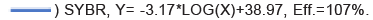Host Specific Eimeria Genus Diagnosis and QPCR Development in Sheeps and Goats
Received: 08-Jun-2023 / Manuscript No. DPO-23-101734 / Editor assigned: 12-Jun-2023 / PreQC No. DPO-23-101734 (PQ) / Reviewed: 26-Jun-2023 / QC No. DPO-23-101734 / Revised: 03-Jul-2023 / Manuscript No. DPO-23-101734 / Accepted Date: 10-Jul-2023 / Published Date: 10-Jul-2023 DOI: 10.4172/2476-2024.8.S13.004
Abstract
Coccidiosis of sheep and goats is caused by protozoa in the genus Eimeria. These protozoa mainly affect young animals, causing a decrease in production and consequent economic losses. Routine diagnosis is made through morphological observation of the oocysts, which has several limitations. The objective of the present study was to develop a real-time PCR (qPCR) technique for the diagnosis of Eimeria spp. in sheep and goats. For this purpose, the 18S rRNA region of the DNA of these parasites was selected because it is a region with low variability among Eimeria spp. The qPCR technique was developed using SYBR Green, resulting in a PCR with high sensitivity, and the ability to amplify samples containing only one oocyst of an Eimeria spp. There was no cross-reaction with other intestinal protozoa, such as Blastocystis, Microsporidia, Cryptosporidium, and Giardia duodenum. The repeatability test showed that the coefficient of variation was less than 2%. This indicated that this method has good sensitivity, specificity, and reproducibility. Thus, the feasibility of using qPCR in the diagnosis of the genus Eimeria was demonstrated in this study. This technique was less laborious and required less skill and diagnostic training compared with the micromorphometry technique conventionally used for this diagnosis
Keywords: 18S rRNA; Sheep; Goat; Coccidiosis; Eimeria; qPCR
Introduction
Coccidiosis is an important parasitic disease of ruminant livestock caused by protozoan parasites of the genus Eimeria, with lambs and kids between 1 and 6 months of age being most susceptible [1]. The occurrence of Eimeria spp. varies among countries from 48.7 to 100% [2-5]. In China, the prevalence of Eimeria spp. in sheep and goats was 92.9% and 78.7%, respectively [6,7].
Eimeria spp. are host specific, meaning that an Eimeria spp. that infects goats does not infect sheep, and vice versa [8]. Thus far, 12 intestinal and one abomasal Eimeria species have been observed in sheep with 10 intestinal species recently found in goats [9-12]. Eimeria ovinoidalis is the most pathogenic species in sheep. Among those found in goats, Eimeria ninakohlyakimovae and Eimeria caprina are the most pathogenic [1]. Others are either less pathogenic or nonpathogenic. Some Eimeria spp. absorb nutrients from their hosts without causing any detectable clinical signs. However, they could be responsible for a decrease in yield, seen in infected animals.
A few studies have developed PCR assays to diagnose Eimeria spp. For example, a primer was obtained for the genus Eimeria based on the 18S rRNA site, and was used to construct a phylogenetic tree comprising the main species in cattle [13]. Yang et al. developed a qPCR technique using primers designed at the 18S rRNA region to quantify the production impacts of Eimeria in sheep [14].
Concerning technological advancements, new assays, such as real-time PCR (qPCR), are now available and capable of developing a more sensitive and specific diagnosis compared with conventional PCR. Hence, the goal of the present study was to develop and standardize a qPCR technique to diagnose Eimeria spp. in sheep and goats.
Materials and Methods
Sample
Positive fecal samples of Eimeria spp. were selected from naturally infected animals and stored in 2.0 mL microtubes at -20ºC in the Veterinary Parasitology Laboratory of Henan Agricultural University.
Positive fecal samples of Giardia duodenalis, Blastocystis, Microsporidia, and Cryptosporidium parvum, detected by PCR, were used to evaluate the specificity of the primers developed in this study [15-18].
