Identification of Haplogroups and Molecular Markers in Skeletal Samples Excavated from the Ancient City of ResuloÄlu (UÄurludaÄ, Ãorum)
Received: 23-Apr-2025 / Manuscript No. DPO‑25‑164777 / Editor assigned: 25-Apr-2025 / PreQC No. DPO‑25‑164777 / Reviewed: 09-May-2025 / QC No. DPO‑25‑164777 / Revised: 16-May-2025 / Manuscript No. DPO‑25‑164777 / Accepted Date: 23-May-2025 / Published Date: 23-May-2025
Abstract
Mitochondrial DNA analyses were carried out in order to determine the haplogroups of 6 individuals obtained from the cemetery of Resuloğlu and dated to the Early Bronze Age (EBA III). The individuals to be included in the bioinformatics analysis were evaluated according to their sequence quality and it was decided that 3 individuals could be used in further analysis. Using bioinformatics tools, it was determined that three individuals belonged to the T2e+152, H2a2a2 and JT haplogroups. These results support a link between the origins of the present‑ day European population and the farmers of the Anatolian Neolithic period. Furthermore, a detailed analysis of single nucleotide polymorphisms revealed T16189C and C150T mutations in the two of three individuals, which are associated with the risk of melanoma and cervical cancer‑HPV infection. These molecular findings are consistent with the health profiles of the excavated skeletons, which indicate that the community struggled with infectious and metabolic diseases. The entire study was carried out in the Ancient DNA and Metagenomics Research Laboratory of the Department of Molecular Biology and Genetics, Istanbul University.
Keywords: Bioinformatics analysis; Anatolian neolithic period; Molecular anthropology; Reactive oxygen species; Polymorphism
Introduction
The resuloğlu settlement and cemetery, located northwest of the village of resuloğlu (Kaleboynu) in the uğurludağ district of the province of corum, in the north of Central Anatolia, dates to the second half of the 3rd millennium BC and is one of the rare cemeteries that can be systematically investigated. According to surface finds in the southeast, north and northwest of the cemetery, it is dated to the last period of the Early Bronze Age (EBA III) [1]. Carbon 14 samples taken from different parts of the settlement yielded dates of 2500/2400–2100/2050 BC [2].
Human mitochondrial DNA, a circular double-stranded structure of 16569 nucleotides and 37 genes, encodes 13 protein subunits of the electron transport chain, 2 rRNAs and 22 tRNAs, in addition to a non-coding control region called the D-loop (displacement loop), which is responsible for transcription and regulation of mtDNA (Taanman, n.d.). This region contains three short sequences (HV1, HV2, HV3), the so-called hyper-variable control region (HVR), which show high population-level variation compared to other regions of the genome [3,4]. HV regions, which are used in ancient DNA (aDNA) analysis due to their comparably high levels of polymorphism, form geographic patterns according to the mutations they contain. Groups of gene sequences from a common ancestor with the same SNPs (single nucleotide polymorphisms) are called haplogroups [5].
Mitochondrial DNA (mtDNA), which is easier to obtain than the nuclear genome, is preferred in molecular anthropology studies due to its high copy number in eukaryotic cells [6]. However, the fact that mtDNA shows material inheritance [7], does not undergo
is quite high are the main factors that have led to the widespread use of the mitochondrial genome in aDNA studies [7,8]. In addition, it has long been hypothesized that the functional diversity of mitochondria, which play an important role in energy metabolism, initiation of apoptosis, and generation of Reactive Oxygen Species (ROS), may influence the development and progression of cancer [9]. It is thought that mutations or inherited polymorphisms in mtDNA can alter the encoded protein subunits of respiratory chain complexes, leading to altered ROS production and accelerating a series of events, including impaired respiratory chain activity. Further ROS production activates a vicious cycle of oxidative stress that may play a role in tumor initiation and progression [10–12]. Somatic mtDNA mutations have been found in numerous malignancies, including breast, ovarian, endometrial, prostate, colon, gastric, thyroid, renal, hepatocellular, esophageal, pancreatic and brain tumors [13–15]. In addition, the D-loop region (nucleotides 16024–516) has been identified as a mutational hotspot in human cancers [16,17]. There is strong evidence that genetic instability in the D-loop region plays a role in carcinogenesis by affecting mtDNA copy number and gene expression [18].
