IL-10 restores MHC class I expression and interferes tumor immunity in papillary thyroid cancer with concomitant Hashimotos thyroiditis
Received: 18-Jul-2019 / Accepted Date: 01-Aug-2019 / Published Date: 12-Aug-2019 DOI: 10.4172/2476-2024.1000152
Abstract
Purpose: The incidence of papillary thyroid cancer (PTC) with concomitant Hashimoto’s thyroiditis (HT) is increasing. Interleukin-10 (IL-10) is a cytokine previously reported to be elevated in this condition. Evidence from multiple human malignancies showed IL-10 participated in tumor immunity and exhibited therapeutic potential. The aim of this study is to investigate whether IL-10 interferes tumor immunity in PTC with concomitant HT.
Method: Expression of IL-10 and major histocompatibility complex (MHC) class I were compared on PTC tissues with or without concomitant HT. PTC cell lines K1 and TPC-1 were stimulated with IL-10 and analyzed for MHC class I expression afterwards. T cell activation, production of Interleukin-2 (IL-2) and IFN-γ and programmed death-1 (PD-1) expression were assessed by coculture of donor peripheral blood lymphocytes (PBLs) with IL-10 pretreated PTC cells. Programmed death-ligand 1 (PD-L1) expression was measured in PTC tissues and IL-10 pretreated cells of K1 and TPC-1.
Results: Increased level of IL-10 and MHC class I were observed in PTC with concomitant HT. IL-10 stimulation increased MHC class I expression of PTC cells in vitro. Coculture of PBLs with IL-10 pretreated PTC cells enhanced T cell activation (%CD25+ of CD3+T cells) and increased IL-2 production along with decreased IFN-γ secretion and PD-1 expression. Reduced PD-L1 expression was seen in PTC+HT tissue samples and IL-10 stimulated PTC cell lines.
Conclusion: Elevated IL-10 expression in PTC with concomitant HT restores MHC class I expression and interferes tumor immunity. IL-10 may facility cancer immunotherapy in MHC class I reduced malignancies.
Keywords: Papillary thyroid cancer; Hashimoto’s thyroiditis; Tumor immunity; MHC Class I Molecule; Interleukin-10
Introduction
Thyroid cancer (TC) is the most common malignancy of the endocrine system with a steadily growing incidence over the past few decades [1]. Papillary thyroid cancer (PTC) is the predominant histotype of TC and is frequently found to be associated with Hashimoto’s thyroiditis (HT) in postoperative pathology reports. The number of patients who suffer from both PTC and HT has increased significantly since Dailey ME et al. first reported the concomitance of the two diseases in 1955 [2-6].
Vast numbers of studies have tried to elucidate the possible relationship between PTC and HT. Despite the dedication devoted to this topic, no consensus could be reached to date. Notably, the majority of those studies, including one completed by our department, considers HT as a protective factor for PTC, which results in better prognosis [3,7-9]. However, the underline mechanism remains unknown.
HT typically presents with diffused infiltrated lymphocytes, which are recruited by cytokine secretion during immune response. We focus on one specific cytokine, interleukin-10 (IL-10), whose expression is found to be elevated in PTC with concomitant HT [10]. More importantly, evidence showed that IL-10 effectively induced activation of CD8+T cells in several mouse tumor models, suggesting its therapeutic potential in cancer immunotherapy [11,12].
The antitumor cluster of differentiation (CD) 8+T cells attack major histocompatibility complex (MHC) class I positive cancer cells. However, human malignancies, including PTC, tend to down regulate MHC class I expression as one of their immune escape strategies [13-15]. Therefore, recovery of MHC class I expression is critical to effective immunotherapy.
The aim of this study is to evaluate the role of IL-10 in PTC patients with concomitant HT, analyzing its potential function from the view of tumor immunity through in vitro experiments.
Materials and Methods
Patients and tissue samples
This study recruited 140 surgically treated PTC patients with/ without HT during Apr. 2014 and Jan. 2016 at the department of Head and Neck Surgery, Fudan University Shanghai Cancer Center. Diagnosis of PTC and HT was based on postoperative pathology reports. Patients with immunodeficiency were excluded from the study. None of the patients had a previous history of any kind of treatment for their thyroid condition before surgery.
Fresh frozen samples were obtained from 69 patients during surgery. Formalin-fixed and paraffin embedded tissue sections of the rest 71 cases were acquired from the hospital tissue bank. Clinical data (sex, age, tumor size, extrathyroidal invasion, lymph node metastasis, multifocality and TNM classification) were collected. TNM classification was determined according to the 8th edition of AJCC/ UICC TNM staging criteria. Each patient signed informed consent for the use of their tissue samples before research. This study acquired Institutional Review Board approval from Fudan University Shanghai Cancer Center.
Cell lines and culture
Normal human thyroid cell line Nthy-ori 3-1 and two PTC cell lines (K1 and TPC-1) were used in this study. Nthy-ori 3-1 was purchased from Sigma-Aldrich, Inc., and cells of K1 and TPC-1 were purchased from University of Colorado Cancer Center Cell Bank. Cells were cultured in RMPI 1640 medium containing 10% FBS (Invitrogen, Carlsbad, CA, USA) at 37 with 5% CO2 in proper humidity.