The primers were designed from the 18S rRNA gene sequences of Eimeria spp. and deposited at Gen Bank with the following accession numbers: MW512853.1 (Eimeria spp. strain 20Q68-2), MN473507.1 (Eimeria parva isolate IQ-Deer No.36), MN473485.1 (E. parva isolate IQ-Deer No.14), MN473478.1 (E. parva isolate IQ-Deer No.7), MN473490.1 (Eimeria intricata isolate IQ-Deer No.19), LC507796.1 (Eimeria hirci), LC507795.1 (E. hirci), MW577425.1 (Eimeria arloingi strain 10), MW577427.1 (Eimeria christenseni strain 12), KX519412.1 (E. ninakohlyakimovae), MW512852.1 (Eimeria sp. isolate 20Q68-1), MT337428.1 (Eimeria sp. isolate 5-12), MN149906.1 (Eimeria sp. isolate TW4), MT801036.1 (Eimeria sp. voucher SX2), MT801027.1 (Eimeria sp. voucher LX1), and MT801017.1 (Eimeria sp. voucher DJY4). After the selection of the sequences, the primers were aligned using the MEGA7 program (www.megasoftware.net). Following the alignment, a specific site was selected and used for primer design, which was performed with Primer Premier 5.0 (www.premierbiosoft.com/primerdesign). The primers obtained were named Eimeria 18S-F (5′-CAGGCTTGTCGCCCTGA-3′) and Eimeria 18S-R (5′-TTCGCAGTAGTTCGTCTTT-3′), with an amplicon of 168 bp.
DNA extraction steps were performed in accordance with product instructions of the Stool DNA Kit (E.Z.N.A Stool DNA Kit, OMEGA, Bozeman, MT, USA).
Endpoint PCR was used with primer sets to obtain the PCR product of Eimeria spp. The reaction mixture (25 μL) contained 2.5 μL PCR buffer (10x), 2.0 μL dNTPs (2 mM each), 1.0 μL MgCl2 (25 mM), 1.0 μL forward and reverse primers (10 μM), 0.2 μL DNA polymerase (1 U•μL-1), 1.0 μL of genomic DNA purified from Eimeria spp. and 17.3 μL of PCR double-distilled water. PCR was carried out under the following conditions: an initial denaturation at 94ºC for 4 min, followed by 35 cycles of 45 s at 94ºC, 35 s at 55ºC, and 1 min at 72ºC. The final extension step was set for 7 min at 72ºC. The PCR amplicon was purified from agarose gel, cloned into a pMD18-T vector, and transformed into Escherichia coli DH5α. Recombinant clones were selected by blue/white screening. The recombinant plasmid DNA was extracted using the SanPrep column plasmid DNA small amount extraction kit (Sangon Biotech, Shanghai, China) and sent to Shanghai Sangon Biotech for sequencing. Recombinant plasmids with the correct sequence of the Eimeria spp. amplicon (hereafter pMD18T-168) was used as the standard in qPCR.
The pMD18T-168 plasmid was used to generate standard dilution series and the development of the qPCR test. The qPCR instrument used in this work was produced by Analytikjena (jean, Germany). The concentration of the plasmid standard solution was measured using Nano Drop 1000 (Thermo Fisher Scientific, Waltham, MA, USA), and the corresponding copy number was calculated. A tenfold dilution series of pMD18T-168, ranging from 3.43 × 103 to 3.43 × 108 copies/µL, was made and used to construct a standard curve. The reaction mixture contained 1.0 μL of plasmid DNA dilution, 1.0 µL Eimeria 18S-F (500 nmol/L), 1.0 µL Eimeria 18S-R (500 nmol/L), 10.0 μL of THUNDERBIRD® Next SYBR® qPCR Mix (TOYOBO, Osaka, Japan), and PCR double-distilled water to a final volume of 20 μL. An initial denaturation at 95ºC for 30 s was followed by 40 cycles of 5 s at 95ºC, 10 s at 56ºC and 15 s at 72ºC. Threshold cycle (Ct) values in dilutions were measured in triplicate and plotted against the logarithm of their initial copy number. Each standard curve was generated by linear regression of the plotted points, and standard curve parameters were obtained. Ct was calculated under default settings for the qPCR soft system Software (ver. 4.0, Analytik).
