Research Article Open Access
IGF-2 Growth Factor Expression in Human Rectal Adenocarcinoma
David Weber1, Daniel Gödde2, Jan Postberg3, Hubert Zirngibl4 and Christian Prinz1*1Department of Internal Medicine, HELIOS Hospital Wuppertal, Wuppertal, 42283, Germany
2Department of Pathology, HELIOS Hospital Wuppertal, Wuppertal, 42283, Germany
3Department of Paediatrics, HELIOS Hospital Wuppertal, Wuppertal, 42283, Germany
4Department of Surgery, HELIOS Hospital Wuppertal, Wuppertal, 42283, Germany
- Corresponding Author:
- Prof. Dr. Christian Prinz
Department of Internal Medicine
HELIOS Hospital Wuppertal, Wuppertal, 42283, Germany
Tel: 0049-202-896-2243
E-mail: christian.prinz@helios-kliniken.de
Received Date: June 20, 2016; Accepted Date: July 01, 2016; Published Date: July 04, 2016
Citation: Weber D, Gödde D, Postberg J, Zirngibl H, Prinz C (2016) IGF-2 Growth Factor Expression in Human Rectal Adenocarcinoma. J Gastrointest Dig Syst 6:451. doi:10.4172/2161-069X.1000451
Copyright: © 2016 Weber D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Background and objective: The study determined growth factor gene expression obtained from patients undergoing curative total mesorectal excision (TME) between 2009 and 2016 at the Helios Klinikum Wuppertal; Germany (total of n=44). Methods: Rectal cancers were grouped according to Lauren’s classification (UICC I-IV stages); overall survival (OS) and progression free survival (PFS). 25 patients were in UICC III/IV stages, while 19 patients were in UICC I/II stages. VEGF-A/B/C/D, PDGF-A, EGF, IGF-1/2, and corresponding receptor subtypes 1/2 mRNA enrichment was determined using quantitative real-time PCR. Data were correlated to clinical outcome. Results: The obtained results show that VEGF-B and IGF-2 mRNA were significantly higher expressed in rectal adenocarcinoma than adjacent normal tissues, while other GF genes were not significantly affected. IGF-2 levels were further upregulated in advanced stages UICC III/IV where patients received radiotherapy. Patients with good response to neoadjuvant radiotherapy (as determined by the histological Dworak regression score) had significant better overall survival. Expression of microRNA miR-483 (a known microRNA molecule previously associated with IGF-2 expression) was evaluated by TaqMAN PCR, but no significant correlation to IGF-2 levels was detected. Conclusions: IGF-2 appears as a biomarker of rectal adenocarcinoma, but does not serve as predictive parameter for tumor progression or survival. The results encourage further development of biomarkers targeting IGF-2 molecules in blood or urine. Furthermore, IGF-2 levels are increased in patients undergoing radiotherapy and thus, IGF related molecules might also serve as predictors for response to neoadjuvant therapy in rectal adenocarcinoma.
Keywords
Rectal cancer; IGF-2; Growth factors; Neoadjuvant therapy
Abbreviations
VEGF: Vascular Epithelial Growth Factor; IGF: Insulin-like Growth Factor; UICC: Union for International Cancer Control; TME: Total Mesorectal Excision; mAB: monoclonal Antibody; MRT: Magnetic Resonance Tomography; CT: Computer Tomography; CRC: Colo Rectal Cancer
Introduction
Rectal cancer is a leading cause of cancer-related death and surgery, i.e. total mesorectal excision with extended lymphadenectomy is the only curative option in locally confined rectal cancer stages [1]. This treatment option is reasonable when the tumour has exceeded the mucosal layer [2]. The implementation of neo-adjuvant therapy in advanced cancer stages reduced the rate of local recurrence rate and sphincter malfunction rate of rectal cancer [3]. Therefore, surgery is immediately done in the case of tumours that are limited to the wall without lymph node invasion (UICC I-II stages), whereas higher stages are usually treated with radiation combined with infusion of 5- fluorouracil (UICC III-IV, each T stage, but N1-3). However, the preoperative evaluation of lymph node infiltration via non-invasive techniques, such as MRT, CT or EUS may not provide an uniquely adequate tool for the prediction of tumour stage In most cases, a final and accurate stage can only be detected postoperatively after tumour removal [4]. Therefore, novel molecular biomarkers and growth factors are of superior clinical interest in order to predict the further course of disease, but also for innovative treatment strategies.
Several growth factors (GFs) may serve as suitable predictive biomarker and target candidates in the resected tumors since elevated expression of many growth factor genes has been found in various cancer tissues. Over the last decade, VEGF-A/-B have been closely related to the progress of colorectal cancer (CRC). Also, vascular endothelial growth factor (VEGF) A and VEGF-B regulate important steps in angiogenesis as well as physiological vascularisation and play a role in gastrointestinal cancers [5]. VEGF-A expression has been shown to be of special importance for neovascularisation in rectal cancer [6]. Also, the VEGFR-1 expression rate gastrointestinal cancer patients is supposed to indicate a high risk group for metastasis [7]. Since neovascularisation may be a novel target to treat advanced tumour stages using monoclonal VEGF-A antibodies (anti-VEGF-A mAb) [8], one anti-VEGF-A mAb, Bevacizumab, has been approved for therapy of CRC in 2005 [9]. two recent studies, however, have pointed out that drugs inhibiting the VEGF pathway promote tumour invasiveness and metastasis in mice [10].