RNA extraction, reverse transcription and quantitative real time PCR (qPCR)
Total RNA of cells and tissue samples was extracted with TRIzol Reagent (Invitrogen, Inc.) and cDNA synthesis was performed by PrimeScriptTM RT Reagent Kit (Takara, Dalian, China). Using SYBR Green Premix Ex TaqTM II (Takara, Dalian, China), quantitative PCR was then conducted in triplicate. Target genes (IL-10, HLA-A, HLA-B, HLA-C and PD-L1) were tested and β-actin served as an internal control for mRNA assays. Results were analyzed according to comparative cycle threshold values (2-ΔΔCt). Primer sequences are listed in Table 1.
| Gene | Forward (5’-3’) | Reverse (5’-3’) |
|---|---|---|
| IL-10 | TCTCCGAGATGCCTTCAGCAGA | TCAGACAAGGCTTGGCAACCCA |
| HLA-A | GTGGCCTCATGGTCAGAGAT | GCAGTTGAGAGCCTACCTGG |
| HLA-B | GTGATCTCCGCAGGGTAGAA | TCCGCAGATACCTGGAGAAC |
| HLA-C | TGATCTCCGCAGGGTAGAAG | CAGATACCTGGAGAACGGGA |
| PD-L1 | CCATACAGCTGAATTGGTCATC | CAGAATTACCAAGTGAGTCCTTTCA |
| β-Actin | CACCATTGGCAATGAGCGGTTC | AGGTCTTTGCGGATGTCCACGT |
Table 1: Primer sequences of target genes for qPCR.
Immunohistochemistry (IHC)
Formalin-fixed and paraffin-embedded tissue sections were deparaffinized in xylene and rehydrated with ethanol. Blocking of endogenous peroxidase activity was conducted using 3% H2O2. After heat-induced antigen retrieval (0.01 mol/L citrate, pH 6.0), 5% BSA was used to block non-specific protein-protein interactions. Sections were then incubated overnight with primary antibodies against IL-10 (20850-1-AP, Proteintech), HLA Class I ABC (ab70328, Abcam) and PD-L1 (13684T, Cell Signaling Technology). Secondary antibody staining and antigen detection was performed using a horseradish peroxidase-conjugated rabbit or mouse IHC kit (KIHC-1, Proteintech). Sections were counterstained with hematoxylin. Images were obtained through an Olympus IX71 inverted microscope with a DP2-BSW Olympus image acquisition software system (Olympus, Japan).
Isolation of Peripheral Blood Lymphocytes (PBLs)
Peripheral blood of healthy donors was drawn through routine venipuncture. PBLs were isolated using human lymphocyte separation medium (Dakewe Biotech Co., Ltd) through differential density gradient centrifugation. Cells were plated in U-shaped bottom 96-well cell culture plates (2 X 105 cells/well) using RMPI 1640 medium containing 10% FBS (Invitrogen, Carlsbad, CA, USA) at 37. Antibodies against CD3 (16-0037-85, eBioscience) and CD28 (16-0289-85, eBioscience) were added into the media at a concentration of 2 μg/ml. After 72 hours, PBLs were dyed with fluorescently conjugated antibodies against CD3 (300308, BioLegend), CD8 (300906, BioLegend) and CD25 (302610, BioLegend) and then sorted by flow cytometer (MoFlo XDP, Beckman Coulter, Inc.). Activated T cells (CD3+CD8+CD25+) were collected.
Pretreatment of PTC cell lines with IL-10
0.1 μg/μL recombinant human IL-10 (200-10, Peprotech) was added into the culture media of K1 and TPC-1 cells for 24 hours. The cells were then collected and washed by phosphate buffer saline (PBS) to remove the residue of IL-10 thoroughly.
Coculture system of activated T cells (CD3+CD8+CD25+) and PTC cell lines
CD3+CD8+CD25+ T cells (effector cells, E) were seeded in flat bottom 24-well cell culture plates (1X105 cells/well) along with PTC cells (targeted cells, T) at an E:T ratio of 10:1 or 30:1 for 24 hours before further experiments.
Flow cytometry
Cells were collected and dyed with fluorescently conjugated antibodies against MHC Class I ABC (ab70328, Abcam), CD3 (300308, BioLegend), CD25 (302610, BioLegend) and PD-1 (329918, Biolegend). Flow cytometry was performed using a Cytomics™ FC 500 cytometer (Beckman Coulter, Inc.). For the detection of MHC class I expression in PTC cell lines, cells were also stained with FITC-labeled goat anti-mouse secondary antibody (555988, BD Pharmingen). Results were analyzed using FlowJo software (Tree Star).
Enzyme-Linked Immunosorbent Assay (ELISA)
The supernatant fluid of coculture systems was analyzed for IL-2 and IFN-γ concentration using precoated Human IL-2 ELISA Kit (12-1020-096, Dakewe Biotech Co., Ltd) and Human IFN- γ ELISA Kit (12-1000-096, Dakewe Biotech Co., Ltd). Briefly, samples and prediluted standards were added to precoated wells. The detection antibody was then added, followed by incubation at room temperature for 1 (IL-2) or 2 (IFN- γ) hours. HRP conjugate was added and incubated at room temperature for 20 minutes. To develop the plate, 3,3'5,5'-tetramethyl benzidine dihydrochloride (TMB) was used. Once the stop solution was added, the absorbance of each well was read at 450 nm by Synergy H4 Hybrid microplate reader (BioTek).
Western blot analysis
Cell lysates were obtained from 1 X 106 cultured cells with a mixture of RIPA protein extraction reagent, protease inhibitor and phosphatase inhibitor (Roche, CA, USA). Thyroid tissue samples were treated with T-PER ™ Tissue Protein Extraction Reagent (Thermo Scientific™) and lysed by Vibra-Cell™ Ultrasonic Liquid Processors (Sonics and Materials, Inc.).
Protein concentration was measured using a bicinchoninic acid assay (BCA). Equal amounts of total protein lysate were separated by 10% SDS-PAGE and transferred onto PVDF membranes. The membranes were then blocked in 5% non-fat milk and probed with primary antibodies against MHC Class I (1:1000, Abcam), PD-L1 (1:1000, Cell Signaling Technology) and GAPDH (1:5000, Abcam) at 4overnight.
After incubation in a solution of goat anti-rabbit or anti-mouse IgG (1:5000 for both; Jackson ImmunoResearch Laboratories) at room temperature for 1 hour, the membranes were treated with enhanced chemiluminescence reagents (Thermo Fisher Scientific). Bands were detected with Alpha Imager (Alpha Innotech, San Leandro, CA, USA).