In our study, the DNA of tooth samples obtained from skeletons excavated in Resuloğlu (Uğurludağ, Çorum) was isolated and the HV1, HV2 and HV3 regions of the d-loop region of the mitochondrial genome were sequenced and bioinformatics analysis was performed. Using the obtained data, mitochondrial haplogroups were determined with the help of MitoTool and hereditary diseases with possible mitochondrial origin were identified.
Materials And Methods
The aim of this study was to amplify and sequence the HV1, HV2 and HV3 regions of the mtDNA D-loop region extracted from the skeletons excavated in the ancient city of Resuloğlu (Uğurludağ/ Çorum) and to perform molecular analyses based on these sequences.
In this study, the appropriate clothes of the person who would be in the sterile room in the laboratory were sterilized with UV and the study was started using a sterile mask and cap. Subsequently, tooth samples from six different individuals were treated with 100% ethanol for 30 minutes, followed by three washes with pure water. The samples were then exposed to UV light in both directions for a total of 30 minutes. After surface sterilization, the root portions of the teeth were cut with a sterile-tipped dremel and pulverized with liquid nitrogen in a sterile environment. Prior to DNA isolation, a decalcification protocol was used to remove calcium from the powdered samples by treating them with a solution of 0.5M EDTA (pH: 7.5) at a ratio of 10 ml EDTA per 10 g tooth powder. After this process, DNA isolation was performed using the Genomic DNA Isolation Kit (LOT: 0721- OY-1464) from HİBRİGEN, with the amounts of certain solutions in the tissue procedure optimized according to the sample amounts. The concentration and purity of the isolated DNA was determined using Thermo Scientific Nanodrop 2000 spectrophotometer. In addition to the ancient individuals, DNA was also isolated from the researcher who conducted the study in the laboratory for control purposes using the HİBRİGEN Saliva DNA Isolation Kit (LOT: MG-TDNA-01). To accomplish amplification of the entire D-loop region of mitochondrial DNA from the isolated DNA samples, six specific primers were designed using the NCBI Primer Blast online program. The amplified region (amplicon) generated by the primers was determined using the Snapgene program, and the primer sequences were constructed as shown in Table 1. PCR experiments were performed separately for each amplicon according to a modified protocol of Kim et al., based on the Tm temperatures of the primers [19]. The reactions were performed according to the specifications listed as shown in Table 2. The sequencing of the amplified D-loop region amplicons was performed by BM laboratuvar sistemleri (BMLabosis BM Lab. Sist. Ltd. Şti.) using the sanger sequencing method. The resulting Sanger sequence data were analyzed according to quality scores and the poor quality ends of the sequences were trimmed. After trimming, the sequence data were aligned to the d-loop region of human mitochondrial DNA obtained from the NCBI database using the NCBI Blast programme. After alignment, the gaps were filled according to the d-loop region and a continuous sequence was obtained. Haplogroup and molecular marker identification was performed on the processed sequence data following these analyses using the mitomaster software.
| OLIGONAME | 5’-3’ |
|---|---|
| P11F | CCCAAAGCTAAGATTCTAAT |
| P11R | CTTTGGAGTTGCAGTTGATG |
| P21F | CACCCTATTAACCACTCACG |
| P21R | GCTGTGCAGACATTCAATTGTT |
| P22F | TATTTATCGCACCTACGTTCA |
| P22R | CTGGTTAGGCTGGTGTTAGG |
| M14F | ACCCCTCACCCACTAGGATA |
| M14R | GAGGATGGTGGTCAAGGGA |
| M15F | CCTCAGATAGGGGTCCCTTG |
| M15R | GGGAACGTGTGGGCTATTTA |
| M31F | TCTTTTGGCGGTATGCACTTT |
| M31R | GTGTCTTTGGGGTTTGGTTG |
Table 1: List of primers used in PCR.
| Time | Loop | Tempature | |
|---|---|---|---|
| Initial denaturation | 30 seconds | 1 | 94°C |
| Denaturation | 1 minute | 40 | 94°C |
| Annealing | 1 minute | P11 - 55°C-51°C | |
| P21 - 57°C | |||
| P22 - 61°C-55°C | |||
| M14 - 59°C | |||
| M15 - 61°C | |||
| M31 - 58°C | |||
| Extension | 1 minute | 72°C | |
| Final extension | 10 minutes | 1 | 72°C |
Table 2: The performed PCR protocol.