Interestingly, recent data shifted insulin-like growth factors (IGFs) into the attention of researchers studying the pathogenesis of rectal cancer. The insulin-like growth factor receptor 1 (IGF-R1) signalling pathway plays a key role in cell survival and transformation [11]. IGFR1 is primarily activated by it endogenous ligands IGF-1 and IGF-2. Activation of IGF-R1 has been shown in several cancers in mouse models [12]. Also, IGF-2 has been related to human rectal adenocarcinoma in a large comprehensive molecular screening assay [13]. As demonstrated in this extensive work, IGF-2 is among the most common gene copy-number amplifications in CRC. This copy-number alteration was found in 7% of the investigated tumours, and was associated with gain of a 100-150 kb region of chromosome 11p15.5 [13].
The current work includes 44 rectal cancer patients, who gave informed consent in order to determine gene expression of several growth factors in surgical specimens. We compared the expression in tumour tissue versus adjacent normal tissue. We aimed to determine the expression of several growth factor mRNAs by quantitative realtime PCR on cDNA libraries obtained by reverse transcription from total RNA isolated from human rectal adenocarcinoma and evaluated their relative expression levels with respect to tumour staging, treatment and clinical outcome in an observation period of 2009-2016. Our results indicate that IGF-2 appears as a biomarker of rectal adenocarcinoma, but does not serve as predictive parameter for tumor progression or survival.
Materials and Methods
Study population and tissues
Rectal cancer tissues were obtained from patients (n=44) who underwent curative surgery between 2009 and 2016 at the Helios Universitätsklinikum Wuppertal, Germany. Formalin-fixed paraffinembedded or fresh tissue samples were investigated after surgery following informed patients consent. Vote of the ethical committee was given by the University of Witten, Germany.
Tissue preparation, RNA extraction and cDNA synthesis by reverse transcription-PCR
Under RNAse-free conditions, formalin-fixed paraffin-embedded (FFPE) tissue samples were sectioned into 10 μm slices. Sections were deparaffinized, rehydrated and stained with haematoxylin. Prior to the extraction of total RNA or genomic DNA tumour or normal mucosa of each section were separated by microdissection. Tissue amounts per slide ranged between 50 mm2 and 300 mm2. Material from small tumour areas was pooled from additional serial sections used. Scrapedoff tissue was deparaffinised with xylene (Sigma-Aldrich) and total RNA and DNA were extracted from the same samples using the Allprep DNA/RNA FFPE Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The RNA was transcribed into cDNA using the Quantitect reverse transcription Kit (Qiagen, Hilden, Germany).
Quantitative RT-PCR
Quantitative real-time PCR (qPCR) was performed using the Quantitect SYBR Green PCR Kit (Qiagen, Hilden, Germany). Primer pairs were designed to span exon junctions (Table 2). Reaction conditions were 15 min at 95°C, then 40 cycles [95°C for 15 sec, 60°C for 30 sec, and 72°C for 60 sec] followed by a temperature gradient from 55°C to 95°C with incremental (per 1°C) measurement of fluorescence for melting curve analyses. All reactions were performed at least as triplicates. For normalization six housekeeping genes were used (β-Actin, GAPDH, OAZ-1, ATP-6, RPL-19, RPL-27). All primers are listed in Table 1. Fold changes for each mRNA analysed were calculated using the delta-delta-Ct method.
| VEGF-A | |
| Forward Primer | 5’-ctc cac cat gcc aag tgg tc-3’ |
| Reverse Primer | 5’-gca gta gct gcg ctg ata ga-3’ |
| VEGF-B | |
| Forward Primer | 5’-ccc tgt ctc cca gcc tga t-3’ |
| Reverse Primer | 5’-cgc gag tat aca cat cta tcc atg a-3’ |
| VEGF-C | |
| Forward Primer | 5’-agg aaa gaa gtt cca cca cca a-3’ |
| Reverse Primer | 5’-gcc ttc tgg cgg ttc gt-3’ |
| VEGF-D | |
| Forward Primer | 5’-aga tcg ctg ttc cca ttc ca-3’ |
| Reverse Primer | 5’-cct gga gat gag agt ggt ctt c-3’ |
| VEGFR-1 | |
| Forward Primer | 5’-tca ctg cca ctc taa ttg tca atg t-3’ |
| Reverse Primer | 5’-aaa cga tga cac ggc ctt tt-3’ |
| VEGFR-2 | |
| Forward Primer | 5’-caa gac agg aag acc aag aaa aga c-3’ |
| Reverse Primer | 5’-ggt gcc aca cgc tct agg a-3’ |
| VEGFR-3 | |
| Forward Primer | 5’-gct gag acc cgt ggt tcc t-3’ |
| Reverse Primer | 5’-cta tgc ctg ctc tct atc tgc tca a-3’ |
| EGF | |
| Forward Primer | 5’-aga ttg ggc tat gcc atc agt -3’ |
| Reverse Primer | 5’-atc tgc tcc tgg ttt tgc ca-3’ |
| EGFR | |
| Forward Primer | 5’-ctt gcc gca aag tgt gta ac-3’ |
| Reverse Primer | 5’-tga tgg agg tgc agt ttt tg-3’ |
| IGF-1 | |
| Forward Primer | 5’-ttt caa caa gcc cac agg gt-3’ |
| Reverse Primer | 5’-ttg agg ggt gcg caa tac at-3’ |
| IGF-2 | |
| Forward Primer | 5’-ctc ctg gag acg tac tgt gc-3’ |
| Reverse Primer | 5’-ctg ggg aag ttg tcc gga ag-3’ |
| IGFR-1 | |
| Forward Primer | 5’-agt cct tcg ctt cgt cat gg-3’ |
| Reverse Primer | 5’-gcg cat cag ttc aaa cag ca-3’ |
| IGFR-2 | |
| Forward Primer | 5’-ccc agg cag ggt ttt ctt tt-3’ |
| Reverse Primer | 5’-acc ggg cca cac aca ttt a-3’ |
| PDGF-A | |
| Forward Primer | 5’-cat gtt ctg gcc gag gaa gc-3’ |
| Reverse Primer | 5’-ccg gat gct gtg gat ctg ac-3’ |
Table 1: PCR Primer sequences used to amplify the signals for different growth factors in tissue specimens of human rectal adenocarcinoma and normal rectal tissues.