Statistical analysis
All data in the study are shown as mean ± SD or SEM as indicated. Independent t-tests were used for continuous variables and Pearson's χ2 tests were used for categorical variables. P<0.05 was considered to indicate a statistically significant difference. Statistical tests were performed using GraphPad Prism 5.01 software (GraphPad Software, Inc.) and IBM SPSS 22.0 (Armonk, NY, USA). Graphs and figures were produced using GraphPad and Abobe Photoshop (Adobe Systems Inc.).
Results
Patient characteristics
Samples from 140 PTC patients were used in this study. Of these patients, 110 (78.6%) were female. 117 (83.6%) patients were aged under 55 years old. About half (76, 54.3%) of the study cohort had cancers that were less than 1 cm. Multifocal lesions were detected in 44 (31.4%) cases. Extrathyroidal invasion only occurred in 18 (12.9%) patients. Lymph node metastasis was positive in 75 (53.6%) patients, while no distant metastasis was identified in the whole cohort. 137 (95.1%) of the patients were classified as stage I or II according to the latest 8th edition of AJCC/UICC TNM staging system.
Based on postoperative pathology reports, 51 (36.4%) patients had HT in addition to PTC. This subgroup consisted more of female patients than PTC group (92.2% vs. 70.8%, P=0.003). The age, multifocal lesions, extrathyroidal invasion, lymph node metastasis and TNM classification between PTC patients with or without HT showed no difference. Notably, tumor size in these two groups varied significantly.
PTC patients with HT had bigger tumors than those without concomitant HT (1.4 ± 1.0 vs. 1.1 ± 0.7, P=0.031). When patients were further divided according to the size of tumor (tumor size ≤ 1 and >1 cm), data revealed more microcarcinoma in PTC group than PTC+HT group (61.8% vs. 41.2%, P=0.018).
Clinical characteristics of the 140 PTC patients in this study are listed in Table 2.
| Clinicopathologic parameters | PTC | PTC+HT | p-value |
|---|---|---|---|
| N (%) | N (%) | ||
| Gender | 0.003** | ||
| Male | 26 (29.2) | 4 (7.8) | |
| Female | 63 (70.8) | 47 (92.2) | |
| Age (years) | 0.159 | ||
| Mean | 42.6 ± 12.4 | 39.5 ± 13.1 | |
| <55 | 73 (82.0) | 44 (86.3) | 0.513 |
| ≥ 55 | 16 (18.0) | 7 (13.7) | |
| Tumor size (cm) | |||
| Mean | 1.1 ± 0.7 | 1.4 ± 1.0 | 0.031* |
| ≤ 1 | 55 (61.8) | 21 (41.2) | 0.018* |
| >1 | 34 (38.2) | 30 (58.8) | |
| Multifocal lesions | 0.060 | ||
| Positive | 23 (25.8) | 21 (41.2) | |
| Negative | 66 (74.2) | 30 (58.8) | |
| Extrathyroidal invasion | 0.200 | ||
| Positive | 9 (10.1) | 9 (17.6) | |
| Negative | 80 (89.9) | 42 (82.4) | |
| Lymph node metastasis | 0.346 | ||
| Positive | 45 (50.6) | 30 (58.8) | |
| Negative | 44 (49.4) | 21 (41.2) | |
| TNM stage 8th | 0.271 | ||
| I, II | 88 (98.9) | 49 (96.1) | |
| III, IV | 1 (1.1) | 2 (3.9) | |
Table 2: Clinical characteristics of all patients in this study (n=140) (*P<0.05; ** P<0.01; PTC: Papillary Thyroid Carcinoma; HT: Hashimoto’s Thyroiditis)
HT is related with higher IL-10 expression in PTC
The effect of HT on IL-10 expression in PTC was measured by qPCR in fresh frozen tissue samples of 69 PTC patients. 37 (53.6%) of these patients had HT in addition to PTC, and they presented higher expression of IL-10 according to the test result (p<0.05, Figure 1A). Comparison of clinical data was carried out between IL-10 low and high expression groups. A total number of 39 (56.5%) patient was attributed to IL-10 high expression group. No remarkable distinction was found in sex, age, multifocal lesions, extrathyroidal invasion, lymph node metastasis and TNM classification between the two groups. Notably, patients in IL-10 high expression group were more vulnerable to tumors larger than 1 cm (66.7% vs. 40%, P=0.027). The clinical information of 69 PTC patients in the qPCR cohort was listed in Table 3.
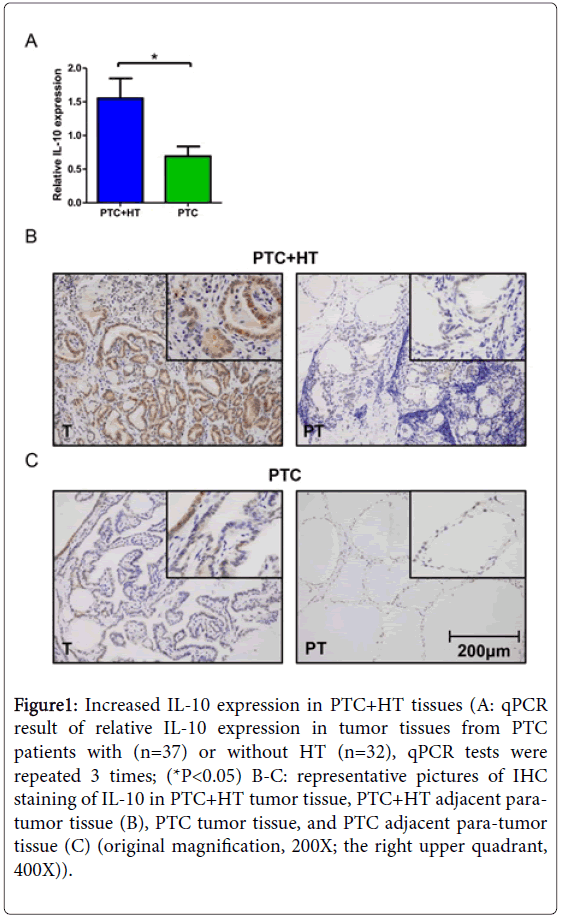
Figure 1: Increased IL-10 expression in PTC+HT tissues (A: qPCR result of relative IL-10 expression in tumor tissues from PTC patients with (n=37) or without HT (n=32), qPCR tests were repeated 3 times; (*P<0.05) B-C: representative pictures of IHC staining of IL-10 in PTC+HT tumor tissue, PTC+HT adjacent paratumor tissue (B), PTC tumor tissue, and PTC adjacent para-tumor tissue (C) (original magnification, 200X; the right upper quadrant, 400X)).