Results
Prior to the determination of haplogroups and molecular markers, the suitability of the raw sequence data obtained for analysis was assessed. After evaluating the usability of the sequence data, it was concluded that only the amplicon sequences obtained with primers P11, M14 and P21 from PCR studies performed with DNA isolated from M68 and M11 individuals could be used. It was also concluded that the amplicon sequences obtained with primers P11, M14, M15, P21 and P31 from the PCR reaction run with the ancient DNA obtained from M196 individual could be used. In the PCR run using DNA isolated from the control individual, the amplicon sequences obtained with primers P11, P13, P22 and M31 were considered suitable for further analysis.
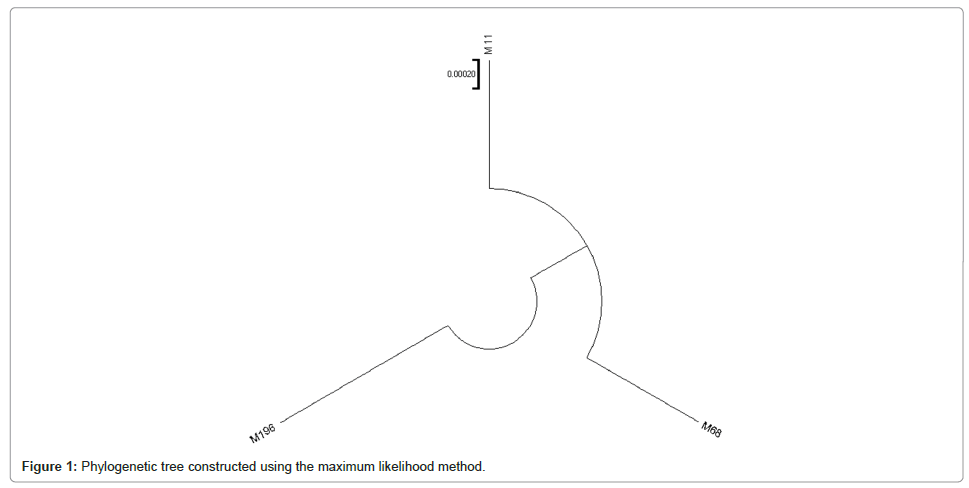
The mitochondrial genome analysis revealed the following haplogroups of the three ancient individuals from six samples: Sample M68 was assigned to haplogroup H2a2a2, M196 to JT and M11 to T2e+152 in Table 3. Three of the six individuals from which raw sequence data were obtained, M7, M17 and M172, were not included in the analysis since the quality scores of the sequence data were very low. The analyses also revealed that the haplogroup of the control individual was H2a2a. In addition to the haplogroups identified, various molecular markers were also detected in individuals as shown in Table 3. To demonstrate the relationships among these ancient individuals, the MEGA11 software was used Maximum Likelihood method is an estimation method that selects the most likely parameters within the statistical model used (Rossi, Richard J. 2018). Phylogenetic tree constructed using the maximum likelihood method as shown in Figure 1.
| Individual | Haplogroup | Variations |
|---|---|---|
| M68 | H2a2a2 | G75GTG(=A73ATG), T152C |
| M196 | JT | A73G, G16049GG, T16126C, |
| T16189C | ||
| M11 | T2e+152 | A73G, C150T, T152C, T16126C, A16254AAAA(=C16251CAAA) |
| Control | H2a2a | A263G, C315CC, G16255GA |
Table 3: Haplogroups and descriptive variations.
