studies involving human patients were in accordance with the ethical standards of the 1964 Helsinki Declaration and its later amendments. Oral informed consent was obtained from all individual participants included in the study.
Results
Patient characteristics
A total of 315 patients were included in this study and the clinicopathological information of patients were shown in Table 1. Invasive ductal carcinoma was diagnosed in 96.51% of the patients and all patients were eligible for NACT according to the guidelines. Approximately 51.11% of patients received the docetaxel+anthracycline+ cyclophosphamide (TAC/TEC × 6) regimen, about 36.19% of patients with Human Epidermal Growth Factor receptor 2 (HER2) expression received docetaxel+ carboplatin+trastuzumab+pertuzumab (TCbHP × 6) or TCbH × 6 regimen and about 6.98% received docetaxel+trastuz umab+pertuzumab (THP × 6) regimen. All patients received radical surgery at our hospital after chemotherapy. Surgical choices included breast-conserving surgery and mastectomy. If axillary lymph node biopsy confirmed metastasis before chemotherapy, axillary lymph node dissection was performed directly, otherwise sentinel lymph node biopsy was performed firstly. For patients accepted breastconserving surgery or had positive axillary lymph nodes, postoperative radiotherapy was required.
| N (315) | Percentage (%) | |
|---|---|---|
| Age | ||
| ≤ 35 | 40 | 12.7 |
| 36-55 | 183 | 58.1 |
| ≥ 56 | 92 | 29.2 |
| BMI | ||
| <24 | 165 | 52.38 |
| ≥ 24 | 150 | 47.62 |
| Menopausal status | ||
| Pre-menopausal | 170 | 53.97 |
| Post-menopausal | 145 | 46.03 |
| T stage | ||
| T1 | 54 | 17.14 |
| T2 | 218 | 69.21 |
| T3,T4 | 43 | 13.65 |
| Axillary lymph node | ||
| Negative | 41 | 13.02 |
| Positive | 224 | 71.11 |
| Uncertain | 50 | 15.87 |
| Histological type | ||
| Invasive ductal carcinoma | 304 | 96.51 |
| Others | 11 | 3.49 |
| Histopathological grade | ||
| I,II | 213 | 67.62 |
| III | 102 | 32.38 |
| ER | ||
| Positive | 185 | 58.73 |
| Negative | 130 | 41.27 |
| PR | ||
| Positive | 152 | 48.25 |
| Negative | 163 | 51.75 |
| HER2 | ||
| Positive | 147 | 46.67 |
| Negative | 168 | 53.33 |
| Ki67 | ||
| ≤ 14% | 11 | 3.49 |
| >14% | 304 | 96.51 |
| Chemotherapy regimens | ||
| TAC × 6 | 161 | 51.11 |
| TCbH/TCbHP × 6 | 114 | 36.19 |
| THP × 6 | 22 | 6.98 |
| others | 18 | 5.72 |
| pCR | ||
| yes | 104 | 33.02 |
| no | 211 | 66.98 |
| pT | ||
| T0/Tis | 116 | 36.83 |
| T ≥ 1 | 199 | 63.17 |
| pN | ||
| N0 | 175 | 55.56 |
| N+ | 140 | 44.44 |
| MP | ||
| 04th May | 160 | 50.79 |
| 01st Mar | 155 | 49.21 |
Note: BMI: Body Mass Index; TAC: docetaxel+adriamycin+cyclophosphamide; TCbHP: docetaxel+carboplatin +trastuzumab+pertuzumab; TCbH: docetaxel+carboplatin+trastuzumab; THP: docetaxel+trastuzumab+pertuzumab; pCR: pathological Complete Response; MP: Miller-Payne grading system.
Table 1: The clinicopathological information of the patients.