Each assay designed included negative and positive controls and the standard curve. The negative control was the PCR reaction without template DNA. The positive control was a PCR reaction containing DNA of Eimeria spp. All controls and samples were assayed at least three times. The specificity of Eimeria spp. qPCR was evaluated by performing the method using gDNA from G. duodenalis, Blastocystis, Microsporidia, and C. parvum simultaneously. The analytical sensitivity (i.e., limit of detection) was established using eight replicates of serially diluted pMD18T-168 plasmid at 3.43 × 107, 106 105, 104 103, 102, 101, and 100 copies/reaction. gDNA was extracted from 10,000 coccidia oocysts, and diluted into five gradients of 10,000, 1,000, 100, 10, and 1 oocyst(s) according to the tenfold dilution series. The established qPCR was used to detect gDNA to determine the sensitivity of the method. The interassay precision of the qPCR was defined as the coefficient of variation (CV) of Ct values obtained for each copy number/reaction in three different assays performed on 3 different days.
The developed qPCR method and McMaster method were used to monitor the Eimeria spp. infection quantity of four naturally infected lambs for 4 weeks and to compare the difference between two methods [19-21].
Data are presented as mean (± SD). The pMD18T-168 DNA levels are presented as a log unit. Graph Pad Prism (www.graphpad-prism.cn) was used for statistical analysis.
Results
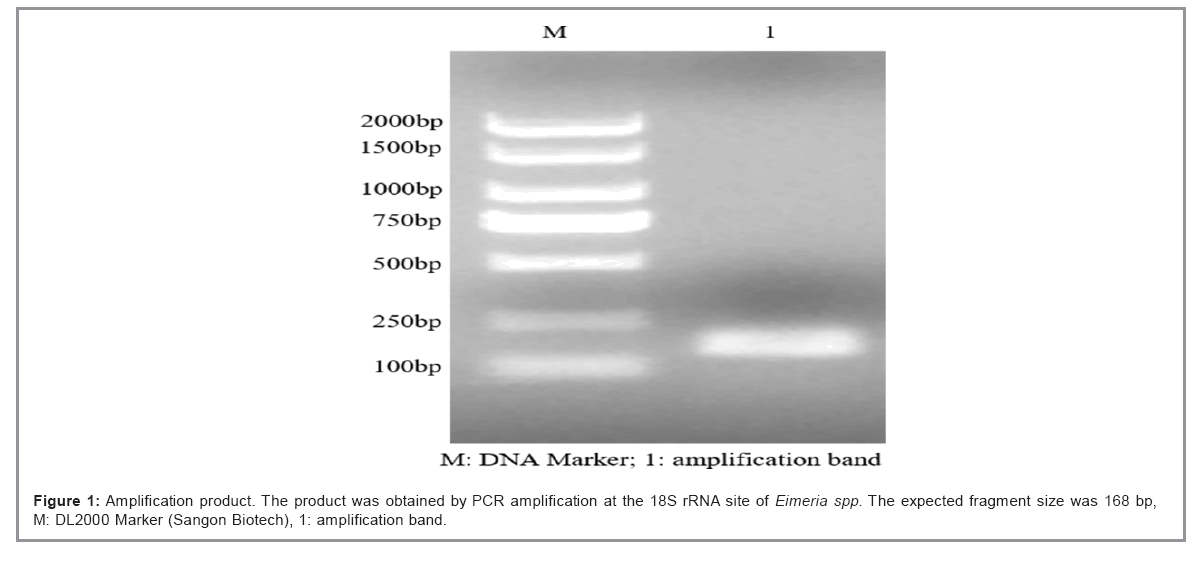
The PCR amplification product of Eimeria spp. 18S rRNA was detected by 1% agarose gel electrophoresis, and the amplified fragment was between 100 bp and 250 bp, which encompassed the expected fragment size (168 bp). The sequencing results of positive clones were compared with sequences (MW512853.1) in GenBank, with a matching rate of 100% (Figure 1 and Table 1).
