Host Status of Interspecific Upland Rice Progenies (Oryza sativa à Oryza barthii) to Meloidogyne incognita
Received: 18-Jul-2018 / Accepted Date: 20-Sep-2018 / Published Date: 25-Sep-2018 DOI: 10.4172/2375-4338.1000198
Keywords: Chlorophyll content; Meloidogyne spp; Oryza spp; Wild rice; Yield loss
Introduction
The genus Oryza includes twenty-three wild species and two cultivated species (cultigens) [1]. The wild species are widely distributed in the humid tropics and sub-tropics of Africa, Asia, central and south America and Australia [2]. The two cultivated species are African rice (O. glaberrima Steud ) confined to West Africa whereas common or Asian rice (O. sativa L.) is now commercially grown worldwide where weather and soils permit [3]. One of the wild species is Oryza barthii A. Chev (1914) also known as Africa annual wild rice, is a plant of wetland and a natural weed of lowland rice which is distributed in tropical Africa from Mauritania east to Ethiopia and south to Botswana and Zimbabwe [4]. Brink and Belay [5] suggested that O. sativa may have been introduced to West Africa much earlier than is usually assumed and that O. barthii could be derived from hybridization between O. sativa and O. glaberrima .
One of the main interests for developing new rice lines is for the purpose of selecting for desirable traits to be developed in varieties. One of such traits is resistance to diseases. Diseases such as those caused by nematodes reduce the productivity of the crop in different ecologies that the rice grows. Plant-parasitic nematodes associated with rice include Meloidogyne spp , Hirshmaniella spp . Aphelenchoides besseyi , Heterodera sacchari and Pratylenchus bracyurus [6,7]. Estimated yield reduction caused by the root knot nematode is 20%-98% [8], white tip nematode is 31% [9], 38% by Cyst nematode and 28% by lesion nematode [10].
The use of resistance as a nematode management option remains one of the cheapest methods for resource poor farmers. Progenies of O. galberrima × O. sativa showed variable resistance to the cyst and rice root knot nematode [11]. Several high yielding cultivars of Oryza sativa have been screened, but not enough genetic variability has been found for resistance to Meloidogyne species causing root-knot disease. The objective of this study was to investigate the reaction of the selected rice lines from Oryza sativa × Oryza barthii for resistance to the root-knot nematode, Meloidogyne incognita .
Material and Methods
The experiment was established at the Roof-top Garden of the Department of Crop Protection and Environmental Biology, University of Ibadan, Nigeria. Nematode extraction, counting and estimation of nematodes from both infected roots and soil were carried out at the Nematology Laboratory of the International Institute of Tropical Agriculture (IITA), Ibadan Nigeria.
Soil sterilization
Sandy-loam topsoil collected from the International Institute of Tropical Agriculture (IITA), Ibadan was sterilized for two hours at 90°C using the soil sterilization machine at the Nematology Laboratory, International Institute of Tropical Agriculture (IITA), Ibadan. The soil was allowed to cool for 24 h, filled into sacks and taken to the Roof-top Garden of the Department of Crop Protection and Environmental Biology, University of Ibadan, where it was transferred into 5 l pots.
Source and extraction of inoculum
Galled roots of Celosia argentea infected with root-knot nematodes were collected from the inoculum plots for M. incognita at the University. The inoculum plots were established from a single eggmass of previously identified M. incognita using morphometric characters and PCR product [12]. Nematode eggs were extracted from the harvested galled roots using 0.5% sodium hypochlorite and collected in a beaker [13]. The number of eggs per ml of the suspension was determined by counting under a dissecting microscope from three aliquots of 2 ml each. The quantity of nematodes in the volume of suspension and the quantity of inoculum carrying the required number of nematodes for inoculation was calculated.
Sources and planting of rice lines
Twenty-two interspecific hybrids of Oryza sativa × Oryza barthii upland rice lines produced at the Africa Rice Center (ARC) formerly known as West Africa Rice Development Association (WARDA) (Table 1) and 11 additional cultivars were evaluated in this study. Rice cultivar CG14 was designated as the resistant check for Meloidogne spp. while TGS3 was the susceptible check. The other cultivars are released varieties and were selected based on their popularity for high yield and resistance to other diseases. The abbreviated names will be used in future presentations. Planting was in perforated pots filled with 5 l of sterilized soil at the Roof-top Garden of the Department of Crop Protection and Environmental Biology, University of Ibadan.