Discussion
During the spread of humans from Africa to other continents, rapid changes in mtDNA at the level of small founder populations, founder events, and genetic drift shaped haplotype frequencies, resulting in haplogroups and sub-haplogroups restricted to specific geographic areas and/or populations. Most likely, with the exception of haplogroups U5 and V, all mtDNA haplogroups common in Europe (H, I, J, K, T, U2e, U3, U4, X and W). Originated in the Middle East and it has been suggested that individuals reaching the Middle/Upper Paleolithic after colonization and subsequent settlement 40-45 thousand years ago mixed through Neolithic dispersal or close contact [20,21]. Haplogroup H, which constitutes approximately 40% of the current European population and has a very high frequency in almost the entire distribution area, originated from haplogroup HV just before the last glacial maximum. This group, which is about 25-30 thousand years old, originated in western Asia and spread from there to central Asia, Europe and East Africa [22,23]. H2, the subhaplogroup of haplogroup H, originated in Central Asia and Eastern Europe between 10,300 and 13,300 years ago, then diverged into haplogroup H2a between 8,900 and 12,2000 years ago and then into haplogroup H2a2 between 7,300 and 11,000 years ago. H2a2a1, a subgroup of H2a2, is thought to be 5,000-9,100 years old [24,25]. The T haplogroup is now thought to have diverged from the JT haplogroup in western Asia about 20,500-29,800 years ago and from the J haplogroup about 29,400-39,100 years ago. The T2 haplogroup, a subgroup of the T haplogroup, diverged about 16,800-21,900 years ago. The J haplogroup first diverged into the J1 subhaplogroup between 21,700 and 32,200 years ago and the J1b haplogroup, derived from this subgroup, emerged during the LGM between about 12,800 and 19,700 years ago. It is not yet clear how many years ago J1b7, a subbranch of J1b, emerged. Previous studies have shown that the majority of present-day European ancestry is derived from three main sources: the Mesolithic hunter-gatherer lineage in Europe, the Neolithic lineage in northwestern Anatolia, which is closely related to the emergence of agriculture in Europe, and the steppe lineage, which is a mixture of upper palaeolithic hunter-gatherers in the Caucasus and early farmers in northern Iran [26–31]. However, in 2018, Mathieson I et al. showed that 105 of 215 new individuals reported from Paleolithic, Mesolithic and Eastern European Neolithic contexts are almost exclusively associated with the hunter-gatherer lineage, 98% of the Balkan Neolithic population is associated with the Northwest Anatolian Neolithic, and Greek Neolithic individuals dating to ca. 4000 BC are more closely related to the upper Paleolithic hunter-gatherer-related lineage in the Caucasus than are Northwest Anatolian Neolithic and Balkan Neolithic individuals [32]. Considering the origin of the modern European population and the spread of the most frequent haplogroups H, T, and J from Western Asia to Europe, the presence of these haplogroups in three individuals and the dating and location of the Resuloğlu cemetery support the migration movement from Asia to Europe through the Black Sea during the Neolithic. This finding is consistent with the general understanding of population history and migration patterns during this period.
In addition, further analysis of the variations found in the individuals revealed the presence of T16189C and C150T mutations in two individuals (M196, M11), which have previously been associated with an increased risk of melanoma and cervical cancer-HPV infection. These findings are in line with studies that have examined diseases that leave traces on skeletal remains to determine the health profile of the population. The studies indicate that the community dealt with various infections and metabolic diseases and our findings clearly show that some of these diseases are based on genetic mutations and variations [33].