| Clinicopathologic parameters | IL-10 Low | IL-10 High | p-value |
|---|---|---|---|
| N (%) | N (%) | ||
| Gender | 0.616 | ||
| Male | 6 (20.0) | 6 (15.4) | |
| Female | 24 (80.0) | 33 (84.6) | |
| Age (years) | |||
| Mean | 38.6 ± 14.2 | 40.8 ± 13.4 | 0.517 |
| <55 | 28 (93.3) | 34 (87.2) | 0.401 |
| ≥ 55 | 2 (6.6) | 5 (12.8) | |
| Tumor size (cm) | |||
| Mean | 1.2 ± 0.8 | 1.3 ± 0.9 | 0.479 |
| ≤ 1 | 18 (60.0) | 13 | 0.027* |
| >1 | 12 (40.0) | 26 | |
| Multifocal lesions | 0.063 | ||
| Positive | 6 (20.0) | 16 | |
| Negative | 24 (80.0) | 23 | |
| Extrathyroidal invasion | 0.435 | ||
| Positive | 4 (13.3) | 8 | |
| Negative | 26 (86.7) | 31 | |
| Lymph node metastasis | 0.512 | ||
| Positive | 13 (43.3) | 20 | |
| Negative | 17 (56.7) | 19 | |
| TNM stage 8th | 0.208 | ||
| I, II | 30 (100.0) | 37 | |
| III, IV | 0 (0.0) | 2 |
Table 3: Clinical characteristics of PTC patients in the qPCR cohort (n=69) (*P<0.05; PTC: Papillary Thyroid Carcinoma; IL-10: Interleukin-10)
Paraffin embedded sections of 71 PTC patients were stained with IL-10 antibody. Results of IHC staining were compared between tumorous (T) and adjacent para-tumor (PT) tissues from PTC patients with/without HT. For each patient group, the tumor tissue exhibited higher expression of IL-10 than adjacent para-tumor tissue. More extensive and higher intensity of IL-10 staining can be seen on tumor samples with PTC+HT compared with PTC (Figures 1B and 1C). However, the difference between adjacent para-tumor tissues from the two patient groups was not clear. Additionally, diffused lymphocytes infiltration which was caused by autoimmune response can be observed on both T and PT sections from PTC+HT.
HT is related with higher MHC Class I expression in PTC
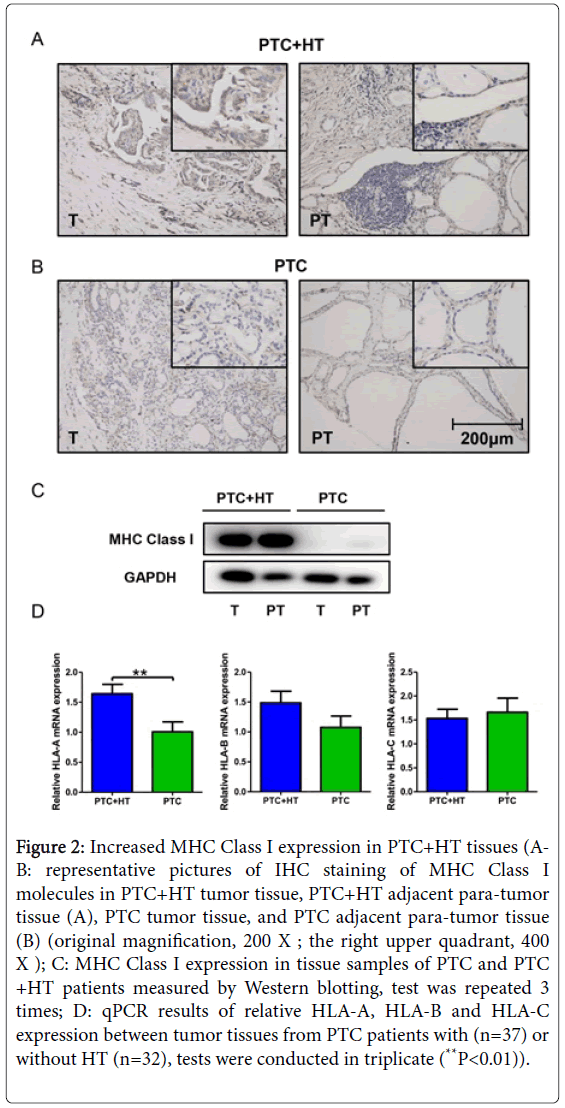
IHC staining and Western blotting were performed to evaluate the effect of concomitant HT on MHC Class I expression in PTC. As shown in Figures 2A and 2B, more abundant expression of MHC Class I was perceived in tissue sections from PTC+HT compared with that from PTC, in both tumor and adjacent para-tumor tissues. Among all four pictures, PTC+HT tumor tissue possessed the highest expression of MHC Class I molecules. Western blotting result in Figure 2C demonstrated a remarkably increased MHC Class I expression in proteins from PTC+HT, which was consistent with the findings of IHC.