Pathological results of radical surgical specimens showed that 116 patients (36.83%) had no residual cancer cells or only ductal carcinoma in situ in the breast. Axillary lymph node metastasis was not found in 175 patients (55.56%). Besides, the Miller-Payne scoring system (MP) also was used to assess chemotherapy response of primary lesion and the results showed that more than half (50.79%) of the patients achieved MP grade 4-5. At last, a total of 104 patients achieved pCR.
Pathological results of radical surgical specimens showed that 116 patients (36.83%) had no residual cancer cells or only ductal carcinoma in situ in the breast. Axillary lymph node metastasis was not found in 175 patients (55.56%). Besides, the Miller-Payne scoring system (MP) also was used to assess chemotherapy response of primary lesion and the results showed that more than half (50.79%) of the patients achieved MP grade 4-5. At last, a total of 104 patients achieved pCR.
Comparisons of BIMs before and after chemotherapy
We first compared the differences in NLR, MLR and PLR at baseline between the pCR and non-pCR groups, but the results showed no statistical difference. However, after chemotherapy, the average NLR, MLR and PLR in the pCR group were significantly lower than those in the non-pCR group (p<0.05) (Table 2). This suggested that patients with lower BIMs after NACT seem to be more likely to reach pCR. In addition, ROC curve analysis was used to determine the optimal cut-off values for the NLR, MLR and PLR. The values of the AUC, sensitivity and specificity in ROC analysis were shown in Table 3. The cut-off values of the NLR, MLR and PLR after NACT were defined as 3.03, 0.29, 143.37 and 0.42 respectively and PLR had the largest AUC with 65% sensitivity and 73% specificity.
| Pre-NACT | Post-NACT | |||||
|---|---|---|---|---|---|---|
| non-pCR | pCR | p | non-pCR | pCR | p | |
| NLR | 2.22 ± 0.07 | 2.15 ± 0.10 | 0.56 | 3.11 ± 0.17 | 2.58 ± 0.19 | 0.05 |
| MLR | 0.21 ± 0.01 | 0.19 ± 0.01 | 0.15 | 0.43 ± 0.02 | 0.32 ± 0.03 | <0.01 |
| PLR | 145 ± 4.25 | 138.7 ± 4.56 | 0.36 | 211.20 ± 10.06 | 148.80 ± 9.89 | <0.01 |
Note: NLR: Neutrophil to Lymphocyte Ratio; MLR: Monocyte-Lymphocyte Ratio; PLR: Platelet to Lymphocyte Ratio; NACT: Neoadjuvant Chemotherapy.
Table 2: Comparisons of BIMs between non-pCR and pCR group.
| Cut-off value | AUC | 95% CI | p | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|
| NLR | 3.03 | 0.57 | 0.51-0.64 | 0.04 | 0.76 | 0.4 |
| MLR | 0.29 | 0.67 | 0.60-0.73 | <0.01 | 0.61 | 0.65 |
| PLR | 143.37 | 0.73 | 0.67-0.79 | <0.01 | 0.65 | 0.73 |
Note: ROC: Receiver Operating Characteristic; AUC: Area Under the Curve; CI: Confidence Interval.
Table 3: ROC curve analyses of BIMs for pCR.
Predictive factors for pCR in univariate and multivariate analyses
Patients were divided into the" ≤ cut-off" group and the ">cut-off" group according to the cut-off value of each BIM. Univariate analysis showed that ER, PR, HER2 status before NACT and BIMs (including NLR, MLR, PLR) after chemotherapy were significantly correlated with pCR (p<0.01). But multivariate logistic regression analysis suggested that only HER2 status (OR, 2.87; 95%CI, 1.54-5.37, p<0.01) and PLR (OR, 0.34; 95%CI, 0.19-0.61, p<0.01) were independent predictors for pCR (Table 4). In addition, subgroup analysis showed that patients in the PLR ≤ cut-off group had a higher pCR rate, which was not affected by the molecular subtype (Table 5).
