Research Article Open Access
Immunogenicity and Efficacy of Coxsackievirus A16 Vaccine Candidate Formulations in a Mouse Model
Jia Lu1, Linlin Zhang1, Taixue An1, Qunying Mao2, Pengfei Li1, Fukun Zhang1, Li Li1, Hui Zhou1, Jiling Wang1, Xiaoqi Chen1, Zejun Wang1*, Zhenglun Liang2and Shuo Shen1
1Department of Viral Vaccine Research, Wuhan Institute of Biological Product (WIBP) Ltd. Co., Wuhan 430207, PR China
2National Institutes for Food and Drug Control, Beijing 100050, PR China
- *Corresponding Author:
- Shuo Shen
Department of Viral Vaccine Research
Wuhan Institute of Biological Product (WIBP) Ltd. Co
Wuhan 430207, PR China
Tel: +86 27 88925340
Fax: +86 27 88842261
E-mail: wangzejun��sinopharm.com
Received Date: October 17, 2016; Accepted Date: January 09, 2017; Published Date: January 13, 2017
Citation: Lu J, Zhang L, An T, Mao Q, Li P, et al. (2017) Immunogenicity and Efficacy of Coxsackievirus A16 Vaccine Candidate Formulations in a Mouse Model. J Mol Immunol 1: 105.
Copyright: ©2017 Lu J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Molecular Immunology
Abstract
Besides enterovirus 71 (EV71), Coxsackie virus A16 (CVA16) is a major etiologic agent of hand, foot and mouth disease (HFMD), causing infections in millions of children under 5 years of age each year. The progress made in the development of inactivated EV71 vaccines encourages research aiming at developing a CVA16 vaccine for better prevention of HFMD and control of spreading of a rapidly evolvingvirus. The immunogenicity and efficacy of a CVA16 vaccine candidate were examined in a mouse model. Several vaccine formulations using different adjuvants and formalin-inactivated or non-inactivated full and empty virus particles were compared. It was observed that the CVA16-P4-L731 vaccine induced strong B and T cell immune responses in mice. The mouse antisera contained the highest titers of neutralizing antibodies reported so far and prevented infection of cells in vitro by prototype A and subgenotype B2b. Pups born to immunized mice were protected from disease and death following challenge by more than 10,000 LD50 of homologous and heterologous viruses. Taken together, the data suggest that the CVA17- P4-L731 is a promising vaccine candidate, either independently or as a valuable component for a combined [EV71+CVA16] bivalent vaccine.
Keywords
CVA16; HFMD; Immunogenicity; Vaccine efficacy; Vaccine formulations; Mouse pup model
Introduction
Coxsackievirus A16 (CVA16) is a serotype of the type species Human enterovirus A, genus Enterovirus in the family Picornaviridae [1,2]. The virus contains a single-stranded, positive-sense RNA genome of approximately 7,400 nucleotides in size packaged inside a non-enveloped icosahedral capsid composed of 60 copies of protomers of each of the proteins VP1 to VP4.
The genome can act as mRNA and is translated in a capindependent manner into a single polyprotein which is subsequently processed by virus-encoded into the structural and nonstructural proteins. The virion becomes infectious following the maturation cleavage of the interim product VP0 into the VP2 and VP4 [3] by an autocatalytic mechanism [4,5].
CVA16 and EV71 are the major pathogens of hand, foot and mouth disease (HFMD) [6] among a dozen of other enteroviruses [7], such as CVA4, 5, 6, 9, 10, 12 and CVB3 [8-15], affecting mainly children under 5 years of age [16]. CVA16 is associated less with severe neurological complications than EV71, although this has been reported [17-19].
Data from the Chinese CDC shows that since the outbreak of HFMD in 2008, the reported cases are increasing and peaked in 2012 at more than two millions in China [19]. EV71 and CVA16 as well as other serotypes of non-polio enteroviruses co-circulated [20] and caused indistinguishable clinical symptoms.
The viruses have evolved rapidly and genome recombination has occurred [21]. The phase 1, 2 and 3 clinical trials of EV71 vaccines are promising [22-29] and EV71 vaccine is available in China. Still, a bivalent or even a multivalent vaccine is required for prevention of the spread of these viruses and complete control of HFMD [16,30-32] since the EV71 vaccine does not provide cross-protection against Coxsackievirus A16 and circulating lineages of other HFMDassociated enteroviruses [29,33].