| Score | Expect | Identities | Gaps | Strand |
|---|---|---|---|---|
| 311 bits (168) |
2.00E-80 | 168/168(100%) | 0/168(0%) | Plus/Plus |
| Query | CAGGCTTGTCGCCCTGAATACTTCAGCATGGAATAATAGGATAGGACCTTGGTTCTATTT | 60 | ||
| Sbjct | CAGGCTTGTCGCCCTGAATACTTCAGCATGGAATAATAGGATAGGACCTTGGTTCTATTT | 753 | ||
| Query | TGTTGGTTTCTAGGACCAAGGTAATGATTAATAGGGACAGTTGGGGGCATTCGTATTTAA | 120 | ||
| Sbjct | TGTTGGTTTCTAGGACCAAGGTAATGATTAATAGGGACAGTTGGGGGCATTCGTATTTAA | 813 | ||
| Query | CTGTCAGAGGTGAAATTCTTAGATTTGTTAAAGACGAACTACTGCGAA | 168 | ||
| Sbjct | CTGTCAGAGGTGAAATTCTTAGATTTGTTAAAGACGAACTACTGCGAA | 861 | ||
Table 1: Sequence alignment. The sequenced sequences were compared with sequences published in Gen Bank.
Development of qPCR for Eimeria spp
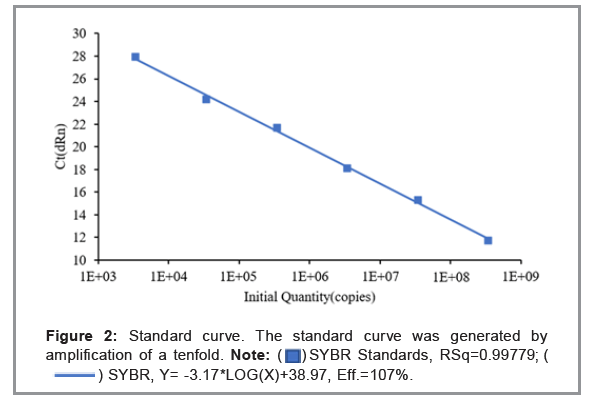
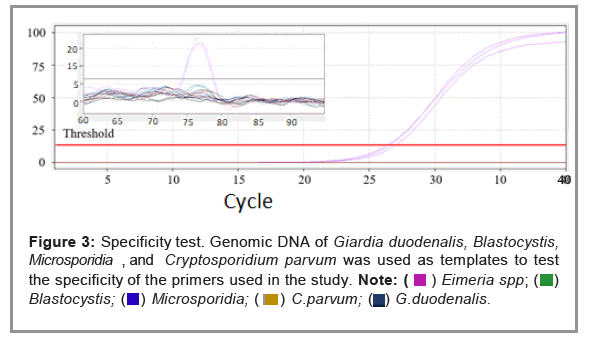
The qPCR was performed using different copy numbers of the pMD18T-168 plasmid. The linear dynamic range of the assay was 6 logs (Figure 2). Assay linearity was established (R2=0.99779) between dilutions containing 3.43 × 103-108 copies/reaction. The standard curve slope was -3.17, which resulted in a high amplification efficiency of 107%. The results confirmed that, over a wide concentration range, the method had a good linearity and can be used for the detection of target DNA. Gradient dilution of the pMD18T-168 plasmid. The standard curve is Y=-3.17*log(x)+38.97, X-axis represents the copy number of template DNA, and the Y-axis represents the Ct value (Figure 3).
Standardization of qPCR for Eimeria spp
The specificity of the qPCR was confirmed because no fluorogenic signal was detected when gDNA of G. duodenalis, Blastocystis, Microsporidia, or C. parvum were used (Figures 3 and 4). The limit of detection of qPCR was 34 copies/reaction, enabling it to detect samples containing a single oocyst of Eimeria spp. The CV of repeatability tests (intra and inter assay) was less than 2%, indicating that the method had good repeatability (Tables 2 and 3).
| Plasmid concentration (copies/μL) | Mean Ct value (n=3) |
|---|---|
| 3.43 × 108 | 11.87 ± 0.75 |
| 3.43 × 107 | 17.04 ± 0.34 |
| 3.43 × 106 | 20.76 ± 0.21 |
| 3.43 × 105 | 25.11 ± 0.32 |
| 3.43 × 104 | 28.1 ± 0.05 |
| 3.43 × 103 | 30.22 ± 0.41 |
| 3.43 × 102 | 30.73 ± 0.66 |
| 3.43 × 101 | 31.87 ± 0.27 |
| 3.43 × 100 | - |
Note: Ct-cycle threshold; "-" - No Ct value
Table 2: Determination of limit of detection for the Eimeria spp. qPCR.
| Oocyst density | Mean Ct value (n=3) |
|---|---|
| 10,000 | 15.95 |
| 1000 | 20.6 |
| 100 | 24.47 |
| 10 | 29.64 |
| 1 | 32.76 |
Note: Ct-cycle threshold
Table 3: Determination of gDNA of oocyst(s) by the Eimeria spp. qPCR.