| Line number | Line names | Line abbreviation |
|---|---|---|
| 101 | ART-15-16-12-3-1-B-1-B-3-1-B | ART-15-B1 |
| 102 | ART-15-16-31-2-1-1-1-B-1-1-B | ART-15-B2 |
| 103 | ART-15-17-7-8-1-1-1-B-1-1-B | ART-15-B3 |
| 104 | ART-15-19-5-4-1-1-1-B-1-1-B | ART-15-B4 |
| 105 | ART-15-21-2-4-1-B-1-B-1-1-B | ART-15-B5 |
| 106 | ART-15-21-56-2-1-1-1-B-1-1 | ART-15-B6 |
| 107 | ART-15-21-56-2-1-1-1-B-1-1-B | ART-15-B7 |
| 108 | ART-15-21-56-2-1-1-1-B-2-2-B | ART-15-B8 |
| 109 | ART-15-22-10-8-1-B-1-B-2-2-B | ART-15-B9 |
| 110 | ART-15-7-16-38-1-B-2 | ART-15-B10 |
| 111 | ART-16-12-1-28-1-1-1-B-1-1 | ART-16-A1 |
| 112 | ART-16-13-13-2-2-B-1-B-1-1 | ART-16-A2 |
| 113 | ART-16-13-13-2-2-B-1-B-1-2-B | ART-16-A3 |
| 114 | ART-16-14-1-33-2-B-1-B-1-1-B | ART-16-A4 |
| 115 | ART-16-16-11-25-1-B-1-B-1-2 | ART-16-A5 |
| 116 | ART-16-17-14-28-3-2-1-B-1-1 | ART-16-A6 |
| 117 | ART-16-21-5-12-3-1-2-B-1-1 | ART-16-A7 |
| 118 | ART-16-4-2-2-2-B-1-B-1-2 | ART-16-A8 |
| 119 | ART-16-5-10-27-1-B-1-B-1-1 | ART-16-A9 |
| 120 | ART-16-5-9-22-3-B-2-1 | ART-16-A10 |
| 121 | ART-16-9-10-15-4-1-1-B-2-1 | ART-16-A11 |
| 122 | ART3-7L9P8-3-B-B-2-1 | ART3-7 |
| CV1 | BRS PRIMAVERA (O. sativa) | BRS PRIMAVERA |
| CV2 | CG 14R (O. glabarrima) | CG 14 |
| CV 3 | MOROBEREKAN (O. sativa) | MOROBEREKAN |
| CV 4 | NERICA 1 | NERICA 1 |
| CV 5 | NERICA 7 | NERICA 7 |
| CV 6 | NERICA 8 | NERICA 8 |
| CV 7 | TGS25 (O. glabarrima) | TGS25 |
| CV 8 | TGS3 (O. glabarrima) | TGS3 |
| CV 9 | WAB 638-1 (O. sativa) | WAB 638-1 |
| CV 10 | WAB 56-104 S (O. sativa) | WAB 56-104 |
| CV 11 | ARICA 4 | ARICA 4 |
CV 1-11=checks; line number 101-122 are the intraspecific crosses; R=resistant, S=susceptible
Table 1: List of Oryza sativa × O. barthhi interspecific lines and checks used for the experiment.
Screening of rice lines for their reaction to root-knot nematode, Meloidogyne incognita
Three seeds per pot were sown in sterile soil contained in 5 l, watered daily and thinned to two seedlings per pot two weeks after planting. Pots to receive inoculum were inoculated with 5000 eggs of M. incognita in 10 ml water three weeks after planting while the control pots were un-inoculated. The experiment was laid out in a completely randomized design and replicated three times. Data collection commenced immediately after inoculation and the data were taken at weekly intervals for three months. The parameters observed and recorded were; the number of leaves and number of tillers taken by physical count, height of the rice plants by meter rule, chlorophyll content using SPAD-chlorophyll meter.
The experiment was terminated 12 weeks after inoculation by which time the nematode would have reproduced sufficiently. Harvesting of rice was conducted when the soil was moist by cutting off the top growth (aerial plant part), upturning the pot and shaking the root free of soil. Shoots, roots and soil were collected separately and collected in labeled polythene bags and taken to the laboratory for further work. At harvest, number of panicles and number of seeds per plant were taken by physical count.
The roots collected in individual polythene bags were washed free of soil and weighed. The roots were then assessed for damage (galling index) using a 1-5 scale modified from [14] where 1=no galls (healthy roots), 2=≤ 10 % roots galled, 3 11-35%, 4=36%-65%, 5=66%-100% of total roots with galls. The roots were then chopped into 1-2 cm pieces from which nematodes were extracted with 0.5% sodium hypochlorite as previously described. Soil from each pot was thoroughly mixed and 200 cm3 was taken out from which nematodes were extracted using the extraction tray method [15]. The total number of eggs/nematodes recovered from roots and number of nematodes recovered from soil were summed to get the final nematode population (Pf). Nematode reproduction factor (RF) was calculated using Pf/Pi where Pf is the final nematode population and Pi is the initial inoculum. Host status was designated based on Resistant = R ≤ 1, GI ≤ 2, no significant yield loss; Tolerant: R>1, GI ≤ 2, no significant yield loss; Hyper-Susceptible: R ≤ 1, GI 2, significant yield loss; Susceptible: R>1, GI>2, significant yield loss [16].