| Patient | Dworak | IGF-2 FC |
| 1 | 0 | 1,88 |
| 2 | 0 | 0,14 |
| 3 | 1 | 1,43 |
| 4 | 1 | 37,87 |
| 5 | 1 | - |
| 6 | 1 | 6,1 |
| 7 | 1 | 3,18 |
| 8 | 1 | 41,83 |
| 9 | 2 | 0,29 |
| 10 | 2 | 1,06 |
| 11 | 2 | 1,95 |
| 12 | 2 | 9,63 |
| 13 | 2 | 52,2 |
| 14 | 2 | 2,59 |
| 15 | 2 | 1,5 |
| 16 | 2 | 128,3 |
| 17 | 2 | 6,99 |
| 18 | 2 | 24,2 |
| 19 | 2 | 5,15 |
| 20 | 3 | 0,73 |
| 21 | 3 | 17,83 |
| 22 | 3 | 16,37 |
| 23 | 3 | 2,7 |
| 24 | 4 | 33,66 |
Table 2: Histological regression scores in patients treated with neoadjuvant radiotherapy in advanced UICC stages, as determined by the Dworak regression score. In parallel, IGF-2 levels in the two different groups were determined and showed increasing values.
Immunohistochemistry
Protein expression of Insulin-like Growth Factor 2 (IGF2, Somatomedian A) was assessed by immunohistochemistry using the DAKO Autostainer plus (DakoCytomation) following the manufacturer´s instructions. 3-5 μm sections of formalin-fixed, paraffin-embedded tissue were dried overnight by 37°C and deparaffinized. Antigen retrieval procedure was performed using the Target Retrieval Solution (Citrate pH 6,0, 10X, DakoCytomation, cat.no. S2369) after which the slides were steamed for 30 min. Endogenous peroxidases were blocked by incubation with Peroxidase- Blocking Solution for 5 min (DAKO REAL TM Peroxidase-Blocking Solution, cat.no. S2023).
The specimen were incubated for 30 min with IGF2-specific primary antibody (Anit-IGF2 , HPA 007993, polyclonal, rabbit, dilution 1:100) followed by subsequent incubations with a visualization-reagent (HIDef Detection TM HRP Polymer System, CELL MARQUE, cat.no. 954D-20). This System includes an antibody-enzyme complex that universally detects mouse and rabbit primary antibodies. The resulting chromogenic reaction is visualized by HRP-compatible chromogens (DAB) (DAB + Chromogen, DAKO, cat.no. K3468) and results in formation of a visible brown reaction product at the antigen site. Finally the slides were counterstained with Mayer´s Hematoxylin for 2 min and coverslipped.
MicroRNA analysis
Quantitative TaqMan real-time PCR was performed with the Rotor- Gene 6000 realtime rotary analyser (Quiagen, Hilden, Germany). MicroRNA expression was assessed using the TaqMan® MicroRNA Assays from Applied Biosystems (Assay IDs: U6 snRNA: 001973, hsamiR- 483-5p: 002338, hsa-miR-483-3p: 002339) following the manufacturer’s instructions. Total RNA was extracted from fresh tissue samples using QIAzol Lysis Reagent (Quiagen, Hilden, Germany) and RNA was transcribed to cDNA using the TaqMan® MicroRNA Reverse Transcription Kit (Applied Biosystems, Cat. # 4366597). A mastermix was prepared on ice using the TaqMan® Fast Universal PCR Master Mix (2X), no AmpErase® UNG (Applied Biosystems, Cat. # 4352042) according to the manufacturer’s instructions. Thermal cycling conditions were initial 10 min at 95°C to activate the AmpliTaq® Fast DNA Polymerase followed by 40 cycles of 95°C for 15 s and 60°C for 60 s.
Statistical analysis
Results are presented as mean ± SEM. The students t-test was used to compare the differences among expression leves of various growth factors in tumors vs. normal adjacent tissue. The Gehan-Breslow- Wilcoxon rank sum test (two groups) was used to compare the different groups when comparing overall survival and progression free survival.
Results
Expression of the growth factors VEGF-A, VEGF-B, VEGF-C and -D, EGF, PDGF-A, IGF-1/2 and corresponding receptors in human rectal adenocarcinoma in different tumor stages (UICC I/II vs UICC III/IV) vs. normal adjacent tissue
Patients’ characteristics are as follows: the average age at surgery was 64,98 +/- 13,57 years; and the range 31-84 years. Average age at diagnose was 64,45 years. 25 patients were male patients, 19 were female. 25 obtained neoadjuvant therapy in UICC stages III and IV, the others (n=19) were not pretreated in stages UICC I and II.