| Univariate analysis | Multivariate analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |||||
| Lower | Upper | Lower | Upper | |||||||
| Age (≤ 45 vs. >45) | 1.62 | 0.96 | 2.64 | 0.07 | - | - | - | - | ||
| Menopausal status (pre vs. post) | 1.51 | 0.94 | 2.42 | 0.09 | - | - | - | - | ||
| BMI (<24 vs. ≥ 24) | 1.03 | 0.64 | 1.65 | 0.91 | - | - | - | - | ||
| T (T1,2 vs. T3,4) | 0.57 | 0.27 | 1.22 | 0.14 | - | - | - | - | ||
| Node (negative vs. positive) | 0.84 | 0.42 | 1.68 | 0.62 | - | - | - | - | ||
| WHO (I-II vs. III) | 1.41 | 0.86 | 2.31 | 0.17 | - | - | - | - | ||
| ER (negative vs. positive) | 0.41 | 0.25 | 0.66 | <0.01 | 0.63 | 0.32 | 1.24 | 0.18 | ||
| PR (negative vs. positive) | 0.34 | 0.21 | 0.55 | <0.01 | 0.51 | 0.26 | 1.02 | 0.06 | ||
| HER2 (negative vs. positive) | 5.35 | 3.19 | 8.99 | <0.01 | 2.87 | 1.54 | 5.37 | <0.01 | ||
| Ki67 (≤ 14% vs. >14%) | 1.33 | 0.35 | 5.11 | 0.68 | - | - | - | - | ||
| NLR (≤ cut-off vs. >cut-off) | 0.48 | 0.28 | 0.81 | <0.01 | 1 | 0.52 | 1.92 | 0.99 | ||
| MLR (≤ cut-off vs. >cut-off) | 0.34 | 0.21 | 0.56 | <0.01 | 0.97 | 0.5 | 1.9 | 0.93 | ||
| PLR (≤ cut-off vs. >cut-off) | 0.2 | 0.12 | 0.33 | <0.01 | 0.34 | 0.19 | 0.61 | <0.01 | ||
Note: OR: Odds Ratio.
Table 4: Univariate and multivariate logistic regression analysis for the predictors of pCR.
| ≤ Cut-off | >Cut-off | OR | 95% CI | p | |
|---|---|---|---|---|---|
| Luminal A/B | - | - | 0.46 | 0.24-0.86 | 0.02 |
| non-pCR | 45 | 99 | - | - | - |
| pCR | 27 | 27 | - | - | - |
| HER2+ (ER-, PR-) | - | - | 0.32 | 0.11-0.94 | 0.04 |
| non-pCR | 13 | 14 | - | - | - |
| pCR | 26 | 9 | - | - | - |
| TNBC | - | - | 0.24 | 0.07-0.89 | 0.04 |
| non-pCR | 7 | 33 | - | - | - |
| pCR | 7 | 8 | - | - | - |
Note: TNBC: Triple Negative Breast Cancer.
Table 5: Correlation analysis between PLR and pCR rate in different molecular subtypes.