Data analysis
Data was processed using Microsoft Excel. Data on nematodes count were transformed using square root transformation √(Χ+0.5). Data were analyzed with Analysis of variance (ANOVA) using SAS 9.1 statistical analysis system package and the mean were separated using Least Significant Difference (LSD) at α=5%.
Results and Discussion
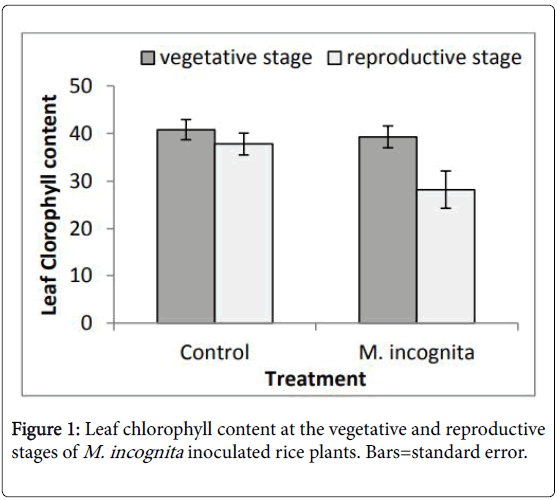
The number of leaves and number of tillers in inoculated plants were significantly (p ≤ 0.05) lower in eight and seven out of the ten ART-15 series lines respectively in comparison to uninoculated plants (Table 2). The number of leaves was reduced significantly in three of the rice lines while the number of tillers was reduced in seven lines of the 11 tested ART-16 series lines. Their reaction was similar to the susceptible check indicating that they may be sensitive to the root-knot nematode. Nematode infection at both low and high initial inoculum densities results in poor plant height, number of tillers, number of panicles and grain yield in rice cultivars [17]. Plant-parasitic nematodes damage roots and the reduced function of the roots influences water and nutrient uptake. The three NERICA cultivars tested also produced fewer numbers of leaves and tillers in inoculated compared to control plants. This was similar to the finding of Akpheokhai and Claudius-Cole [18] where nematode inoculated NERICA varieties showed reduced number of leaves and tillers and in addition were shorter than uninoculated plants. Most of the plants evaluated produced similar number of panicles when comparing inoculated and control plants however, ART-15-B5, ART-16-A4 , BRSPrimavera and WAB56-104 had significantly (p ≤ 0.05) fewer panicles compared to plants that were not inoculated. Chlorophyll content of inoculated plants taken at the vegetative stage of the plants were lower (not significant) compared to the healthy plants but at maturity there was a marked (p ≤ 0.05) reduction in leaf chlorophyll content of inoculated plants. All the ART-15 series lines showed a significant reduction in chlorophyll content in inoculated plants (Figure 1).
| Accessions | No. of leaves | No. of tillers | No. of panicles | |||
|---|---|---|---|---|---|---|
| Control | M. incognita | Control | M. incognita | Control | M. incognita | |