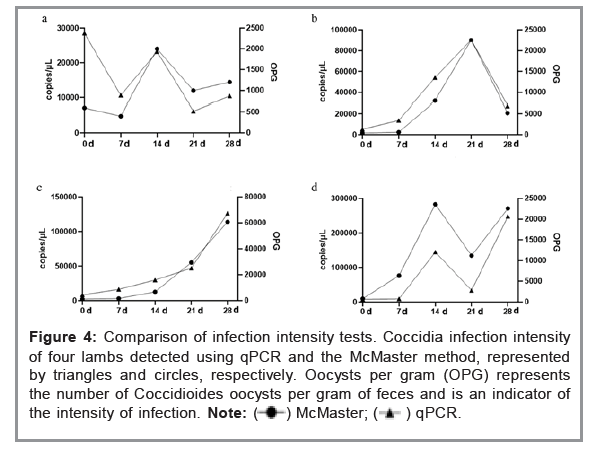
Figure 4 illustrates of the comparison of the established fluorescence-based quantitative method and McMaster method used to measure the infection intensity of four lambs naturally infected with coccidia for four consecutive weeks. According to the results, the trend in changes of oocyst infection intensity in the four lambs detected by the two methods was roughly consistent, indicating that the fluorescence-based quantitative method can be used for quantitative detection of coccidia infection in sheep (Figure 4 and Table 4).
Figure 4: Comparison of infection intensity tests. Coccidia infection intensity
of four lambs detected using qPCR and the McMaster method, represented
by triangles and circles, respectively. Oocysts per gram (OPG) represents
the number of Coccidioides oocysts per gram of feces and is an indicator of
the intensity of infection. 
| Plasmid concentration (copies/μL) | Intraassay | Interassay | ||||
|---|---|---|---|---|---|---|
| Mean Ct value | SD | CV | Mean Ct value | SD | CV | |
| 3.43 × 108 | 11.68 | 0.22 | 1.88% | 11.77 | 0.1 | 0.81% |
| 3.43 × 107 | 17.4 | 0.12 | 0.69% | 17.14 | 0.23 | 1.33% |
| 3.43 × 106 | 20.62 | 0.24 | 1.16% | 20.82 | 0.23 | 1.11% |
| 3.43 × 105 | 24.82 | 0.29 | 1.16% | 24.96 | 0.15 | 0.58% |
| 3.43 × 104 | 27.24 | 0.32 | 1.17% | 27.82 | 0.5 | 1.80% |
Note: Ct-Cycle Threshold; SD-Standard Deviation
Table 4: Intra- and interassay reproducibility tests of qPCR.
Discussion
In the present study, the 18S rRNA site of the DNA from several species of Eimeria spp. derived from sheep and goats was used to design primers because this is a highly conserved region among species [22].
A standardized qPCR is expected to provide sensitive, accurate, and reproducible results. One essential requirement for developing a standardized PCR test is the availability of a common standard that is easy to produce in large amounts, is stable, and shows consistent quality across different production batches [23]. Recombinant plasmids are stable and easy to standardize, and are often used as standard materials to construct qPCR standard curves [24,25]. In this study, a plasmid DNA of Eimeria spp., pMD18T-168, was constructed and used as a calibrator to develop a reliable absolute quantitative qPCR for quantifying parasite load in biological samples.
The results of tests revealed that the assay could reliably detect 34 copies of the cloned Eimeria amplicon per μL of fecal DNA extract, and it was possible to find samples containing a single oocyst of Eimeria spp. This demonstrated that the assay showed high sensitivity and could be a useful tool for routine diagnosis. The results resemble those obtained by Kokuzawa, et al. who used a conventional PCR, which was also based on the 18S rRNA site, to amplify a single oocyst of this protozoan, with the goal of subsequent sequencing and evaluation of genetic variability among oocysts from the same species [13]. The method established in this study is more sensitive than the qPCR established to diagnose Eimeria spp. in sheep, with a sensitivity of 80 copies per μL of fecal DNA extract reported by Yang, et al. [14]. In addition, the established method is also suitable for goats, because the primers were designed from the 18S rRNA gene sequences of Eimeria spp. of both sheep and goats.