Immunohistochemical analysis
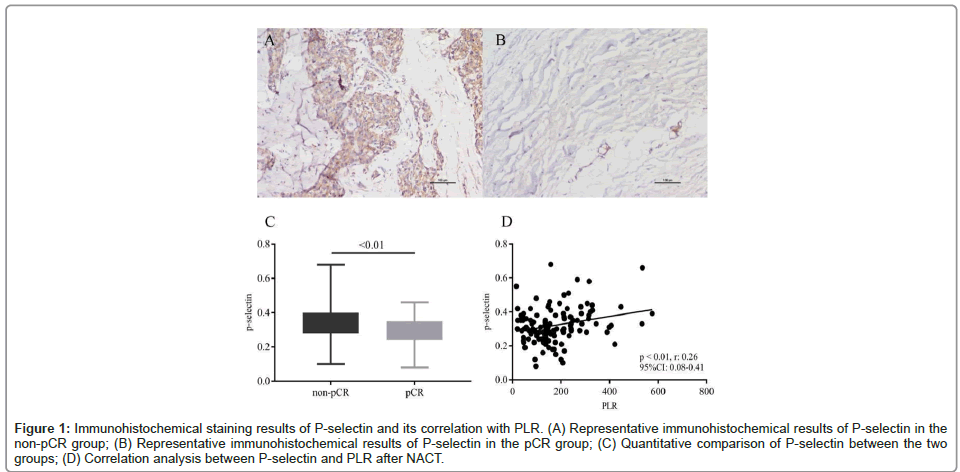
Immunohistochemical staining was performed on the tissue sections of 125 patients, including 75 patients in the non-pCR group and 50 patients in the pCR group. The stained proteins included the lymphocyte marker CD3 and the activated platelet marker P-selectin. Protein expression levels were measured by Image J software. The results showed that there were few CD3+ cells in these tissue sections, but the P-selectin expression varied in different sections. Besides, the average expression level of P-selectin in the non-pCR group was significantly higher than that in the pCR group (0.34 ± 0.01 vs. 0.29 ± 0.01, p<0.01) (Figure 1). Correlation analysis showed that the P-selectin expression was positively correlated with the PLR after NACT (r: 0.26, 95% CI: 0.08-0.41, p<0.01), but had no obvious correlation with the platelet count in peripheral blood.
Figure 1: Immunohistochemical staining results of P-selectin and its correlation with PLR. (A) Representative immunohistochemical results of P-selectin in the non-pCR group; (B) Representative immunohistochemical results of P-selectin in the pCR group; (C) Quantitative comparison of P-selectin between the two groups; (D) Correlation analysis between P-selectin and PLR aft er NACT.
Immunohistochemical staining results of P-selectin and its correlation with PLRA: A: Representative immunohistochemical results of P-selectin in the non-pCR group; B: Representative immunohistochemical results of P-selectin in the pCR group; C: Quantitative comparison of P-selectin between the two groups; D: Correlation analysis between P-selectin and PLR after NACT.
Survival analysis
The median follow-up time was 32 months (range 7-64 months). During the follow-up period, there were 6 deaths (1.90%), 20 lost to follow-up (6.35%) and 29 local recurrence or distant metastasis (9.20%) without death. The number of deaths, follow-up losses and relapses was 2, 10, 11 cases, respectively in the PLR ≤ cut-off group, compared with 4, 10, 18 cases in the PLR>cut-off group. Survival analysis showed no significant difference in DFS and OS between the two groups. Besides, we also investigated the effect of NLR and MLR on DFS, but no meaningful results were obtained (Table 6).
| DFS (≤ cut-off vs. >cut-off) | OS (≤ cut-off vs. >cut-off) | |||||
|---|---|---|---|---|---|---|
| Ratio | 95% CI | p | Ratio | 95% CI | p | |
| NLR | 1.06 | 0.50-2.27 | 0.88 | 1.22 | 0.23-6.32 | 0.82 |
| MLR | 1.4 | 0.66-2.94 | 0.38 | 0.98 | 0.18-5.33 | 0.98 |
| PLR | 0.95 | 0.45-2.02 | 0.89 | 1.12 | 0.19-6.43 | 0.9 |
Note: DFS: Disease-Free Survival; OS: Overall Survival.
Table 6: Survival curves obtained with Kaplan-Meier analysis.
Discussion
Blood routine test results, which reflect the individual’s system or local status, also have certain application value for cancer diagnosis [26]. For example, neutrophils can produce cytokines, chemokines and growth factors that promote angiogenesis, tumor cell proliferation and migration in the tumor microenvironment [27]. Platelets, an important part of the blood clotting system, also can contribute to cancer-favored inflammation response and high platelet counts were reported to be associated with adverse outcomes in BC [28,29]. In contrast, lymphocytes in the blood are thought to can reflect the status of anti-tumor immunity [30]. Based on the precious findings, NLR, MLR and PLR, which reflect the balance between inflammatory and immune response in cancer, have attracted more and more attention from researchers [31]. However, with the increase of the number of studies, the value of these BIMs in BC especially in predicting the response to NACT, has become controversial. Furthermore, few studies have simultaneously compared the value of these BIMs in predicting response to NACT.