| ARICA 4 | 35.3 | 24.6* | 9 | 7.9* | 6.5 | 7.7 |
| ART-15-B1 | 39.7 | 27.8* | 9.6 | 8.3* | 8.3 | 6.7 |
| ART-15-B2 | 38.3 | 21.9* | 10 | 6.9* | 5.3 | 4.7 |
| ART-15-B3 | 36.8 | 27.2* | 9.6 | 7.9* | 7 | 4.8 |
| ART-15-B4 | 22.6 | 28.6* | 8.1 | 6.3* | 6.5 | 6 |
| ART-15-B5 | 25.6 | 22.3* | 7.9 | 6.4* | 8.7 | 4.3* |
| ART-15-B6 | 28.8 | 21.1* | 8.3 | 6.2* | 5.2 | 7.8 |
| ART-15-B7 | 34.6 | 23.2* | 8.6 | 6.3* | 5.5 | 6.5 |
| ART-15-B8 | 40 | 30.9* | 10.3 | 10.1 | 9.7 | 6.7 |
| ART-15-B9 | 28.4 | 26.5 | 8.3 | 6.9* | 9.2 | 6.7 |
| ART-15-B10 | 31.8 | 29.4 | 10 | 8.4 | 5.8 | 4.8 |
| ART-16-A1 | 30.4 | 32.4 | 10.4 | 10.3 | 6.8 | 7 |
| ART-16-A2 | 33.9 | 29.9 | 8.7 | 8.8 | 7.8 | 5.7 |
| ART-16-A3 | 31.8 | 33.7 | 10.3 | 8.3* | 6.3 | 5.8 |
| ART-16-A4 | 34.3 | 24.5* | 10.1 | 7.4* | 6.5 | 4.2* |
| ART-16-A5 | 19.8 | 22.4 | 5.7 | 6.8 | 5.8 | 4.3 |
| ART-16-A6 | 40 | 28.5* | 11.9 | 8.0* | 6.8 | 5.2 |
| ART-16-A7 | 27.9 | 29.4 | 9 | 8.1 | 5.8 | 5.3 |
| ART-16-A8 | 28 | 22.4* | 8.2 | 6.6* | 4.8 | 5.3 |
| ART-16-A9 | 27.5 | 26.3 | 9.1 | 7.1* | 4.3 | 4.5 |
| ART-16-A10 | 35.4 | 29.4 | 8.4 | 6.8* | 5.3 | 4.7 |
| ART-16-A11 | 26.4 | 21.8 | 8.3 | 6.8* | 5.3 | 4.5 |
| ART3-7 | 25.7 | 21.2 | 6.9 | 6.7 | 6.2 | 4.1 |
| BRS PRIMAVERA | 25.4 | 24.1 | 8.1 | 8.4 | 9 | 5.8* |
| CG 14 | 44.9 | 48.2 | 19.1 | 17.9 | 18 | 14.2 |
| MOROBEREKAN | 19.9 | 17.9 | 6.1 | 5.2 | 6 | 5.2 |
| NERICA 1 | 30.4 | 26.3* | 7.1 | 6.6 | 6.3 | 4.8 |
| NERICA 7 | 32.7 | 25.7* | 7.9 | 6.5* | 8.3 | 5.3 |
| NERICA 8 | 40.7 | 25.9* | 10.9 | 8.0* | 10.7 | 6.5 |
| TGS25 | 67.7 | 55.4 | 19.9 | 17.3 | 16.9 | 14 |
| TGS3 | 75.4 | 54.3* | 19.8 | 14.7* | 18 | 13.7* |
| WAB 56-104 | 29.9 | 27.6 | 9.1 | 8.4* | 6.7 | 6.2 |
| WAB 638-1 | 39.2 | 36.3 | 12.7 | 8.3* | 9.8 | 8.3* |
| LSD | 8.8 | 5.2 | 1.6 | 1.3 | 4.3 | 3.7 |
Values are means of six plants; *=significant difference between inoculated and control plants at p = 0.05 using LSD
Table 2: Effect of Meloidogyne incognita on number of leaves, tillers and panicles of O. sativa × O. barthii accessions.
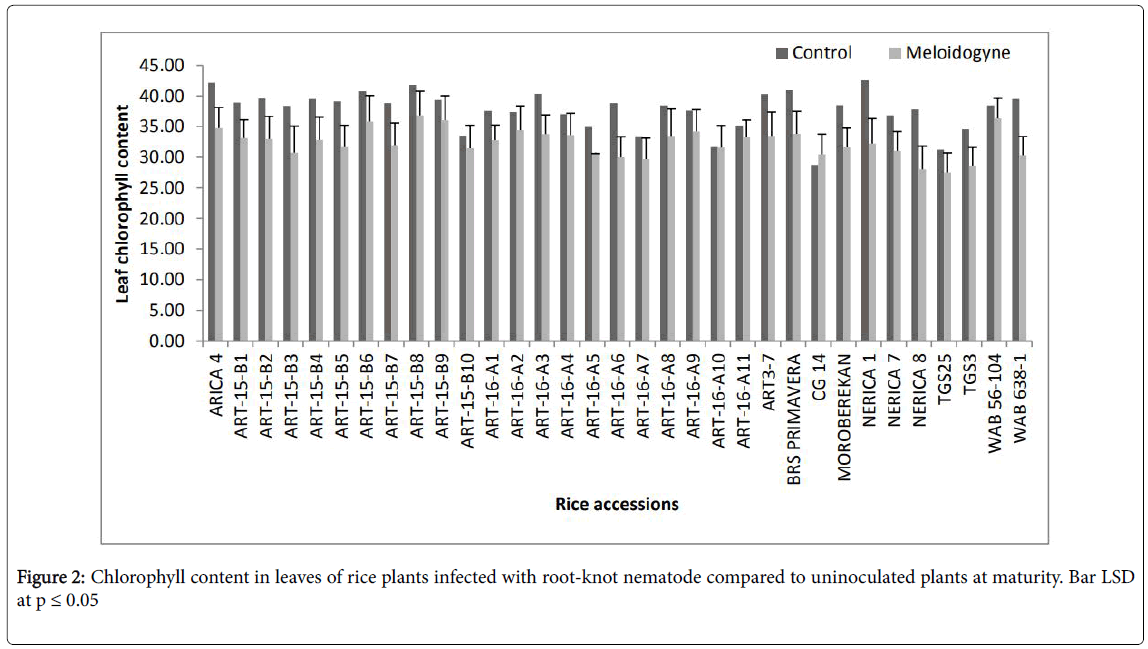
Among the ART-16 series lines, only ART-16-A9 had a nonsignificant amount of reduction in chlorophyll content of leaves and was similar in reaction to the resistant check and WAB 56-104 (Figure 2).