Figure 2: Increased MHC Class I expression in PTC+HT tissues (AB: representative pictures of IHC staining of MHC Class I molecules in PTC+HT tumor tissue, PTC+HT adjacent para-tumor tissue (A), PTC tumor tissue, and PTC adjacent para-tumor tissue (B) (original magnification, 200 X ; the right upper quadrant, 400 X ); C: MHC Class I expression in tissue samples of PTC and PTC +HT patients measured by Western blotting, test was repeated 3 times; D: qPCR results of relative HLA-A, HLA-B and HLA-C expression between tumor tissues from PTC patients with (n=37) or without HT (n=32), tests were conducted in triplicate (**P<0.01)).
Tissue samples were then further examined for HLA-A, HLA-B and HLA-C expression by qPCR. Data were analyzed between PTC patients with/without HT. We detected significantly higher expression of HLA-A in tumors from PTC+HT (P<0.01, Figure 2D). As for the HLA-B and HLA-C gene, no difference in expression was discovered between the two groups.
IL-10 induces MHC Class I expression in PTC cells in vitro
We have demonstrated that concomitant HT was related with increased expression of IL-10 and MHC Class I, respectively. To evaluate the correlation between IL-10 and MHC Class I expression, normal human thyroid cell line Nthy-ori 3-1 and PTC cell lines K1 and TPC-1 were used in this study.
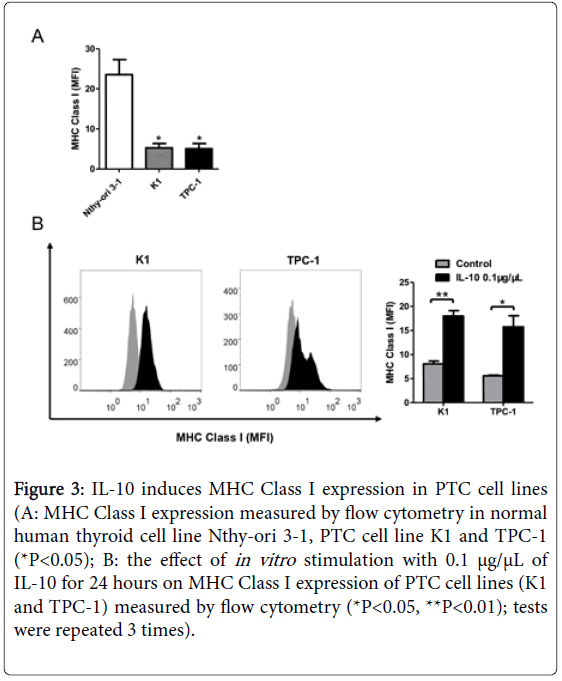
We first examine the baseline expression of MHC Class I molecules in each cell line using flow cytometry. Compared with Nthy-ori 3-1, cells of K1 and TPC-1 both presented reduced mean fluorescence intensity (MFI) of MHC Class I (P<0.05, Figure 3A). The PTC cells were then stimulated with 0.1 μg/μL recombinant human IL-10 for 24 hours. Flow cytometry results showed significantly increased expression of MHC Class I molecules in both cell lines after IL-10 stimulation (P<0.01 and P<0.05, Figure 3B).
Figure 3: IL-10 induces MHC Class I expression in PTC cell lines (A: MHC Class I expression measured by flow cytometry in normal human thyroid cell line Nthy-ori 3-1, PTC cell line K1 and TPC-1 (*P<0.05); B: the effect of in vitro stimulation with 0.1 μg/μL of IL-10 for 24 hours on MHC Class I expression of PTC cell lines (K1 and TPC-1) measured by flow cytometry (*P<0.05, **P<0.01); tests were repeated 3 times).
IL-10 enhances T cell activation in vitro
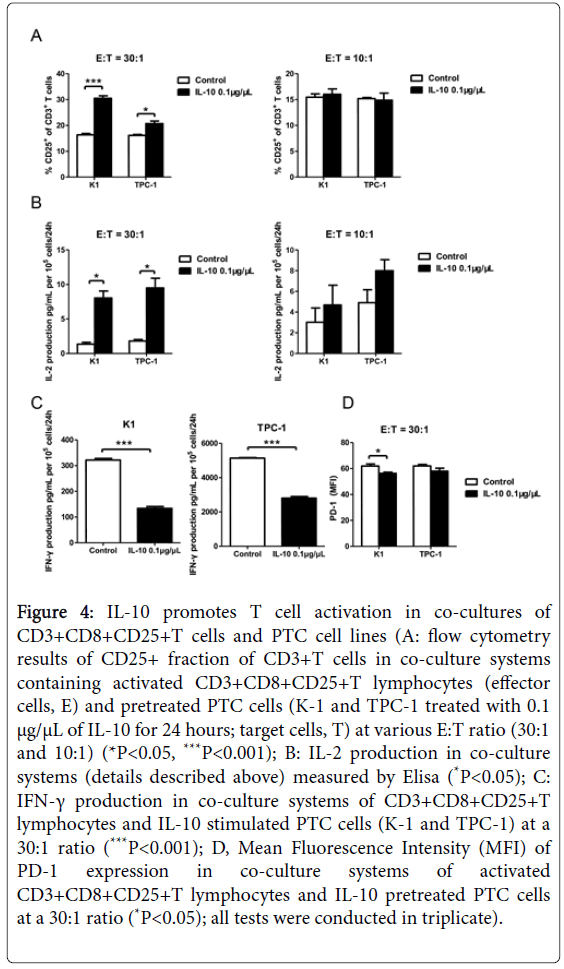
CD3+CD8+CD25+T cells isolated from heathy donors were cocultured with IL-10 pretreated cells of K1 and TPC-1 for 24 hours. The effector cells (E, T cells) and targeted cells (T, PTC cells) were planted at an E:T ratio of 30:1 or 10:1. The proportion of CD25+T cells of all CD3+T cells was determined by flow cytometry. As shown in Figure 4A, higher proportion of activated CD3+CD8+CD25+T cells appeared in both cell lines after IL-10 administration when the E:T ratio was set at 30:1 (P<0.001 and P<0.05). However, when the ratio was lower to 10:1, no noticeable distinction could be observed.