In this study, we found that there was no difference in the BIMs before chemotherapy between the pCR group and the non-pCR group. However, the NLR, MLR and PLR in the pCR group all were significantly lower than those in the non-pCR group after NACT. This suggested that pCR might be easier to achieve in patients with weaker systemic inflammatory response after chemotherapy. ROC analysis showed that PLR, with 65% sensitivity and 73% specificity, had the largest AUC compared to NLR and MLR. Univariate analysis showed that parameters such as ER, PR, HER2, NLR, MLR, PLR were significantly associated with pCR. But multivariate logistic regression analysis showed that only HER2 and PLR were independently predictive factors of pCR. It is well known that HER2 was a positive predictor for BC patients with the application of anti-HER2 targeted drugs [32]. Especially for patients receiving trastuzumab plus pertuzumab during NACT, the pCR rate even can reach more than 60% [33]. Our results also support this conclusion. Although NLR and MLR after chemotherapy were associated with pCR rates in univariate analysis, they did not show statistical significance in multivariate analysis and survival analysis. The insignificance of their results might be attributed to the fact that the neutrophil and monocyte counts could be disturbed because of most patients were injected with granulocyte stimulating factor to prevent the neutropenia after chemotherapy. The incidence of thrombocytopenia during NACT was relatively low in this study and patients were less likely to be treated for thrombocytopenia. Therefore, the platelet count could reflect the patient's actual condition.
In addition to the role in hemostasis, platelets have been also thought to play an important role in promoting tumor progression and metastasis. First, platelets can produce a variety of cytokines such as platelet-derived growth factors and vascular endothelial growth factor A, to stimulate tumor progression and dissemination. Second platelets can induce the epithelial-mesenchymal switch of tumor cells, reduce tumor cell anoikis in the blood. Third, platelets can protect tumor cells from being recognized by immune cells through forming a cell-fibrinplatelet aggregate surrounding tumor cells in the circulation. This is thought to play an important role in contributing to tumor metastasis [10,34]. Therefore, the dynamics of PLR represents the disorder of inflammatory immune status of patients. Prior to the present study, there have been some studies that used PLR as a predictive marker for NACT in BC. Some reported that increased PLR during chemotherapy predicted higher pCR rate, while others had the opposite conclusions [35,36]. A meta-analysis has demonstrated that increased PLR during NACT could be as a prognostic biomarker for poor overall survival and DFS [37]. Our results supported that increased PLR suggested poor efficacy of NACT.
Some researchers believed that cell component in blood might can provide information about the status of tumor microenvironment, since most inflammation and immune cells in tumor microenvironment were derived from peripheral blood [16]. However, there are few studies to investigate the relationship between peripheral blood cells and corresponding infiltrating cells in tumor stroma. One important reason is the lack of a specific marker to label the same type of cells in both blood and stroma. P-selectin, also known as CD62P, is stored in α-granules and Weibel-Palade bodies of platelets and endothelial cells, respectively. P-selectin expression is considered to be a marker of platelet activation. P-selectin on activated platelets can mediate interactions with monocytes, instigating paracrine-signaling mechanisms that lead to enhanced inflammation, as well as inducing factors that trigger thrombosis [38]. In this study, P-selectin staining was performed on the specimens of 125 patients. The results showed that the expression of P-selectin in tumor microenvironment was significantly positively correlated with PLR, suggesting that the expression of P-selectin in stroma might be affected by platelets in peripheral blood, but the mechanism needs to be further studied. There are still some limitations in this study. First, this is a single-center retrospective study with a small sample size and the follow-up time is short, which makes some conclusions less convincing. Second, although P-selectin is thought to be expressed mainly on activated platelets, it is not a platelet-specific marker. The expression levels of P-selectin in tissues were not exactly equivalent to TEPs. Therefore, the significance of correlation analysis between P-selectin and PLR remains to be further explored.