The reduction of chlorophyll in leaves is evidence that the nematode hampered root function and that the gall formation by the nematode is a nutrient sink that draws assimilates from the plant [19]. The consequence of reduced chlorophyll content is that the plant is further unable to sequester assimilates for storage, thus leading to yield reduction.
Generally, the majority (17) of the O. sativa × O. barthi lines produced significantly more grains per panicle and were comparable to the high yielding NERICA and ARICA 4 cultivars (Table 3). This implies that they have good agronomic and yield traits. However, when inoculated with Meloidogyne incognita , number of grains per panicle was reduced significantly (p ≤ 0.05) in all except one (ART-15-B9 ) of the ART-15 series lines. In the ART-16 series lines the reduction in number of grains per panicle was not significant for ART-16-A1 , ART-16-A3 , ART-16-A5 , ART-16-A10 , ART-16-A11 , and ART3-7 . The 100 grain weight was significantly lower in four out of the ten ART-15 series lines and five out of the ART-16 series lines. The 100 grain weight in the susceptible TGS3 check was the lowest among all inoculated lines while ART-16-A3 had the highest 100 seed weight among the inoculated progenies followed by ART-16-A2 , ART-16-A7 , and ART-16-A10 . Line ART-15-B3 when inoculated had among the highest values for 100 grain weight but it was significantly lower than the value from the control plants. Among the cultivars NERICA 7 was among the highest yielding in terms of 100 grain weight irrespective of nematode inoculation.
| Accessions | Number of grains per panicle | 100 grain weight (g) | ||
|---|---|---|---|---|
| Control | M. incognita | Control | M. incognita | |
| ARICA 4 | 110.7 | 83.0* | 2.6 | 2.5 |
| ART-15-B1 | 108.7 | 96.0* | 2.9 | 2.7 |
| ART-15-B2 | 102.3 | 88.3* | 2.8 | 2.2* |
| ART-15-B3 | 82.3 | 56.3* | 3.5 | 2.8* |
| ART-15-B4 | 76 | 64.7* | 2.6 | 2.2* |
| ART-15-B5 | 96.7 | 56.0* | 2.9 | 2.6 |
| ART-15-B6 | 100 | 84.7* | 2.9 | 1.7* |
| ART-15-B7 | 117.7 | 74.3* | 2.9 | 2.5 |
| ART-15-B8 | 121 | 87.3* | 2.8 | 2.5 |
| ART-15-B9 | 102 | 75.7 | 2.8 | 2.4 |
| ART-15-B10 | 70 | 42.3* | 3.7 | 3.1 |
| ART-16-A1 | 88 | 77.3 | 2.8 | 2.6 |
| ART-16-A2 | 114.3 | 71.7* | 2.9 | 2.8 |
| ART-16-A3 | 107 | 77.3 | 3.4 | 2.9 |
| ART-16-A4 | 109.3 | 79.3* | 2.7 | 2.4* |
| ART-16-A5 | 108 | 94.7 | 3.1 | 2.4* |
| ART-16-A6 | 110 | 74.7* | 2.8 | 2.5 |
| ART-16-A7 | 115 | 82.7* | 2.9 | 2.8 |
| ART-16-A8 | 110.7 | 93.3* | 3.3 | 2.6* |
| ART-16-A9 | 113 | 90.7 | 2.7 | 2.1 |
| ART-16-A10 | 135 | 103.7* | 3.2 | 2.8 |
| ART-16-A11 | 87 | 68.3* | 3.1 | 2.5* |
| ART3-7 | 103.3 | 74.3* | 2.9 | 2.6* |
| BRS PRIMAVERA | 144.7 | 79.3* | 2.4 | 2.0* |
| CG 14 | 85.7 | 78 | 3 | 2.5 |
| MOROBEREKAN | 88 | 65.7 | 2.8 | 2.5 |
| NERICA 1 | 101 | 84.0* | 3 | 2.7 |
| NERICA 7 | 83.3 | 62.7* | 3.5 | 3.2 |
| NERICA 8 | 111 | 92.0* | 2.7 | 2.2 |
| TGS25 | 72 | 65.3 | 2.2 | 1.8 |
| TGS3 | 82 | 51.3* | 2.3 | 1.6* |
| WAB 56-104 | 87.7 | 72.7 | 2.5 | 2.4 |
| WAB 638-1 | 96.3 | 79.7* | 2.9 | 2.1* |
| LSD | 28.1 | 24 | 0.6 | 0.5 |
Values are means of six plants; *=significant difference between inoculated and control plants at p = 0.05 using LSD
Table 3: Effect of Meloidogyne incognita on number of grains per panicle and 100 grain weight of 33 rice genotypes.