The T16189C variant in the human mitochondrial DNA control region has been associated with various diseases, including endometrial cancer [34], as well as several other multifactorial diseases [35–37]. The T to C substitution at position 16189 often results in the formation of a continuous poly-C tract between nucleotides 16180 and 16195 within the D-loop region, leading to heteroplasmic length variations of the poly-C tract in different mtDNA molecules [38]. Lial, et al., showed that different poly-C variants show differences in the average mtDNA copy number [39]. Since the 16189 nucleotide is very close to the termination-associated sequence of the D-loop region, it is suggested that the T16189C variant may affect mtDNA replication [10,14]. A case-control study conducted by Ebner et al., [42] in 2011 showed that the T16189C variant has a higher incidence in melanoma patients compared to controls. The C150T variation has been identified in tumor sequences in a number of studies [43,44]. The C150T polymorphism was found to increase the risk of cervical cancer in a study conducted by Zhai K et al. in 2011, which examined D-loop sequence variations in Chinese women, including 142 cervical cancer patients and 136 controls, both HPV-positive and HPV-negative. In addition, HPV-positive individuals were found to be more likely to carry the C150T polymorphism than HPV-negative individuals [45]. However, although the C150T variant has been associated with longevity in several previous studies, the mechanism behind this association remains uncertain [46– 48]. The general perception is that cancer is a disease of the modern era. However, the oldest evidence of hominin cancer was found in the skeleton of Australopithecus sediba, dated to 1.98 million years ago in the Malapa region of South Africa. Cancer cases from ancient times contradict this perception [49,50]. Certain types of tumors can leave their mark on bone [51]. Apart from a case of prostate cancer detected at the protein level in a study by [52], previously reported cases of ancient carcinoma have been identified by morphological observations of the effects of cancer on bones [53–57]. Here we report possible cases of malignancy identified at the molecular level in ancient samples.
Conclusion
This study is one of the first aDNA studies conducted in the region in terms of determining the origins of ancient civilizations and the first detection of malignancy at the molecular level in ancient individuals obtained form the site. It is a groundbreaking study for its encouraging interdisciplinary work in providing supportive data to the fields of archaeology and anthropology.
Acknowledgements
This work was supported by The Scientific and Technological Research Council of Türkiye, TÜBİTAK (Grant Number: 1919B012002870) and Istanbul University Scientific Research Projects Coordination Office (Grant Number: FLO-2021-37908)
Data availability
The data that support the findings of this study are openly available in figshare at https://doi.org/10.6084/m9.figshare.23733588.v1.
References
- Atamtürk D, Duyar İ (2009) Resuloğlu (uğurludağ, Çorum) iskeletlerinin antropolojik analizi. Arkeometri Sonuçları Toplantısı 25:311-328.
- Dardeniz G, Yıldırım T (2022) Metal consumption of a middle-range society in the late 3rd millennium BC Anatolia: A new socioeconomic approach. Plos One 17:e0269189.
[Crossref] [Google Scholar] [PubMed]
- Brandstätter A, Niederstätter H, Parson W (2004). Monitoring the inheritance of heteroplasmy by computer-assisted detection of mixed base calls in the entire human mitochondrial DNA control region. Int J Leg Med. 118: 47-54.
[Crossref] [Google Scholar] [PubMed]
- Krebs JE, Goldstein ES, Kilpatrick ST, Bartlett J (2017) LEWIN’S GENES XII.
- Carelli V, Achilli A, Valentino ML, RengoC, Semino O, et al. (2006) Haplogroup effects and recombination of mitochondrial DNA: Novel clues from the analysis of leber hereditary optic neuropathy pedigrees. Am J Hum Genet 78.
[Crossref] [Google Scholar] [PubMed]
- Pakendorf B, Stoneking M (2005) Mitochondrial DNA and human evolution. Annu Rev Genom Hum Genet 6:165-183.
[Crossref] [Google Scholar] [PubMed]
- Manfredi G, Thyagarajan D, Papadopoulou LC, Pallotti F, Schon EA, et al. (1997) The fate of human sperm-derived mtdna in somatic cells. Am J Hum Genet 61.
[Crossref] [Google Scholar] [PubMed]
- Ingman M, Kaessmann H, Paabo S, Gyllensten U (2000) Mitochondrial genome variation and the origin of modern humans. Nature 408.
[Crossref] [Google Scholar] [PubMed]
- Carew JS, Huang P (2002) Mitochondrial defects in cancer. Mol Cancer 9:1.
[Crossref] [Google Scholar] [PubMed]
- Birch Machin MA (2006) The role of mitochondria in ageing and carcinogeninis. Clin Exp Dermatol 31:548-552.
[Crossref] [Google Scholar] [PubMed]
- Ishikawa K, Takenaga K, Akimoto M, Koshikawa N, Yamaguchi A, et al. (2008) ROS-Generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science 320.