Rectal cancer tissues (n=44) were obtained from patients who underwent curative surgery. Tumours were grouped according to Lauren’s UICC classification and to the pathohistological TNM-stage. Copy numbers of each gene were normalized to 106 house keeping gene copies (incl. β-Actin, GAPDH, OAZ-1, ATP-6, RPL-19, RPL-27), and relative expression of tumor vs. normal tissue is shown in a logarithmic manner. Growth factor and corresponding receptor mRNA levels were determined in rectal cancer tissue using qPCR, and fold changes of each gene were determined after normalization to the six housekeeping genes. Expression of growth factors in patients was determined in patients receiving surgery immediately (UICC I/II, n=19) or patients receiving neoadjuvant protocols and subsequently operated (UICC III/IV patients, n=25).
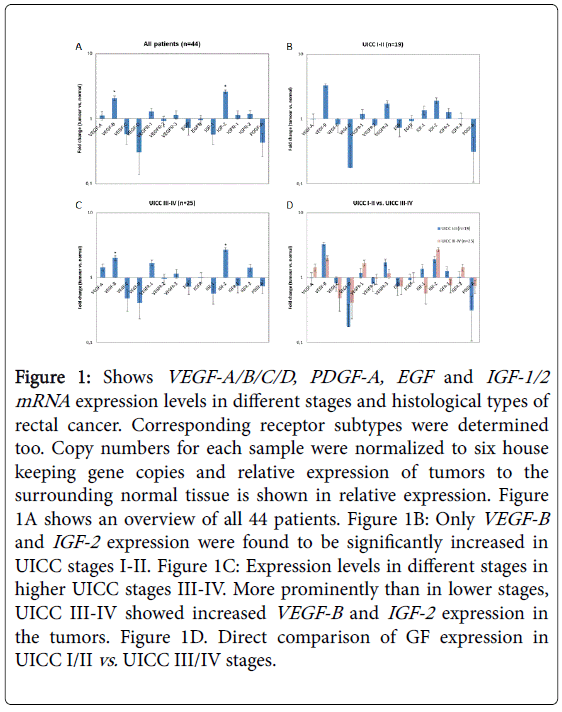
Figure 1A shows the relative VEGF/A-D, PDGF-A, EGF-1/2 or IGF-1/2 expression in all n=44 patients at time point zero in the resected surgical specimens. Corresponding VEGF-receptors, EGFreceptors, and IGF-R1/2 receptor mRNA levels were also determined in rectal cancer tissue and an overview of the results in given in Figure 1A.
Figure 1: Shows VEGF-A/B/C/D, PDGF-A, EGF and IGF-1/2 mRNA expression levels in different stages and histological types of rectal cancer. Corresponding receptor subtypes were determined too. Copy numbers for each sample were normalized to six house keeping gene copies and relative expression of tumors to the surrounding normal tissue is shown in relative expression. Figure 1A shows an overview of all 44 patients. Figure 1B: Only VEGF-B and IGF-2 expression were found to be significantly increased in UICC stages I-II. Figure 1C: Expression levels in different stages in higher UICC stages III-IV. More prominently than in lower stages, UICC III-IV showed increased VEGF-B and IGF-2 expression in the tumors. Figure 1D. Direct comparison of GF expression in UICC I/II vs. UICC III/IV stages.
Figures 1B and 1C present VEGF-A/B/C/D, EGF and IGF-1/2 mRNA expression levels in different stages and histological types of rectal cancer. Figure 1B shows the relative GF expression in patients at time point zero in the resected surgical specimens without previous treatment (UICC I-II). VEGF-B and IGF-2 expression were found to be significantly increased in UICC stages I-II (p=0.05 using students unpaired t-test). As shown in Figure 1C, expression levels in advanced cancer stages were also determined in higher UICC stages III-IV in patients that were treated with neoadjuvant radiochemotherapy.
More prominently than in lower stages, we found that in UICC IIIIV patients the VEGF-A and VEGF-R1 receptors were slightly, but not significantly increased (Figure 1C). More prominently, VEGF-B and IGF-2 expression was found to be highly significantly increased when comparing UICC I/II vs. III/IV stages (p=0.01 using the paired students t test). In Figure 1D, a direct comparison of low vs. high stages is presented. VEGF-C , VEGF-D as well as EGF and PDGF-A were not significantly increased in advanced tumor stages and did not reveal statistically significant differences among the two groups (using students t-tests).
IGF-2 production in tumor cells
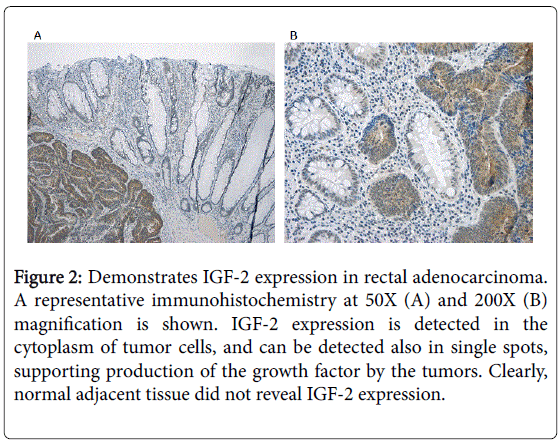
Figure 2 demonstrates IGF-2 expression in tumor cells rectal adenocarcinoma, as investigated by immunocytochemistry. A representative immunohistochemistry at 50X (A) and 200X (B) magnification is shown. IGF-2 expression is detected in the cytoplasm of tumor cells, and can be detected also in single spots, supporting production of the growth factor by the tumors. Normal adjacent tissue did not reveal IGF-2 expression.