Fluorescence-based quantitative PCR is widely used in parasite detection. For example, RT-PCR was used to quantitatively detect the amount of Cryptosporidium oocysts in mouse fecal samples to study the ovulation rule of immunosuppressed and non-immunosuppressed mice infected with Cryptosporidium mouse [26]. A quantitative RT-PCR was established to study the growth inhibition effect of drugs on Cryptosporidium parvum [27].
Conclusion
In this study, the developed qPCR method was used to detect the intensity of coccidia infection in lambs naturally infected with Eimeria. The results were similar to those of routine detection, indicating that the established qPCR can be used to detect the intensity of intestinal coccidia infection in lambs. A plasmid DNA of Eimeria spp. pMD18T-168, was constructed and used as a calibrator to develop a reliable qPCR for quantifying parasite load in biological samples. The method was shown to be highly sensitive, accurate, and reproducible. The qPCR method was successfully applied for quantifying parasite load of Eimeria spp. in lamb feces. The method could be used to evaluate the effect of anticoccidial drugs and growth and reproduction of Coccidia in vitro and in vivo.
Declarations
Acknowledgements
We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Funding
This work was supported by China Agriculture Research System of MOF and MARA (CARS-38)
Disclosure
The authors declare no competing interests.
Data availability
All data generated or analyzed during this study are included in this published article.
Ethics approval
All experimental procedures were reviewed and approved by the Henan Agriculture University Animal Care and Use Committee (license number SCXK (Henan) 2013-0001).
Consent to participate
Not applicable.
Consent for publication
All authors consent to be published.
References
- Bangoura B, Bardsley KD (2020) Ruminant coccidiosis. Vet Clin North Am Food Anim Pract 36: 187-203.
- Balicka-Ramisz A, Ramisz A, Vovk S, Snitynskyj V, (2012) Prevalence of coccidia infection in goats in Western Pomerania (Poland) and West Ukraine region. Ann Parasitol 58: 167-171.
- Cavalcante AC, Teixeira M, Monteiro JP, Lopes CW (2012) Eimeria species in dairy goats in Brazil. Vet Parasitol 183: 356-358.
- Mohamaden WI, Sallam NH, Abouelhassan EM (2018) Prevalence of Eimeria species among sheep and goats in Suez Governorate, Egypt. Int J Vet Sci Med 6: 65-72.
- Saratsis A, Joachim A, Sotiraki S, 2011. Lamb coccidiosis dynamics in different dairy production systems. Vet Parasitol 181: 131-138.
- Diao NC, Zhao B, Chen Y, Wang Q, Chen ZY, et al. (2022) Prevalence of Eimeria Spp. Among Goats in China: A Systematic Review and Meta-Analysis. Front Cell Infect Microbiol 12: 806085.
- Wang CR, Xiao JY, Chen AH, Chen J, Wang Y, et al. (2010). Prevalence of coccidial infection in sheep and goats in north eastern China. Vet Parasitol 174: 213-217.
- Nicole KST, Navarre CB (2018) Coccidiosis in Large and Small Ruminants. Vet Clin North Am Food Anim Pract 34: 201-108.
- Ammar SI, Watson AM, Craig LE, Cope ER, Schaefer JJ, et al. (2019) Eimeria gilruthi-associated abomasitis in a group of ewes. J Vet Diagn Invest 31: 128-132.
- Dittmar K, Mundt HC, Grzonka E, Daugschies A, Bangoura B (2010) Ovine coccidiosis in housed lambs in Saxony-Anhalt (central Germany). Berli Munch Tierarztl Wochenschr 123: 49-57.
- Das M, Laha R, Goswami A, Goswami A (2017) Gastrointestinal parasitism of goats in hilly region of Meghalaya, India. Vet world 10: 81-85.