Conclusion
This study confirmed that systemic inflammatory-immune imbalance after NACT was associated with poor chemotherapy response and PLR after chemotherapy could serve as a potential predictor. In addition, we found that the expression of P-selectin in tumor microenvironment might be affected by PLR in peripheral blood, but the exact mechanism needs to be further studied.
Authors’ Contributions
WW, YZY contributed to the design of the research, to the analysis of the data and to the writing of the manuscript. WW, CH, RYZ and WJZ contributed to the material preparation and data collection. All authors read and approved the final manuscript.
Funding
This work was supported by the Natural Science Foundation of Jiangsu Province (grant number BK20190126).
Competing Interests
The authors declare that they have no competing interests.
Acknowledgments
We acknowledge all the patients participated in the study. We are also grateful to all the staff who helped us in the study.
References
- Gampenrieder SP, Rinnerthaler G, Greil R (2013) Neoadjuvant chemotherapy and targeted therapy in breast cancer: Past, present and future. J Oncol 2013:1-12.
[Crossref] [Google Scholar] [PubMed]
- Kong X, Moran MS, Zhang N, Haffty B, Yang Q, et al. (2011) Meta-analysis confirms achieving pathological complete response after neoadjuvant chemotherapy predicts favourable prognosis for breast cancer patients. Eur J Cancer 47:2084-2090.
[Crossref] [Google Scholar] [PubMed]
- Provenzano E (2021) Neoadjuvant chemotherapy for breast cancer: Moving beyond pathological complete response in the molecular age. Acta Med Acad 50:88-109.
[Crossref] [Google Scholar] [PubMed]
- Fowler AM, Mankoff DA, Joe BN (2017) Imaging neoadjuvant therapy response in breast cancer. Radiology 285:358-375.
[Crossref] [Google Scholar] [PubMed]
- Chen S, Ibrahim NK, Yan Y, Wong ST, Wang H, et al. (2017) Complete metabolic response on interim (18) F-fluorodeoxyglucose positron emission tomography/computed tomography to predict long-term survival in patients with breast cancer undergoing neoadjuvant chemotherapy. Oncologist 22:526-534.
[Crossref] [Google Scholar] [PubMed]
- Fan H, Li C, Xiang Q, Xu L, Zhang Z, et al. PIK3CA mutations and their response to neoadjuvant treatment in early breast cancer: A systematic review and meta-analysis. Thorac Cancer 9:571-579.
[Crossref] [Google Scholar] [PubMed]
- Hanahan D (2022) Hallmarks of cancer: New dimensions. Cancer Discov 12:31-46.
[Crossref] [Google Scholar] [PubMed]
- Cadwell K. Crosstalk between autophagy and inflammatory signalling pathways: Balancing defence and homeostasis. Nat Rev Immunol 16:661-675.
[Crossref] [Google Scholar] [PubMed]
- Jaillon S, Ponzetta A, di Mitri D, Santoni A, Bonecchi R, et al. (2020) Neutrophil diversity and plasticity in tumour progression and therapy. Nat Rev Cancer 20:485-503.
[Crossref] [Google Scholar] [PubMed]
- Best MG, Wesseling P, Wurdinger T (2018) Tumor-educated platelets as a noninvasive biomarker source for cancer detection and progression monitoring. Cancer Research 78:3407-3412.
[Crossref] [Google Scholar] [PubMed]
- Christofides A, Strauss L, Yeo A, Cao C, Charest A, et al. (2022) The complex role of tumor-infiltrating macrophages. Nat Immunol 23:1148-1156.