Figure 2: Demonstrates IGF-2 expression in rectal adenocarcinoma. A representative immunohistochemistry at 50X (A) and 200X (B) magnification is shown. IGF-2 expression is detected in the cytoplasm of tumor cells, and can be detected also in single spots, supporting production of the growth factor by the tumors. Clearly, normal adjacent tissue did not reveal IGF-2 expression.
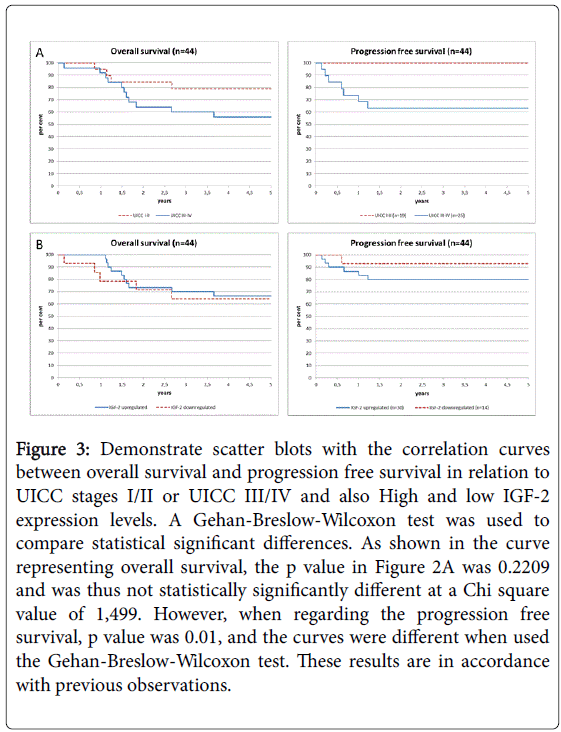
Overall survival (OS) depending on UICC stage (3A) and IGF-2 expression (3B).
As shown in Figure 3, OS and PFS were determined in regard to clinical cancer stages (3A) and IGF-2 levels (3B). As shown in Figure 3A, OS and progression free survival were decreased in patients with UICC stages III-IV, as expected (p=0.01 using the Chi-square or Gehan-Breslow-Wilcoxon test). As shown in Figure 3B, we found that an elevated expression of IGF-2 (compared to normal tissues at logarithmical scales above or below 1) seemed to predict a worse prognosis and early relapse. Overall survival at 5 years was not significantly affect. However, as demonstrated in the Kaplan Meier analysis, increased expression of IGF-2 was associated with early relapse and decreased progression free survival. 6 patients had a relapse in the patient group with IGF-2 expression (n=30), while only one a relapse in the low expression group (n=14). Due to the small and differing patient numbers in the two groups, this difference was not statistically significant different (p=0.40). Since no difference was observed regarding the overall survival and PFS, these data do not support a critical role of IGF-2 for further survival and metastasis.
Figure 3: Demonstrate scatter blots with the correlation curves between overall survival and progression free survival in relation to UICC stages I/II or UICC III/IV and also High and low IGF-2 expression levels. A Gehan-Breslow-Wilcoxon test was used to compare statistical significant differences. As shown in the curve representing overall survival, the p value in Figure 2A was 0.2209 and was thus not statistically significantly different at a Chi square value of 1,499. However, when regarding the progression free survival, p value was 0.01, and the curves were different when used the Gehan-Breslow-Wilcoxon test. These results are in ccordance with previous observations.
MicroRNA miR-483 expression and correlation with IGF-2 expression
The cancer genome study of CRC previously found a focal amplification of the IGF-2 gene in 7% of the studied tumors [13]. The study also reported an increased expression of the microRNA miR-483. MicroRNA molecules are 20-22 bp short RNA molecules that are considered to play an important regulatory role in cancer and may serve as predictors of rectal cancer not only in tumors, but also blood samples of patients.
Thus, we investigated expression of the microRNA in correlation with IGF-2. In identical groups, RNA was extracted and expression of miR-483 was determined by TaqMan.
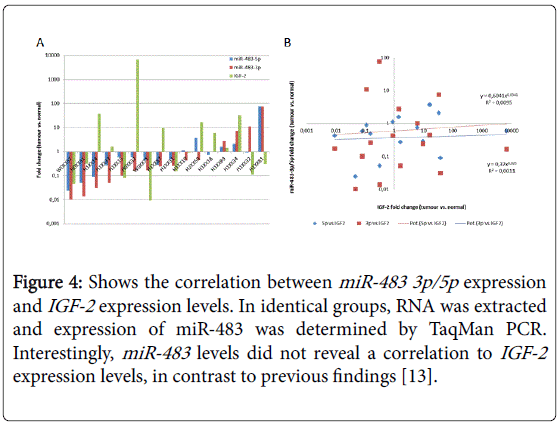
Figure 4 shows the correlation between miR-483 3p/5p expression and IGF-2 expression levels as determined by TaqMan PCR. Interestingly, miR-483 levels did not reveal a correlation to IGF-2 expression levels, in contrast to previous findings [13].
Figure 4: Shows the correlation between miR-483 3p/5p expression and IGF-2 expression levels. In identical groups, RNA was extracted and expression of miR-483 was determined by TaqMan PCR. Interestingly, miR-483 levels did not reveal a correlation to IGF-2 expression levels, in contrast to previous findings [13].