- Silva LM, Vila-Viçosa MJ, Nunes T, Taubert A, Hermosilla C, et al. (2014) Eimeria infections in goats in Southern Portugal. Rev Bras Parasitol Vet 23: 280-286.
- Kokuzawa T, Ichikawa-Seki M, Itagaki T (2013) Determination of phylogenetic relationships among Eimeria species, which parasitize cattle, on the basis of nuclear 18S rDNA sequence. J Vet Med Sci 75: 1427-1431.
- Yang R, Jacobson C, Gardner G, Carmichael I, Campbell A, et al. (2014). Longitudinal prevalence, oocyst shedding and molecular characterisation of Eimeria species in sheep across four states in Australia. Exp Parasitol 145: 14-21.
- Buckholt MA, Lee JH, Tzipori S (2002) Prevalence of Enterocytozoon bieneusi in swine: an 18-month survey at a slaughterhouse in Massachusetts. Appl Environ Microbiol 68: 2595-2599.
- Jiang J, Alderisio KA, Xiao L (2005) Distribution of cryptosporidium genotypes in storm event water samples from three watersheds in New York. Appl Environ Microbiol 71, 4446-4454.
- Lalle M, Pozio E, Capelli G, Bruschi F, Crotti D, et al. (2005) Genetic heterogeneity at the beta-giardin locus among human and animal isolates of Giardiaduodenalis and identification of potentially zoonotic subgenotypes. Int J Parasitol 35: 207-213.
- Scicluna SM, Tawari B, Clark CG, (2006). DNA barcoding of blastocystis. Protist 157: 77-85.
- Bauer BU, Pomroy WE, Gueydon J, Gannac S, Scott I, et al. (2010) Comparison of the FLOTAC technique with the McMaster method and the Baermann technique to determine counts of Dictyocaulus eckerti L1 and strongylid eggs in faeces of red deer (Cervus elaphus). Parasitol Res 107: 555-560.
- Sandoval E, Morales G, Ybarra N, Barrios M, Borges J (2011) Comparison between two different models of McMaster chambers used for the counting in the diagnosis of nematode infections gastroenteric in ruminants. Zootec. Trop. 29: 495-501.
- Taylor M, Coop RL, Wall RL (2007) Veterinary parasitology. (3rd edn). Hoboken: Blackwell Publishing United States.
- Ogedengbe JD, Hanner RH, Barta JR, (2011) DNA barcoding identifies Eimeria species and contributes to the phylogenetics of coccidian parasites (Eimeriorina, Apicomplexa, Alveolata). Int J Parasitol 41: 843-850.
- Berizi M, Babaie J, Fard-Esfahani P, Enshaeieh M, Noordin R, et al. (2021) Development of a Quantitative Real-Time PCR Assay for Detection of Toxoplasma gondii in Brain Samples. Iran J Parasitol 16: 621-630.
- Nunes BTD, de Mendonça MHR, Simith DB, Moraes AF, Cardoso CC, et al. (2019) Development of RT-qPCR and semi-nested RT-PCR assays for molecular diagnosis of hantavirus pulmonary syndrome. PLoS Negl Trop Dis 13: e0007884.
- Xu MY, Liu SQ, Deng CL, Zhang QY, Zhang B (2016) Detection of Zika virus by SYBR green one-step real-time RT-PCR. J Virol Methods 236: 93-97.
- Huang Y, Song Y, You Y, Mi R, Han X, et al. (2021) Development of an immunocompetent mouse model susceptible to Cryptosporidium tyzzeri infection. Parasite Immunol 43: e12800.
- Zhang H, Zhu G (2020) High-Throughput Screening of Drugs Against the Growth of Cryptosporidium parvum In Vitro by qRT-PCR. Methods Mol Biol 2052: 319-334.
Citation: Li S, Jian Y, Zhang K, Li X, Wang R, et al. (2023) Host Specific Eimeria Genus Diagnosis and qPCR Development in Sheep and Goats. Diagnos Pathol Open S13:004. DOI: 10.4172/2476-2024.8.S13.004
Copyright: © 2023 Li S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 1583
- [From(publication date): 0-2023 - Dec 10, 2025]
- Breakdown by view type
- HTML page views: 1219
- PDF downloads: 364