[Crossref] [Google Scholar] [PubMed]
- Presti D, Dall'Olio FG, Besse B, Ribeiro JM, di Meglio A, et al. (2022) Tumor Infiltrating Lymphocytes (TILs) as a predictive biomarker of response to checkpoint blockers in solid tumors: A systematic review. Crit Rev Oncol Hematol 177:103773.
[Crossref] [Google Scholar] [PubMed]
- Wilson BE, Gorrini C, Cescon DW (2022) Breast cancer immune microenvironment: From pre-clinical models to clinical therapies. Breast Cancer Res Treat 191:257-267.
[Crossref] [Google Scholar] [PubMed]
- Denkert C, Loibl S, Noske A, Roller M, Muller BM, et al. (2010) Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 28:105-113.
[Crossref] [Google Scholar] [PubMed]
- Ilie M, Hofman V, Ortholan C, Bonnetaud C, Coelle C, et al. (2012) Predictive clinical outcome of the intratumoral CD66b-positive neutrophil-to-CD8-positive T-cell ratio in patients with resectable nonsmall cell lung cancer. Cancer 118:1726-1737.
[Crossref] [Google Scholar] [PubMed]
- Sahin AB, Cubukcu E, Ocak B, Deligonul A, Orhan OS, et al. (2021) Low pan-immune-inflammation-value predicts better chemotherapy response and survival in breast cancer patients treated with neoadjuvant chemotherapy. Sci Rep 11:14662.
[Crossref] [Google Scholar] [PubMed]
- Corbeau I, Thezenas S, Maran-Gonzalez A, Colombo PE, Jacot W, et al. (2020) Inflammatory blood markers as prognostic and predictive factors in early breast cancer patients receiving neoadjuvant chemotherapy. Cancers (Basel) 12:1.
[Crossref] [Google Scholar] [PubMed]
- Ethier JL, Desautels D, Templeton A, Shah PS, Amir E, et al. (2017) Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: A systematic review and meta-analysis. Breast Cancer Res 19:2.
[Crossref] [Google Scholar] [PubMed]
- Lusho S, Durando X, Mouret-Reynier MA, Kossai M, Lacrampe N, et al. (2021) Platelet-to-lymphocyte ratio is associated with favorable response to neoadjuvant chemotherapy in triple negative breast cancer: A study on 120 patients. Front Oncol 11:678315.
[Crossref] [Google Scholar] [PubMed]
- Gianni C, Palleschi M, Schepisi G, Casadei C, Bleve S, et al. (2022) Circulating inflammatory cells in patients with metastatic breast cancer: Implications for treatment. Front Oncol 12:882896.
[Crossref] [Google Scholar] [PubMed]
- Xue LB, Liu YH, Zhang B, Yang YF, Yang D, et al. (2019) Prognostic role of high neutrophil-to-lymphocyte ratio in breast cancer patients receiving neoadjuvant chemotherapy: Meta-analysis. Medicine (Baltimore) 98:e13842.
[Crossref] [Google Scholar] [PubMed]
- Li X, Dai D, Chen B, Tang H, Xie X, et al. (2018) The value of neutrophil-to-lymphocyte ratio for response and prognostic effect of neoadjuvant chemotherapy in solid tumors: A systematic review and meta-analysis. J Cancer 9:861-871.
[Crossref] [Google Scholar] [PubMed]
- Li J, Jiang Z (2022) Chinese Society of Clinical Oncology Breast Cancer (CSCO BC) guidelines in 2022: Stratification and classification. Cancer Biol Med 19:769.
[Crossref] [Google Scholar] [PubMed]
- Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, et al. (2018) Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol 36:2105-2122.
[Crossref] [Google Scholar] [PubMed]
- Litiere S, Collette S, de Vries EG, Seymour L, Bogaerts J, et al. (2017) RECIST-learning from the past to build the future. Nat Rev Clin Oncol 14:187-192.