IGF-2 levels in patients with neoadjuvant radiotherapy
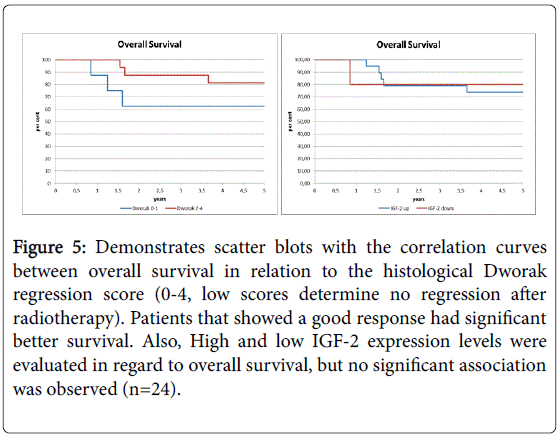
In 24 patients treated with neoadjuvant radiochemotherapy, histological response to radiation was determined and histological response rate was determined by a pathological score. Survival curves are shown in Figure 5. As expected, survival was worse in patients that did not respond to radiotherapy as determined by the histological Dworak regression score. In 6 patients, no response was seen in histological since tumor cells were highly present. In 18 patients, a clear response to radiation was observed (Figure 5 and Table 2). Next, IGF-2 levels were compared among the different groups. IGF-2 levels increased in patients that showed a good histological regression as determined by higher Dworak scores.
Figure 5: Demonstrates scatter blots with the correlation curves between overall survival in relation to the histological Dworak regression score (0-4, low scores determine no regression after radiotherapy). Patients that showed a good response had significant better survival. Also, High and low IGF-2 expression levels were evaluated in regard to overall survival, but no significant association was observed (n=24).
Discussion
Colon and rectal cancers comprise the third-most common cancers in Europe and in the United States. The 5-year survival rate for the 150,000 individuals diagnosed each year with CRC is 65.2% for all stages, but drops to 11.3% in those patients with metastatic disease. Introduction of neoadjuvant therapy and growth factor (GF)-related drugs for the treatment of metastatic CRC has improved the outcome significantly. Treatments of metastatic CRC, which are currently under approval, include VEGF-targeting compounds or anti-epidermal growth factor receptor (EGFR) mAbs, which may be used for first-, second- or third-line treatment in patients with metastatic disease [14]. K-ras mutations have been shown being important predictors for the outcome in EGF-related treatment. A predictive parameter related to the (other) growth factors, however, is still missing. Therefore, we investigated the relative expression of GF and GF-receptor mRNAs in rectal cancer tissue. We used FFPE tumour specimens (obtained through surgery) from different stages of rectal cancer in order to evaluate the importance of EGF-independent factor during further tumour invasion and lymph node metastasis.
First, being an overall “snapshot”, the current study did not detect significant alterations in the levels of EGF, VEGF-C/D, PDGF-A, as well as the corresponding receptors in rectal cancers (Figure 1A, overview of all tumors). Our data reveal an unchanged expression pattern of these factors in more advanced tumour stages and may thus limit the potential use of EGF mRNA levels as predictive biomarkers or EGF-related mABs for use in modern therapy of rectal cancer. This differential expression, however, may be explained by the diversity of the tumours. Gene expression profiles of the patients at different stages in each patient were not available here, but-in line with our data- other authors have presented data in which a decreased growth factor expression in advanced stages was observed [15,16]. Our observation, however, is in line with these last data, and may be explained by the observation that tumor progress may be associated with genetic instability, loss of genes. Also, appears that certain factors may have crucial importance at different time points of progression, for example during early lymphangiogenesis as reported for VEGF-C and VEGF-D.
Interestingly, we found that VEGF-B expression was increased in early and advanced tumour stages compared to the adjacent tissue at the time point of surgery. These new data thus suggest new implications of potential high clinical importance. One explanation for this finding is that VEGF-B might be mainly produced by the tumour cells themselves. VEGF-B appeared to be downregulated in advanced stages. This observation may be explained by the fact that although a progress of tumour cell mass would occur, there could be reduced expression or production of this growth factor, for example due to a loss of differentiation and chromosomal instability, leading to a generally reduced gene expression profile. It has been shown that VEGF-B is expressed in almost every kind of solid tumour and has been associated with progression and survival in different types of cancer [17-21]. Thus, this finding supports previous findings, but underlines the important role of VEGF-B in this tumor entity.
Most importantly, we report a prominent elevation of IGF-2 expression in early and advanced cancer stages. We found increased expression of IGF-2 on a very high level in tumour tissues versus very low levels in adjacent normal tissues up at 100-fold expression. Thus, IGF-2 mRNA represents a novel biomarker that is clearly up-regulated in the rectal tumour tissue. A predictive role for survival, however, was not detected, limiting the value of this marker for the further prognosis.
Our data are in accordance with the previous comprehensive molecular characterisation on CRC, revealing a special role for IGF-2 in CRC [13]. The cancer genome study of CRC previously found a focal amplification of the IGF-2 gene in 7% of the studied tumors, the most common amplification observed in that study and was associated with gain of a 100–150 kb region of chromosome 11p15.5 [13]. The authors specifically report an elevated expression of IGF-2 but not of Insulin and Tyrosine hydroxylase (TH). Chromosome 11 contains the genes encoding insulin (INS), insulin-like growth factor 2 (IGF-2), and tyrosine hydroxylase (TH). IGF2 overexpression through loss of imprinting has been implicated in the promotion of CRC [22,23]. In this group, a subset of tumours (15%) without IGF-2 amplification also had dramatically higher levels (as much as 100X) of IGF-2 gene expression, an effect not attributable to methylation changes at the IGF-2 promoter.