The highest yielding lines (5) were ART-15-B1, ART-15-B8, ART-15-B9 , ART-16-A2 , and ART-16-A7 , which were in a similar yield range with the high yielding ARIKA, BRS Primavera and the resistant CG14 cultivar (Table 4). The percentage yield reduction was significant in majority (23) of the lines and cultivars evaluated. Significant yield reduction ranged 20%-59% with the highest yield reduction of 59% occurring in ART-15-B6 and ART-15-B10 . Yield was significantly reduced in 22 lines out of the 30 rice lines evaluated [20]. Galls on the rice plants were generally small, with majority occurring at root tips which resulted in reduced root volume. Similar observation was made by Adebayo and Salawu [21].
| Genotype | Yield (grain weight) (g) | Yield Reduction (g) (% Reduction) | Gall index | Total nematode population | Reproductive Factor (RF) | Host Rating | |
|---|---|---|---|---|---|---|---|
| Control | M. incognita | ||||||
| ARICA 4 | 18.3 | 12.9 | 5.4 (29.5)* | 2 | 181.3 | 6.6 | Susceptible |
| ART-15-B1 | 20.8 | 16.8 | 4.0 (19.5)* | 2 | 203.9 | 8.3 | Susceptible |
| ART-15-B2 | 16.6 | 7.6 | 9.0 (54.2)* | 2 | 238.1 | 11.3 | Susceptible |
| ART-15-B3 | 11.9 | 7.3 | 4.6 (38.7)* | 2 | 150.5 | 4.5 | Susceptible |
| ART-15-B4 | 16.9 | 9.8 | 7.1 (42.0)* | 2 | 256.2 | 13.1 | Susceptible |
| ART-15-B5 | 13.8 | 13.2 | 0.6 (4.3) | 2 | 168.3 | 3.7 | Tolerant |
| ART-15-B6 | 13.7 | 5.6 | 8.1 (59.1)* | 3 | 234.5 | 11 | Susceptible |
| ART-15-B7 | 14.5 | 11.8 | 2.7 (18.6) | 2 | 200.5 | 8 | Tolerant |
| ART-15-B8 | 22.3 | 12.2 | 10.1(45.3)* | 3 | 220.6 | 9.7 | Susceptible |
| ART-15-B9 | 18.2 | 9.3 | 8.9 (48.9)* | 2 | 274.4 | 15.1 | Susceptible |
| ART-15-B10 | 16.1 | 6.6 | 9.5 (59.0)* | 3 | 260.7 | 13.6 | Susceptible |
| ART-16-A1 | 10.9 | 9.5 | 1.4 (12.8) | 2 | 228.5 | 10.4 | Tolerant |
| ART-16-A2 | 19.7 | 10.9 | 8.8 (44.7)* | 2 | 291.6 | 17 | Susceptible |
| ART-16-A3 | 12.6 | 8.9 | 3.7 (29.4) | 2 | 277.1 | 15.4 | Tolerant |
| ART-16-A4 | 13.1 | 9.1 | 4.0 (30.5)* | 2 | 203.9 | 8.3 | Susceptible |
| ART-16-A5 | 14.6 | 8.2 | 6.4 (43.8)* | 2 | 150.4 | 4.5 | Susceptible |
| ART-16-A6 | 16.9 | 9.6 | 7.3 (43.2)* | 2 | 205.4 | 8.4 | Susceptible |
| ART-16-A7 | 20.4 | 11.3 | 9.1 (44.6)* | 3 | 121.5 | 3 | Susceptible |
| ART-16-A8 | 9.9 | 9.9 | 0.0 (0.0) | 2 | 107 | 2.8 | Tolerant |
| ART-16-A9 | 9.6 | 8.4 | 1.2 (12.5) | 2 | 159.8 | 5.1 | Tolerant |
| ART-16-A10 | 15.3 | 10.3 | 5.0 (32.7)* | 2 | 234.5 | 11 | Susceptible |
| ART-16-A11 | 16.2 | 9.3 | 6.9 (2.6)* | 2 | 199 | 7.9 | Susceptible |
| ART3-7 | 11.9 | 10.3 | 1.6 (13.4) | 2 | 125.3 | 5.2 | Tolerant |
| BRS PRIMAVERA | 18.1 | 9.8 | 8.3 (45.9)* | 3 | 141.6 | 4 | Susceptible |
| CG 14 | 20.7 | 17.5 | 3.2 (15.5) | 2 | 14.1 | 0.4 | Resistant |
| MOROBEREKAN | 13.1 | 9.5 | 3.6 (27.5)* | 2 | 162.8 | 5.3 | Susceptible |
| NERICA 1 | 11.9 | 10.5 | 1.4 (11.8) | 2 | 204.3 | 8.3 | Tolerant |
| NERICA 7 | 15.8 | 7.9 | 7.9 (50.0)* | 2 | 176.9 | 6.3 | Susceptible |
| NERICA 8 | 16.5 | 7.8 | 8.7 (52.7)* | 3 | 164.1 | 5.4 | Susceptible |
| TGS25 | 12.5 | 11.4 | 1.1 (8.8) | 2 | 119.8 | 2.9 | Tolerant |
| TGS3 | 12.4 | 6.9 | 5.5 (44.5)* | 2 | 190.7 | 7.3 | Susceptible |
| WAB 56-104 | 18.5 | 15.8 | 2.7 (14.6) | 2 | 121 | 3 | Tolerant |
| WAB 638-1 | 13.1 | 9.1 | 4.0 (30.5)* | 2 | 198.6 | 7.8 | Susceptible |