Figure 4: IL-10 promotes T cell activation in co-cultures of CD3+CD8+CD25+T cells and PTC cell lines (A: flow cytometry results of CD25+ fraction of CD3+T cells in co-culture systems containing activated CD3+CD8+CD25+T lymphocytes (effector cells, E) and pretreated PTC cells (K-1 and TPC-1 treated with 0.1 μg/μL of IL-10 for 24 hours; target cells, T) at various E:T ratio (30:1 and 10:1) (*P<0.05, ***P<0.001); B: IL-2 production in co-culture systems (details described above) measured by Elisa (*P<0.05); C: IFN-γ production in co-culture systems of CD3+CD8+CD25+T lymphocytes and IL-10 stimulated PTC cells (K-1 and TPC-1) at a 30:1 ratio (***P<0.001); D, Mean Fluorescence Intensity (MFI) of PD-1 expression in co-culture systems of activated CD3+CD8+CD25+T lymphocytes and IL-10 pretreated PTC cells at a 30:1 ratio (*P<0.05); all tests were conducted in triplicate).
ELISA was used to measure IL-2 secretion of activated T cells from cocultures. Results showed IL-10 successfully increased amount of IL-2 production in both K1 and TPC-1 cocultures. Significance appeared at 30:1 ratio (P<0.05, Figure 4B) while the E:T ratio of 10:1 failed to show difference. IFN-γ secretion in those cocultures at an E:T ratio of 30:1 were also evaluated by ELISA. Data showed T cells cocultured with TPC-1 cells possessed greater ability of IFN-γ secretion than that with K1 cells, regardless of IL-10 stimulation. With the pretreatment of IL-10, IFN-γ secretion of T cells dropped dramatically in cocultures of both PTC cell lines (P<0.001, Figure 4C).
PD-1 expression on activated T cells in cocultures was measured by flow cytometry at an E:T ratio of 30:1. Compared with control groups, the extra treatment of IL-10 in K1 cocultures effectively lower the expression of PD-1 on T cells (P<0.05, Figure 4D). Unfortunately, the expression difference in TPC-1 cocultures was not conclusive.
IL-10 regulates T cell activation through PD-1/PD-L1 pathway in vitro
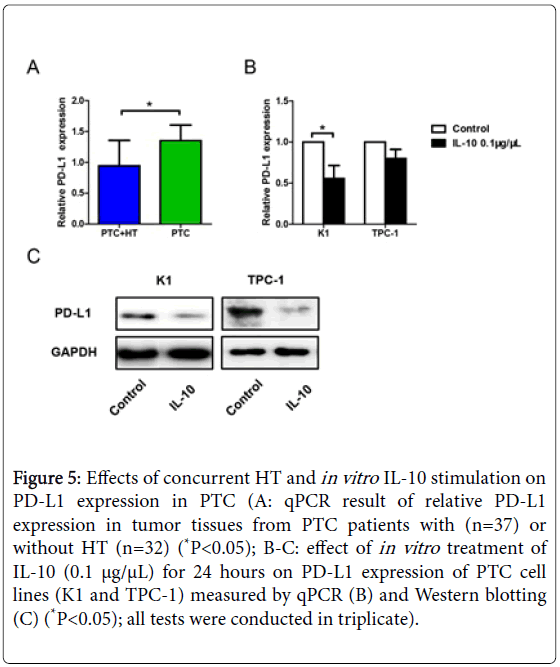
Our previous results showed PD-1 expression was reduced in cocultures of activated CD3+CD8+CD25+T cells and IL-10 pretreated K1 cells at an E:T ratio of 30:1. As the other half of the co-stimulatory molecules, expression of PD-L1 was then tested by qPCR. Data analysis revealed reduced PD-L1 expression in tumor tissues from patients with PTC+HT (P<0.05, Figure 5A), indicating the down regulation of T cell apoptosis.
Figure 5: Effects of concurrent HT and in vitro IL-10 stimulation on PD-L1 expression in PTC (A: qPCR result of relative PD-L1 expression in tumor tissues from PTC patients with (n=37) or without HT (n=32) (*P<0.05); B-C: effect of in vitro treatment of IL-10 (0.1 μg/μL) for 24 hours on PD-L1 expression of PTC cell lines (K1 and TPC-1) measured by qPCR (B) and Western blotting (C) (*P<0.05); all tests were conducted in triplicate).
Cells of K1 and TPC-1 were then stimulated with 0.1 μg/μL recombinant human IL-10 for 24 hours and measured their expression of PD-L1 using qPCR and Western blotting. The qPCR result in Figure 5B showed down regulated PD-L1 expression on K1 cells after IL-10 stimulation (P<0.05). However, the change of PD-L1 expression on TPC-1 cells was not quite clear. Western blotting results of both K1 and TPC-1 cells revealed reduced PD-L1 expression under the influence of IL-10 administration (Figure 5C).
Discussion
The relationship between Hashimoto’s thyroiditis and papillary thyroid cancer has always been intriguing [4]. According to extensive researches in this field, PTC with concomitant HT normally had a better prognosis [7-9,16]. A former study conducted in our institute pointed out that the presence of HT, though a risk factor for PTC diagnosis, served as a protective factor for central compartment lymph node metastasis [3]. However, contradictory opinions also occur where HT may act as a risk factor or harbor no potential contribution to PTC prognosis at all [5,6]. In our study, clinical statistics showed PTC patients with concomitant HT had bigger tumors than PTC patients while the TNM classification of the two groups revealed no significant difference. Tumor size relates directly to T classification and is generally considered a risk factor in PTC. Therefore, the inconsistency between advanced T classification and the overall TNM classification in PTC+HT group indicates the protective role of HT, to some degree.