Towards understanding the IGF system during cancer growth and progression, progressive prostate cancer models, such as SV40 large T antigen immortalized human prostate epithelial cells (P69, M2182, M2205, and M12) and LNCaP sublines (C4, C4-2, and C4-2B4), have been used previously. As shown in these studies [24], IGF-2 mRNA levels progressively increase as prostate cancer cells become more tumorigenic and metastatic, suggesting that IGF-2 contributes in part to cancer progression. Retroviral mediated transient expression of IGF-2-specific ribozyme (RZ) caused extensive cell death. In stably cloned cell lines, both RZ and mutant ribozyme (MRZ) inhibited cancer cell growth, suggesting that inhibition of apoptosis through elevated IGF-2 levels may be relevant for cell growth.
IGF-2 and associated molecules, however, may play a role for the detection of rectal cancer in blood ahead of the tumor tissue, as other reports have demonstrated. Recent data suggest data hypo- or hypermethylation of the IGF-2 promoter sequences in conditions of high alcohol consumption could play a role for colorectal cancer, since alcohol seems act as a methyl group antagonist. Nutritional studies, have not yet delivered a clear conclusion in this context, but underline the role of IGF-2 in colorectal cancer too [25].
The cancer genome study [13] also reported an increased expression of the microRNA miR-483 in close correlation to IGF-2 expression. MicroRNA molecules are 20-22 bp short RNA molecules that are considered to play an important regulatory role in cancer. Thus, we investigated expression of the microRNA in correlation with IGF-2. We therefore aimed to detect a correlation between miR-483 3p/5p expression and IGF-2 expression levels as determined by TaqMan PCR. In identical groups, RNA was extracted and expression of miR-483 was determined by TaqMan. Interestingly, miR-483 levels did not reveal a correlation to IGF-2 expression levels, in contrast to previous findings. miR-483-5p specifically is located in one of the IGF-2 introns and has been shown to regulated IGF-2 expression, possibly by exerting a feedback loop through DHX9, a RNA helicase, leading to increasing transcription numbers. Our current study, however, did not reveal a clear correlation between the two factors; which may be explained by the fact that other micro RNA molecules might play additional roles, or that our data in rectal cancer might show difference to colon cancer cells.
In other clinical studies, biomarkers in serum of CRC patients were identified among a large panel of biomarkers, and the study revealed that Insulin like growth factor binding protein 2 (IGFBP2), Dickkopf-3 (DKK3), and Pyruvate kinase M2(PKM2) showed a 73% sensitivity at 95% specificity as a biomarker of CRC in an Australian patient data base [26]. The authors report that a 3 biomarker panel had a higher sensitivity and specificity for early stage (Stage I and -II) disease than the faecal occult blood test, raising the possibility for its use as a noninvasive blood diagnostic or screening test.
Also, Loss of imprinting (LOI), an epigenetic alteration affecting the insulin-like growth factor II gene (IGF2 ), is found in normal colonic mucosa of about 30% of colorectal cancer (CRC) patients, but it is found in only 10% of healthy individuals. A pilot study in 172 patients [22] investigated the utility of LOI as a marker of CRC risk, and the adjusted odds ratio for LOI in lymphocytes was 5.15 for patients with a positive family history [95% confidence interval (95% CI), 1.70 to 16.96; probability P=0.002], and 21.7 for patients with CRC (95% CI, 3.48 to 153.6; P=0.0005). LOI of IGF-2 can be assayed with a DNAbased blood test, and it may be a valuable predictive marker of an individual's risk for CRC [22].
Most interestingly, recent data also point out that IGF-1 receptor expression is important for the response the neoadjuvant radiotherapy [27]. In this work, subjects treated with preoperative radiotherapy and radical resection of rectal carcinoma were investigated using immunohistochemistry and RT-PCR to detect IGF-1R expression in pre-treatment and postoperative colorectal cancer specimens. Radiosensitivity for rectal cancer specimens was evaluated by observing rectal carcinoma mass regression combined with fibrosis on HE staining, degree of necrosis and quantity of remaining tumor cells, similar to our current work. A high IGF-1R protein hyper-expression was significantly correlated with a poor response to radiotherapy, and thus, the authors concluded that IGF-1R expression level may serve as a predictive biomarker for radiosensitivity of rectal cancer. Similar to this work, we find increased expression of IGF-2 in the tumor samples in advanced stages that have been pretreated with radiotherapy. It may be speculate that this response is due to the autocrine secretion of IGF-2 in the tumor cells following hypoxia induced by radiation. Nevertheless, the data point towards a critical role of the IGF-1 receptor and the corresponding ligand as a potential marker for a presumative response for patients undergoing neoadjuvant radiation.
Overall, our data are in accordance with our current findings that IGF-2 may be of special importance in the pathogenesis, but also in determination of rectal cancer cancer presence. Especially in patients avoiding endocopy, testing for IGF-2 levels, LOI of IGF-2, or IBFbinding protein 2 in blood could be an important finding to strongly support immediate staging. Our results may also support development of gene therapies targeting IGF-2 in rectal cancer treatment.
Acknowledgments, Competing Interests, Funding
No conflict of interest exists. This work was performed by A.W. as PhD thesis at the Helios Universitätsklinikum Wuppertal der Privaten Universität Witten/Herdecke, under the supervision of Prof. C. Prinz. Histopathological experiments were performed by A.D.; H.Z. provided surgical specimens, and J. P. provided scientif expertise and quality control of the experiments.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, et al. (2011) Global cancer statistics. CA Cancer J Clin 61: 69-90.