| 8.9 | 6.5 | 16.3 | 2.8 | ||||
Values are means of 6 plants; same letters in a row indicate no significant difference using LSD at P = 0.05; Galling index on a scale of 1-5 with 1=no galls and 5=65-100% galling; Host status based on Resistant=R = 1, GI = 2, no significant yield loss; Tolerant: R>1, GI = 2, no significant yield loss; Hyper-Susceptible: R = 1, GI>2, significant yield loss; Susceptible: R>1, GI>2, significant yield loss; *= significant yield difference
Table 4: Yield, nematode damage and host resistance of the O. sativa × O. barthii progenies inoculated with Meloidogyne incognita .
The number of nematodes were in multiples of the initial inoculum, which is reflected in the reproductive factor (RF) that was more >2 in all genotypes except for the resistant check. The lowest range of RF was 2.8, 3.0, and 30 in WAB 56-104 , ART-16-A8 , and ART-16-A7 respectively. The ability of the lines to permit multiplication of the nematodes indicates that they are good hosts of M. incognita . In a similar study by Adebayo and Salawu [21] and Afolami and Orisajo [22] galling index and reproductive factor were high showing sensitivity of the tested lines to M. incognita . Effective inoculation is reported to result in moderate to high galling severity and high multiplication of Meloidogyne graminicola in susceptible rice and wheat plants [14]. Except for the resistant check, none of the lines and cultivars tested were designated as resistant. Of the 32 cultivars that were compared to the resistant check, 22 were susceptible while 10 were tolerant. This is similar to the findings of Afolami and Orisajo [22] who identified five tolerant lines and 15 susceptible of the twenty lines evaluated. The status of WAB 56-104 in this study is similar to the findings by De Waele et al. [20] where it was categorized as one of the less susceptible cultivars among those evaluated in their study. Tolerance is a good trait for the grower on the short term because the crop is able to produce yield that is not significantly lower compared to those not challenged with the nematode. In the long term however, such crops build up root-knot nematode populations in the soil to a point where the tolerance trait is unable to sustain and the plant becomes susceptible. In addition, such field will have a high nematode load in the soil such that production of any other crop that is susceptible becomes challenging. A preferable trait would be one in which the nematode populations are reduced rather than increased. The promising lines among the crosses are ART 16A8 and ART-16-A9 . The use of WAB 56-105 can also be encouraged in areas with low nematode densities. These lines can also be used as parents for further breeding research for developing resistant lines.
Acknowledgement
The authors appreciate the provision of the upland rice lines and laboratory support received from Africa Rice.
References
- Vaughan DA, Morishma (2003) Biosystematics of the Genus Oryza. In: Smith CW, Dilday RH (eds) Rice: Origin, History, Technology, and Production. John Wiley & Sons, pp 27-66.
- Akimoto M, Shimamoto Y, Morishima H (1999) The extinction of genetic resources of Asian wild rice, Oryza rufipogon Griff.: A case study in Thailand. Genetic Resources and Crop Evolution 46: 419-425.