[Crossref] [Google Scholar] [PubMed]
- Modica-Napolitano JS, Kulawiec M, Singh KK (2007) Mitochondria and human cancer. Curr Mol Med 7.
- Chatterjee A, Mambo E, Sidransky D (2006) Mitochondrial DNA mutations in human cancer. Oncogene 25:4663-4674.
[Crossref] [Google Scholar] [PubMed]
- Kulawiec M, Owens KM, Singh KK (2009) Cancer cell mitochondria confer apoptosis resistance and promote metastasis. Cancer Biol Ther 8:1378-1385.
[Crossref] [Google Scholar] [PubMed]
- Penta JS, Johnson FM, Wachsman JT, Copeland WC (2001) Mitochondrial DNA in human malignancy. Mutat Res 488.
[Crossref] [Google Scholar] [PubMed]
- Parsons TJ, Muniec DS, Sullivan K, Woodyatt N, Greiner AR., et al. (1997) A high observed substitution rate in the human mitochondrial DNA control region. Nat Genet 15:4.
[Crossref] [Google Scholar] [PubMed]
- Yoneyama H, Hara T, Kato Y, Yamori T, Matsuura ET, et al. (2005a) Nucleotide sequence variation is frequent in the mitochondrial DNA displacement loop region of individual human tumor cells. Mol Cancer Res 3:14-20.
- Lee HC, Li SH, Lin JC, Wu CC, Yeh DC, et al. (2004) Somatic mutations in the D-loop and decrease in the copy number of mitochondrial DNA in human hepatocellular carcinoma. Mutat Res 547:71-78.
[Crossref] [Google Scholar] [PubMed]
- Kim NY, Lee HY, Park SJ, Yang WI, Shin KJ, et al. (2013). Modified midi- and mini-multiplex pcr systems for mitochondrial DNA control region sequence analysis in degraded samples. J Forensic Sci 58:3.
[Crossref] [Google Scholar] [PubMed]
- Richards M, Macaulay V, Hickey E, Vega E, Sykes B, et al. (2000) Tracing European founder lineages in the near Eastern mtDNA pool. Am J Hum Genet 67:1251-1276.
[Google Scholar] [PubMed]
- Torroni A, Schurr TG, Cabell MF, Brown MD, Neel JV, et al. (1993) Asian affinities and continental radiation of the four founding Native American mtDNAs. Am J Hum Genet 53:563-590.
[Google Scholar] [PubMed]
- Achilli A, Rengo C, Magri C, Battaglia V, Olivieri A, et al. (2004) The molecular dissection of mtDNA haplogroup H confirms that the Franco-cantabrian glacial refuge was a major source for the European gene pool. Am J Hum Genet 75:910-918.
[Crossref] [Google Scholar] [PubMed]
- Roostalu U, Kutuev I, Loogväli EL, Metspalu E, Tambets K, et al. (2007) Origin and expansion of haplogroup h, the dominant human mitochondrial DNA lineage in West Eurasia: The near eastern and caucasian perspective. Mol Biol Evol 24:436-448.
[Crossref] [Google Scholar] [PubMed]
- Behar DM, Harmant C, Manry J, Oven VM, Haak W, et al. (2012) The basque paradigm: Genetic evidence of a maternal continuity in the Franco-Cantabrian Region since Pre-Neolithic times. Am J Hum Genet 90.
[Crossref] [Google Scholar] [PubMed]
- Behar DM, Oven VM, Rosset S, Metspalu M, Loogväli EL, et al. (2012) A “Copernican” reassessment of the human mitochondrial DNA tree from its root. Am J Hum Genet 90.
[Crossref] [Google Scholar] [PubMed]
- Haak W, Lazaridis I, Patterson N, Rohland N, Mallick S, et al. (2015) Massive migration from the steppe was a source for Indo-European languages in Europe. Nature 522:7555.
[Crossref] [Google Scholar] [PubMed]
- Hofmanová Z, Kreutzer S, Hellenthal G, Sell C, Diekmann Y, et al. (2016). Early farmers from across Europe directly descended from Neolithic Aegeans. Proc Natl Acad Sci USA 113.