Development of HT involves a series of complicate immune responses which ultimately lead to cytokine release, lymphocyte infiltration and cell destruction in the thyroid gland. Of special interest is IL-10, whose polymorphism was associated with a threefold increasing risk of HT [17]. Notably, IL-10 has also been reported to contribute in tumorigenesis in multiple human malignancies including colorectal and cervical carcinoma [18,19]. Stanciu AE et al. reported that serum IL-10 levels were substantially higher in PTC+HT patients with persistent/recurrent disease compared with PTC patients (with or without recurrence) [10]. Unfortunately, we were unable to conduct the similar comparison due to our insufficient survival information of the patient cohort. Nevertheless, our data confirmed an elevated IL-10 expression in tumor tissue from PTC patients with concomitant HT both by qPCR and IHC staining. When patients were classified by their IL-10 expression, difference appeared in tumor size. The IL-10 low expression group harbored more microcarcinoma compared with IL-10 high expression group. This result combined with our previous finding that PTC patients had smaller tumors than PTC+PT patients, suggesting the importance of IL-10 in HT.
Although widely considered as an anti-inflammatory cytokine, IL-10 could also induce the cytotoxicity of CD8+ T cells in immune response to cancer [11]. Mumm JB et al. demonstrated that transgenic overexpression of IL-10 protected mice from carcinogenesis [20]. The basis for CD8+ T cell cytotoxicity in cancer is the recognition of MHC class I molecules, which carry a cancer-derived peptide. However, loss of MHC class I expression is a frequent encountered mechanism of immune escape in malignant diseases [13,14]. The restore of MHC class I expression, on the other hand, could potentiate tumor immunity [13,21]. Downregulation of MHC class I in both PTC tumor and adjacent para-tumor tissue was clearly showed on the IHC staining pictures. Results in our study also revealed significantly higher expression of MHC class I in PTC+HT patients compared with PTC patients, with HLA-A gene served as the major contributor.
Our previous results demonstrated that concomitant HT resulted in higher MHC class I expression in PTC. Moreover, administration of IL-10 effectively induced MHC class I expression on PTC cell lines K1 and TPC-1. Coculture systems containing IL-10 pretreated PTC cells and CD3+CD8+CD25+T cells were employed to further examine the possible impact of IL-10 on tumor immunity. T cells cocultured with IL-10 stimulated cells of K1 and TPC-1 exhibited increased cell activation (%CD25+ of CD3+T cells) and IL-2 production at a E:T ratio of 30:1, which was evidence for promotion in tumor immunity in PTC. The insignificant results in cocultures at E:T ratio of 10:1 emphasized the importance of the amount of CD8+T cells. Naturally, with More CD8+T cells, comes greater cytotoxicity. In contrary to IL-2, the concentration of IFN- γ in IL-10 stimulated cocultures dropped dramatically compared with untreated groups. IFN-γ secreted by activated T cells is essentially regarded as an anti-tumor cytokine and a key factor to the induction of cytotoxic T lymphocytes. Nevertheless, evidence demonstrating IFN- γ facilitated carcinogenesis of colorectal carcinoma and melanoma indicates the opposite effect of this molecule [22,23]. Our result was another proof for the association of IFN- γ and immune escape, which may lead to tumor progression. The PD-1 expression on activated T cells cocultured with IL-10 pretreated K1 cells was downregulated significantly, revealing a suppressed T cell apoptosis as expected.
As the other half of the co-stimulatory molecules, PD-L1 expression was also investigated. Tissue samples showed that HT reduced PD-L1 expression in PTC patients, which may consequently promote T cell cytotoxicity. Consistent with the coculture results, IL-10 stimulation successfully induced down regulation of PD-L1 expression in PTC cells. IFN- γ was previously reported to induce PD-L1 expression in ovarian cancer [24]. Based on our results, IFN- γ may also serve as a barrier to tumor immunity in PTC.
The current study has some limitations which could compromise our findings. The sample number is relatively small, which warrants further investigations with expanded patient cohorts for this topic. Moreover, we were unable to analyze the recurrence between PTC patients with or without HT and patients with a high IL-10 or low IL-10 expression due to insufficient survival data. The result should be interpreted with caution because cytokine IL-10 is not an equivalent for HT. We discovered an induced expression of IL-10 by HT and further revealed an enhanced tumor immunity associated with elevated IL-10. Whether or not this phenomenon could improve PTC prognosis has not been proved. Also, the relationship between IL-10 induced MHC class I expression and PD-1/PD-L1 pathway needs further confirmation.
Conclusion
In conclusion, IL-10 successfully recovers MHC class I expression and enhances tumor antigenicity in PTC with concomitant HT. This study may provide new insights for cancer immunotherapy.
Acknowledgements
Author contributions
ZW.L and JQ.H performed the experiments, LT.H and TT.Z collected the specimens, WJ.W and ZW.L analyzed data, ZW.L and JQ.H wrote the paper, JQ.H and T.L revised the paper, T.L and QH.J designed the study, QH.J, YL.W and Y.W supervised the study.
Funding
This work was supported by the National Natural Science Foundation of China (81272934, 81572622 to Qing-Hai JI and 81702753 to Tian Liao).
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Lim H, Devesa S, Sosa J, Check D, Kitahara C (2017) Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA 317: 1338-1348.
- Caturegli P, De Remigis A, Chuang K, Dembele M, Iwama A, et al. (2013) Hashimoto's thyroiditis: Celebrating the centennial through the lens of the Johns Hopkins hospital surgical pathology records. Thyroid 23: 142-150.
- Zhang L, Li H, Ji QH, Zhu YX, Wang ZY, et al. (2012) The clinical features of papillary thyroid cancer in Hashimoto’s thyroiditis patients from an area with a high prevalence of Hashimoto’s disease. BMC Cancer 12: 610.