- Bertin P, Bouharmont J (1997) Use of somaclonal variation and in vitro selection for chilling tolerance improvement in rice. Euphytica 96: 135-142.
- Brink M, Belay G (2006) Plant Resources of Tropical Africa 1. Cereals and Pulses. (PROTA Foundation: Wageningen, The Netherlands; Backhuys Publishers: Leiden, The Netherlands.
- Nayar NM (2012) Evolution of the African Rice: A Historical and Biological Perspective. Crop Sci 52: 505-516.
- Babatola JO (1984) Rice nematode problems in Nigeria: Their occurrence, distribution and pathogenesis. Tropic Pest Manag 30: 256-265.
- Coyne DL, Plowright RA (2004) Nematode Parasites of Rice. In: Luc M, Sikora RA, Bridge J (editors). Plant parasitic nematodes in subtropical and tropical agriculture, CABI.
- Soriano IRS, Prot JC, Matias DM (2000) Expression of Tolerance for Meloidogyne graminicola in Rice Cultivars as Affected by Soil Type and Flooding. J Nematol 32: 309-317.
- Tulek A, Ates SS, Akin K, Surek H, Kaya R, et al. (2014) Determining yield losses in rice cultivars resulting from rice white tip nematode Aphelenchoides besseyi in field condition. Pak J Nematol 32: 149-154.
- Prasad JS, Panwar MS, Rao YS (1987) Nematode problems of rice in India. Tropical Pest Management 33: 127-136.
- Jones M, Nash P, Plowright R, Coyne D (1999) Resistance to the rice nematodes Heterodera sacchari, Meloidogyne graminicola and M. incognita in Oryza glaberrima and O. glaberrima x O. sativa interspecific hybrids. Nematology 1: 745-751.
- Claudius-Cole AO, Muntala A, Fawole B (2017) Characterization of Meloidogyne species and the reaction of tomato Solanum lycopersicum L. cultivars to Meloidogyne incognita and Meloidogyne javanica. J Crop Protect 6:167-179.
- Southey JF (1986) Laboratory Methods for Work with Plant and Soil Nemadodes. Ministry of Agriculture, Fisheries and Food, Her Majesty’s Stationary Office, London, 202.
- Pokharel RR, Duxbury JM, Abawai G (2012) Evaluation of Protocol for Assessing the Reaction of Rice and Wheat Germplasm to Infection by Meloidogyne graminicola. J Nematol 44: 274-283.
- Coyne DL, Nicol JM, Claudius-Cole B (2007) Practical plant nematology: a field and laboratory guide. International Institute of Tropical Agriculture, Cotonou, Benin.
- Afolami SO (2000) Suggestions for improvement of current methods of studying and reporting resistance to root-knot nematodes. International Journal of Nematology 10: 94-100.
- Akpheokhai LI, Udoyong JU (2017) Influence of Heterodera sacchari (Luc And Merny) population densities on growth and yield components and of some interspecific upland rice genotypes. Nigerian J Agri, Food Environ 13: 86-92.
- Akpheokhai LI, Claudius-Cole AO (2017) Effect of Heterodera sacchari on growth and yield of selected upland NERICA rice in Nigeria. Tropic Plant Pathol 42: 431-442.
- Akpheokhai LI, Fawole B, Claudius-Cole AO (2014) Effects of Heterodera sacchari on Leaf Chlorophyll Content and Root Damage of some Upland NERICA Rice Cultivars. IOSR J Agri Vet Sci 7: 49-57.
- De Waele D, Das K, Zhao D, Tiwari RKS, Shrivastava DK, et al. (2013) Host response of rice genotypes to the rice rootknot nematode (Meloidogyne graminicola) under aerobic soil conditions. Arch Phytopathol Plant Prot 46: 670-681.
- Adebayo RA, Salawu EO (2010) Response of Some Selected Rice Varieties to Infestation by a Root-Knot Nematode, Meloidogyne incognita, Chitwood (1949)-SciAlert Responsive Version. Int J Agri Res 5: 453-459.
- Afolami SO, Orisajo SB (2003) Searching for resistance to root-knot nematodes: the case of NERICA upland rice compared with some elite Asian varieties in Nigeria. Nigerian J Plant Prot 20: 25-40.
Citation: Claudius-Cole AO, Salam AO, Mande S (2018) Host Status of Interspecific Upland Rice Progenies (Oryza sativa × Oryza barthii) to Meloidogyne incognita. J Rice Res 6: 198 DOI: 10.4172/2375-4338.1000198
Copyright: © 2018 Claudius-Cole AO, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3892
- [From(publication date): 0-2018 - Oct 05, 2025]
- Breakdown by view type
- HTML page views: 2979
- PDF downloads: 913