[Crossref] [Google Scholar] [PubMed]
- Jones ER, Gonzalez-Fortes G, Connell S, Siska V, Eriksson A, et al. (2015) Upper palaeolithic genomes reveal deep roots of modern Eurasians. Nat Commun 6:1.
[Crossref] [Google Scholar] [PubMed]
- Kılınç GM, Omrak A, Özer F, Günther T, Büyükkarakaya AM, et al. (2016) The demographic development of the first farmers in anatolia. Curr Biol 26:19.
[Crossref] [Google Scholar] [PubMed]
- Lazaridis I, Nadel D, Rollefson G, Merrett DC, Rohland N, et al. (2016) Genomic insights into the origin of farming in the ancient Near East. Nature 536:419-424.
[Crossref] [Google Scholar] [PubMed]
- Mathieson I, Lazaridis I, Rohland N, Mallick S, Patterson N, et al. (2015) Genome-wide patterns of selection in 230 ancient Eurasians. Nature 528:499-503.
- Mathieson I, Roodenberg AS, Posth C, Nagy SA, Rohland N, et al. (2018). The genomic history of southeastern Europe. Nature 555:197-203.
[Crossref] [Google Scholar] [PubMed]
- Atamtürk D, Duyar İ (2010) Resuloğlu Erken Tunç Çağı Topluluğunda Ağız ve Diş Sağlığı (Oral Healn the Human Skeletons from the Cemetery of Resuloğlu (Early Bronze Age). J Faculty of Letters Cilt 27:33-52.
- Liu VWS, WangY, Yang HJ, Tsang PCK, Ng TY, et al. (2003) Mitochondrial DNA variant 16189T>C is associated with susceptibility to endometrial cancer. Hum Mutat 22:173-174.
[Crossref] [Google Scholar] [PubMed]
- Khogali SS, Mayosi BM, Beattie JM, McKenna WJ, Watkins H et al. (2001) A common mitochondrial DNA variant associated with susceptibility to dilated cardiomyopathy in two different populations. Lancet 357:9264.
[Crossref] [Google Scholar] [PubMed]
- Weng SW, Liou CW, Lin TK, Wei YH, Lee CF, et al. (2005) Association of mitochondrial deoxyribonucleic acid 16189 variant (T→C Transition) with metabolic syndrome in Chinese adults. J Clin Endocrinol Metab 90:5037-5040.
[Crossref] [Google Scholar] [PubMed]
- Mueller EE, Eder W, Ebner S, Schwaiger E, Santic D, et al. (2011) The mitochondrial T16189C polymorphism is associated with coronary artery disease in middle European populations. PLoS ONE, 6:1.
[Crossref] [Google Scholar] [PubMed]
- Berger C, Grubwieser HP, Hohoff C, Parson W (2011). Evaluating sequence-derived mtDNA length heteroplasmy by amplicon size analysis. Forensic Sci Int Genet 5.
[Crossref] [Google Scholar] [PubMed]
- Liou CW, Lin TK, Chen JB, Tiao MM, Weng SW, et al. (2010) Association between a common mitochondrial DNA D-loop polycytosine variant and alteration of mitochondrial copy number in human peripheral blood cells. J Med Genet 47:11.
[Crossref] [Google Scholar] [PubMed]
- Poulton J (2002) Type 2 diabetes is associated with a common mitochondrial variant: Evidence from a population-based case-control study. Hum Mol Genet 11:13.
[Crossref] [Google Scholar] [PubMed]
- Roberti M, Musicco C, Polosa PL, Milella F, Gadaleta MN, et al. (1998). Multiple protein-binding sites in the TAS-region of human and rat mitochondrial DNA. Biochem Biophys Res Commun 243:36-40.
[Crossref] [Google Scholar] [PubMed]
- Ebner S, Lang R, Mueller EE, Eder W, Oeller M, et al. (2011) Mitochondrial haplogroups, control region polymorphisms and malignant melanoma: A study in middle European caucasians. PLoS ONE 6.
[Crossref] [Google Scholar] [PubMed]
- Chen JZ, Kadlubar FF (2004) Mitochondrial mutagenesis and oxidative stress in human prostate cancer. J Environ Health 22:1-12.