[Crossref] [Google Scholar] [PubMed]
- Dupre A, Malik HZ (2018) Inflammation and cancer: What a surgical oncologist should know. Eur J Surg Oncol 44:566-570.
[Crossref] [Google Scholar] [PubMed]
- Wu L, Saxena S, Awaji M, Singh RK (2019) Tumor-associated neutrophils in cancer: Going pro. Cancers (Basel) 11:1.
[Crossref] [Google Scholar] [PubMed]
- Schmied L, Hoglund P, Meinke S (2021) Platelet-mediated protection of cancer cells from immune surveillance-possible implications for cancer immunotherapy. Front Immunol 12:640578.
[Crossref] [Google Scholar] [PubMed]
- Taucher S, Salat A, Gnant M, Kwasny W, Mlineritsch B, et al. (2003) Impact of pretreatment thrombocytosis on survival in primary breast cancer. Thromb Haemost 89:1098-1106.
[Google Scholar] [PubMed]
- Kravtsov DS, Erbe AK, Sondel PM, Rakhmilevich AL (2022) Roles of CD4+ T cells as mediators of antitumor immunity. Front Immunol 13:972021.
[Crossref] [Google Scholar] [PubMed]
- Caziuc A, Schlanger D, Amarinei G, Dindelegan GC (2020) Neutrophils-to-lymphocytes, lymphocytes to-monocytes and platelets-to-lymphocytes ratios-predictive biomarkers for response to neoadjuvant chemotherapy in breast cancer. J BUON 25:182-187.
[Google Scholar] [PubMed]
- Loibl S, Gianni L (2017) HER2-positive breast cancer. Lancet 389:2415-2429.
[Crossref] [Google Scholar] [PubMed]
- Takada M, Toi M (2020) Neoadjuvant treatment for HER2-positive breast cancer. Chin Clin Oncol 9:32.
[Crossref] [Google Scholar] [PubMed]
- Braun A, Anders HJ, Gudermann T, Mammadova-Bach E (2021) Platelet-cancer interplay: Molecular mechanisms and new therapeutic avenues. Front Oncol 11:665534.
[Crossref] [Google Scholar] [PubMed]
- Hu Y, Wang S, Ding N, Li N, Huang J, et al. (2020) Platelet/lymphocyte ratio is superior to neutrophil/lymphocyte ratio as a predictor of chemotherapy response and disease-free survival in luminal b-like (HER2) breast cancer. Clin Breast Cancer 20:e403-e409.
[Crossref] [Google Scholar] [PubMed]
- Cuello-Lopez J, Fidalgo-Zapata A, Lopez-Agudelo L, Vasquez-Trespalacios E (2018) Platelet-to-lymphocyte ratio as a predictive factor of complete pathologic response to neoadjuvant chemotherapy in breast cancer. PLoS One 13:e0207224.
[Crossref] [Google Scholar] [PubMed]
- Zhang M, Huang XZ, Song YX, Gao P, Sun JX, et al. (2017) High platelet-to-lymphocyte ratio predicts poor prognosis and clinicopathological characteristics in patients with breast cancer: A meta-analysis. Biomed Res Int 2017:9503025.
[Crossref] [Google Scholar] [PubMed]
- Nasti TH, Bullard DC, Yusuf N (2015) P-selectin enhances growth and metastasis of mouse mammary tumors by promoting regulatory T cell infiltration into the tumors. Life Sci 131:11-18.
[Crossref] [Google Scholar] [PubMed]
Citation: Wang W, Chen H, Zhao R, Yao Y (2024) High Platelet-Lymphocyte Ratio may Predict Poor Therapeutic Response in Breast Cancer Patients Treated with Neoadjuvant Chemotherapy. Diagnos Pathol Open 9:243
Copyright: © 2024 Wang W, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 1290
- [From(publication date): 0-0 - Dec 23, 2025]
- Breakdown by view type
- HTML page views: 991
- PDF downloads: 299