- Langenbach MR, Sauerland S, Kröbel KW, Zirngibl H (2010) Why so late?!-delay in treatment of colorectal cancer is socially determined. Langenbecks Arch Surg 395: 1017-1024.
- Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, et al. (2004) Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351: 1731-1740.
- Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, et al. (2004) Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging--a meta-analysis. Radiology 232: 773-783.
- Carmeliet P (2003) Angiogenesis in health and disease. Nat Med 9: 653-660.
- Du JR, Jiang Y, Zhang YM, Fu H (2003) Vascular endothelial growth factor and microvascular density in esophageal and gastric carcinomas. World J Gastroenterol 9: 1604-1606.
- Martins SF, Garcia EA, Luz MA, Pardal F, Rodrigues M (2013) Clinicopathological correlation and prognostic significance of VEGF-A, VEGF-C, VEGFR-2 and VEGFR-3 expression in colorectal cancer. Cancer genomics & proteomics 10:55-67.
- Shah MA, Ramanathan RK, Ilson DH, Levnor A, D'Adamo D, et al. (2006) Multicenter phase II study of irinotecan, cisplatin, and bevacizumab in patients with metastatic gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol 24: 5201-5206.
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, et al. (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350: 2335-2342.
- Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, et al. (2009) Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell 15: 232-239.
- Pollak MN, Schernhammer ES, Hankinson SE (2004) Insulin-like growth factors and neoplasia. Nat Rev Cancer 4: 505-518.
- Moorehead RA, Sanchez OH, Baldwin RM, Khokha R (2003) Transgenic overexpression of IGF-II induces spontaneous lung tumors: a model for human lung adenocarcinoma. Oncogene 22: 853-857.
- Cancer Genome Atlas Network (2012) Comprehensive molecular characterization of human colon and rectal cancer. Nature 487: 330-337.
- Jonker DJ, O'Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, et al. (2007) Cetuximab for the treatment of colorectal cancer. N Engl J Med 357: 2040-2048.
- Cabuk D, Basaran G, Celikel C, Dane F, Yumuk PF, et al. (2007) Vascular endothelial growth factor, hypoxia-inducible factor 1 alpha and CD34 expressions in early-stage gastric tumors: relationship with pathological factors and prognostic impact on survival. Oncology 72:111-117.
- Kasem M, Tuncer I, Kotan C, Ibilolu I, Oztrk M, et al. (2009) Significance of VEGF and microvascular density in gastric carcinoma. Hepatogastroenterology 56: 1236-1240.
- Goncharuk IV, Vorobjova LI, Lukyanova NY, Chekhun VF (2009) Vascular endothelial growth factor exression in uterine cervical cancer: correlation with clinicopathologic characteristics and survival. Experimental oncology 31:179-181.
- Kolev Y, Uetake H, Iida S, Ishikawa T, Kawano T, et al. (2007) Prognostic significance of VEGF expression in correlation with COX-2, microvessel density, and clinicopathological characteristics in human gastric carcinoma. Annals of surgical oncology 14:2738-2747.
- Lee JC, Chow NH, Wang ST, Huang SM (2000) Prognostic value of vascular endothelial growth factor expression in colorectal cancer patients. European journal of cancer (Oxford, England: 1990) 36:748-753.
- Nam DH, Park K, Suh YL, Kim JH (2004) Expression of VEGF and brain specific angiogenesis inhibitor-1 in glioblastoma: prognostic significance. Oncology reports 11:863-869.
- Sun B, Zhang S, Zhang D, Yin X, Wang S, et al. (2007) Doxycycline influences microcirculation patterns in B16 melanoma. Experimental biology and medicine (Maywood, NJ) 232:1300-1307.
- Cui H, Cruz-Correa M, Giardiello FM, Hutcheon DF, Kafonek DR, et al. (2003) Loss of IGF2 imprinting: a potential marker of colorectal cancer risk. Science 299: 1753-1755.
- Nakagawa H, Chadwick RB, Peltomaki P, Plass C, Nakamura Y, et al. (2001) Loss of imprinting of the insulin-like growth factor II gene occurs by biallelic methylation in a core region of H19-associated CTCF-binding sites in colorectal cancer. Proceedings of the National Academy of Sciences of the United States of America 98:591-596.
- Guo N, Ye JJ, Liang SJ, Mineo R, Li SL, et al. (2003) The role of insulin-like growth factor-II in cancer growth and progression evidenced by the use of ribozymes and prostate cancer progression models. Growth hormone & IGF research: official journal of the Growth Hormone Research Society and the International IGF Research Society 13:44-53.
- Nishihara R, Wang M, Qian ZR, Baba Y, Yamauchi M, et al. (2014) Alcohol, one-carbon nutrient intake, and risk of colorectal cancer according to tumor methylation level of IGF2 differentially methylated region. The American journal of clinical nutrition 100:1479-1488.
- Fung KY, Tabor B, Buckley MJ, Priebe IK, Purins L, et al. (2015) Blood-based protein biomarker panel for the detection of colorectal cancer. PLoS One 10: e0120425.
- Wu XY, Wu ZF, Cao QH, Chen C, Chen ZW, et al. (2014) Insulin-like growth factor receptor-1 overexpression is associated with poor response of rectal cancers to radiotherapy. World J Gastroenterol 20: 16268-16274.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 15962
- [From(publication date):
August-2016 - Aug 08, 2025] - Breakdown by view type
- HTML page views : 15073
- PDF downloads : 889