[Crossref] [Google Scholar] [PubMed]
- Yoneyama H, Hara T, Kato Y, Yamori T, Matsuura ET, et al. (2005b). Nucleotide sequence variation is frequent in the mitochondrial DNA displacement loop region of individual human tumor cells. Mol Cancer Res 3:14-20.
- Zhai K, Chang L, Zhang Q, Liu B, Wu Yv, et al (2011). Mitochondrial C150T polymorphism increases the risk of cervical cancer and HPV infection. Mitochondrion 11:559-563.
[Crossref] [Google Scholar] [PubMed]
- MITOMAP. (2009). MITOMAP: A Human Mitochondrial Genome Database. [Crossref] [Google Scholar] [PubMed]
- Santoro A, Salvioli S, Raule N, Capri M, Sevini F, et al. (2006) Mitochondrial DNA involvement in human longevity. Biochim Biophys Acta 1757:1388-1399.
[Crossref] [Google Scholar] [PubMed]
- Zhang J, Asin-Cayuela J, Fish J, Michikawa Y, Bonafé M, et al. (2003). Strikingly higher frequency in centenarians and twins of mtDNA mutation causing remodeling of replication origin in leukocytes. Proc Natl Acad Sci USA 100:1116-1121.
[Crossref] [Google Scholar] [PubMed]
- Lieverse AR, Temple DH, Bazaliiskii VI (2014a) Paleopathological description and diagnosis of metastatic carcinoma in an early bronze age (4588+34 Cal. BP) forager from the Cis-Baikal region of eastern sberia. PLoS ONE 9:12.
[Crossref] [Google Scholar] [PubMed]
- Randolph-Quinney PS, Williams SA, Steyn M, Meyer MR, Smilg JS, et al. (2016) Osteogenic tumour in Australopithecus sediba: Earliest hominin evidence for neoplastic disease. S Afr J Sci 112:1-7.
- Bass WM (1978) Paleopathological diagnosis and interpretation: Bone diseases in ancient human populations. Am J Phys Anthropol 60:31.
- Schultz M, Parzinger H, Posdnjakov DV, Chikisheva TA, Schmidt-Schultz TH, et al. (2007) Oldest known case of metastasizing prostate carcinoma diagnosed in the skeleton of a 2,700-year-old Scythian king from Arzhan (Siberia, Russia). Int J Cancer121:2591-2595.
[Crossref] [Google Scholar] [PubMed]
- Lieverse AR, Temple DH, Bazaliiskii VI (2014b). Paleopathological description and diagnosis of metastatic carcinoma in an early bronze age (4588+34 Cal. BP) forager from the cis-baikal region of eastern siberia. PLoS ONE 9:12.
[Crossref] [Google Scholar] [PubMed]
- Mekik SE, Sekmen B, Yavuz S, Arıcan E, Duyar I, et al. (2023) Identification of haplogroups and molecular markers in skeletal samples excavated from the ancient city of resuloğlu (uğurludağ, çorum) - sequencing chromatogram data. Authorea.
- Modica-Napolitano JS, Kulawiec M, Singh KK (2007) Mitochondria and human cancer. Curr Mol Med 7.
- Rossi Richard J (2018) Mathematical statistics: An introduction to likelihood based inference. New York, John Wiley & Sons. 227. ISBN 978-1-118-77104-77114.
- Taanman JW (1999) The mitochondrial genome: Structure, transcription, translation and replication. Biochim Biophys Acta 1410:103-123.
[Crossref] [Google Scholar] [PubMed]
Citation: Sekmen B, Yavuz S, Mekik SE, Atamtürk D, Duyar I, Arıcan E, et al. (2025) Identification of Haplogroups and Molecular Markers in Skeletal Samples Excavated from the Ancient City of ResuloÄlu (UÄurludaÄ, Ãorum). Diagnos Pathol Open 10:248.
Copyright: © 2025 Sekmen B, Yavuz S, Mekik SE, Atamtürk D, Duyar I, Arıcan E, et al. This is an open‑access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 839
- [From(publication date): 0-0 - Dec 14, 2025]
- Breakdown by view type
- HTML page views: 712
- PDF downloads: 127