- Dailey ME, Lindsay S, Skahen R (1955) Relation of thyroid neoplasms to Hashimoto disease of the thyroid gland. AMA Arch Surg 70: 291-297.
- Konturek A, Barczyński M, Wierzchowski W, Stopa M, Nowak W (2013) Coexistence of papillary thyroid cancer with Hashimoto thyroiditis. Langenbecks Arch Surg 398: 389-394.
- Alcântara-Jones DMD, Alcântara-Nunes TFD, Rocha BdO, Oliveira RDD, Santana ACP, et al. (2015) Is there any association between Hashimoto’s thyroiditis and thyroid cancer? A retrospective data analysis. Radiol Bras 48: 148-153.
- Nam HY, Lee HY, Park GC (2016) Impact of coâ€existent thyroiditis on clinical outcome in papillary thyroid carcinoma with high preoperative serum antithyroglobulin antibody: A retrospective cohort study. Clin Otolaryngol 41: 358-364.
- Jeong JS, Kim HK, Lee CR, Park S, Park JH, et al. (2012) Coexistence of chronic lymphocytic thyroiditis with papillary thyroid carcinoma: Clinical manifestation and prognostic outcome. J Korean Med Sci 27: 883-889.
- Liang J, Zeng W, Fang F, Yu T, Zhao Y, et al. (2017) Clinical analysis of Hashimoto thyroiditis coexistent with papillary thyroid cancer in 1392 patients. Acta Otorhinolaryngol Ital 37: 393-400.
- Stanciu AE, Serdarevic N, Hurduc AE, Stanciu MM (2015) IL-4, IL-10 and high sensitivity-CRP as potential serum biomarkers of persistent/recurrent disease in papillary thyroid carcinoma with/without Hashimoto's thyroiditis. Scand J Clin Lab Invest 75: 539-548.
- Emmerich J, Mumm JB, Chan IH, Laface D, Truong H, et al. (2012) IL-10 directly activates and expands tumor-resident CD8(+) T cells without de novo infiltration from secondary lymphoid organs. Cancer Res 72: 3570.
- Gu T, Jesus MD, Gallagher HC, Burris TP, Egilmez NK (2017) Oral IL-10 suppresses colon carcinogenesis via elimination of pathogenicCD4+ T-cells and induction of antitumor CD8+ T-cell activity. Oncoimmunology 6: e1319027.
- Angell TE, Lechner MG, Jang JK, LoPresti JS, Epstein AL (2014) MHC class I loss is a frequent mechanism of immune escape in papillary thyroid cancer that is reversed by interferon and selumetinib treatment in vitro. J Clin Cancer Res 20: 6034-6044.
- Paulson KG, Tegeder A, Willmes C, Iyer JG, Afanasiev OK, et al. (2014) Downregulation of MHC-I expression is prevalent but reversible in Merkel cell carcinoma. Cancer Immunol Res 2(11): 1071-1079.
- Zhang J, Xu Z, Zhou X, Zhang H, Yang N, et al. (2014) Loss of expression of MHC class I-related chain A (MICA) is a frequent event and predicts poor survival in patients with hepatocellular carcinoma. Int J Clin Exp Pathol 7: 3123.
- Zhu F, Shen Y, Li F, Fang Y, Hu L, et al. (2016) The Effects of Hashimoto Thyroiditis on lymph node metastases in unifocal and multifocal papillary thyroid carcinoma: A retrospective chinese cohort study. Medicine (Baltimore) 95.
- Gerenova J, Stanilova S (2016) IL-12B and IL-10 gene polymorphisms in the development of Hashimoto's thyroiditis. Int J Immunogenet 43: 397-403.
- Shi YH, Zhao DM, Wang YF, Xue L, Ji MR, et al. (2016) The association of three promoter polymorphisms ininterleukin-10 gene with the risk for colorectal cancer and hepatocellular carcinoma: A meta-analysis. Sci Rep 6: 30809.
- Berti FC, Pereira AP, Cebinelli GC, Trugilo KP, Brajão dOK (2017) The role of interleukin 10 in human papilloma virus infection and progression to cervical carcinoma. Cytokine Growth Factor 34: 1-13.
- Mumm JB, Emmerich J, Zhang X, Chan I, Wu L, et al. (2011) IL-10 elicits IFNγ-dependent tumor immune surveillance. Cancer Cell 20: 781-796.
- Luo N, Nixon M, Gonzalez-Ericsson P, Sanchez V, Opalenik S, et al. (2018) DNA methyltransferase inhibition upregulates MHC-I to potentiate cytotoxic T lymphocyte responses in breast cancer. Nat Commun 9: 248.
- Hanada T, Kobayashi T, Chinen T, Saeki K, Takaki H, et al. (2006) IFNgamma-dependent, spontaneous development of colorectal carcinomas in SOCS1-deficient mice. J Exp Med 203: 1391.
- Taniguchi K, Petersson M, Hoglund P, Kiessling R, Klein G, et al. (1987) Interferon γ induces lung colonization by intravenously inoculated B16 melanoma cells in parallel with enhanced expression of class I major histocompatibility complex antigens. Proc Natl Acad Sci USA 84: 3405-3409.
- Abiko K, Matsumura N, Hamanishi J, Horikawa N, Murakami R, et al. (2015) IFN-γ from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br J Cancer 112: 1501-1509.
Citation: Lu ZW, Hua JQ, Han LT, Zhang TT, Wei WJ, et al. (2019) IL-10 Restores MHC Class I Expression and Interferes Tumor Immunity in Papillary Thyroid Cancer with Concomitant Hashimoto’s Thyroiditis. Diagn Pathol Open 4: 152. DOI: 10.4172/2476-2024.1000152
Copyright: © 2019 Lu ZW, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 3787
- [From(publication date): 0-2019 - Dec 19, 2025]
- Breakdown by view type
- HTML page views: 2863
- PDF downloads: 924