Like other RNA viruses, enteroviruses are known to have a high mutation rate due to the low fidelity of the virus-encoded RNAdependent RNA polymerase (RdRp) and due to frequently occurring recombination events [21,34]. CVA16 has evolved quickly since it was first associated with HFMD in 1959 [9,35].
The genotype B and its subgenotypes and clusters have emerged [20]. Additional antigen variants might exist within several serotypes of enteroviruses on the basis of reduced or nonreciprocal crossneutralization between variants [13,14]. Therefore, a vaccine candidate against HFMD should stimulate immune responses against the prevailing subgenotypes as well as the homologous vaccine strain [3]. In addition, for an inactivated vaccine high yields in a cell culture system and formulation with an optimal adjuvant are also very important [36].
The aim of this study was to screen a CVA16 vaccine candidate against different isolates and their variants in Vero cells, aiming at fulfillment of the criteria mentioned above. The CVA16-P4-L731 was selected from plaque-purified clones after serial passaging of clinical isolates in Vero cells.
Inactivated and non-inactivated full particles (FP) and empty particles (EP) were administrated to Balb/C mice at different dosages and with different adjuvants. The vaccine candidate induced high titers of neutralizing antibodies against itself, genotype A and subgenotypes of B. The vaccine protected one-day-old mouse pups born to immunized mothers passively from disease and death after challenge with CVA16 viruses at extremely high doses of LD50.
Materials and Methods
Ethics statement
Animal experiments were performed in accordance with the guidelines of Chinese Council on Animal Care. The research protocol was approved by Animal Care and Use Committee of WIBP.
Cells and viruses
African green monkey kidney (Vero) and human rhabdomyosarcoma (RD) cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% of fetal calf serum (FCS) (Gibco, Sera), 100 U/ml penicillin and 0.1 mg/ml streptomycin. Virus stocks were produced in flasks at an MOI of 0.01 in DMEM without FCS.
The 2- and 10-stack cell factories (Corning Inc) were used for a large-scale propagation of viruses at an MOI of 0.001. The supernatant of infected cells was harvested when a cytopathic effect (CPE) of 80% was reached. Viruses were titrated by the method of 50% tissue culture infective dose (TCID50)/ml as described previously [37].
Isolation, passaging and plaque purification of a vaccine candidate
The CVA16 P4 strain was isolated from a clinical sample of a patient with HFMD in Hubei in 2010 and passaged serially 11 times in Vero cells at 0.1 MOI. Plaque-to-plaque purification was performed 3 times using 0.5% agarose-DMEM overlay and an identical overlay containing 0.01% of Neutral Red at 3-4 days post-infection. One of clones, referred to as CVA16-P4-L731, was passaged 3 more times in Vero cells for further characterization.
Viral RNA amplification and sequencing
Viral RNA was extracted from virus infected Vero cells using QIAamp® Viral RNA Mini Kit (Qiagen) following the manufacturer’s instructions. Reverse transcription-polymerase chain reactions (RTPCR) were performed using random and CVA16-specific primers, respectively, following the manufacturers’ protocols (TaKaRa). Nine pairs of primers were used to generate overlapping PCR fragments covering the whole genome for sequencing. Sequencing was performed by Genescript Com.
Virus purification
The supernatant of infected cells was clarified by centrifugation at 5°C at 10,940 g in a Beckham JA10 rotor for 15 min and by microfiltration through 0.45 μm filter (Sartorius Intec). Viruses were concentrated by ultrafiltration using a 100 kDa tangential flow filter capsule (Sartorius Intec) to the 1/10 of the original volume. The concentrate was centrifuged through 5 ml of 20% (W/V) sucrose cushion in a Beckman SW28 rotor at 141,000 g for 6 hr at 5°C.
The viruses were dissolved in 0.1 mM CaCl2-10 mM phosphate buffered saline (PBS) at 4°C overnight and centrifuged at 5°C on a 15-55% (W/W) of a sucrose gradient in a Beckman SW41 rotor at 288,000 g for 3 hr. The 80S- and 160S-particles, referred to as empty and full particles (EP and FP), respectively [33], were further purified by centrifugation of a virus mixture at a density of 1.31 g/ml of CsCl at 5°C in a Beckman Ti-90 Rotor at 214,197 g for 18 h.
CsCl was removed from diluted virus fractions by centrifugation in a Beckman SW41 rotor at 288,000 g for 2 h at 5°C. The concentration and purity of viral proteins were determined by SDS-PAGE using a BSA standard (Thermal, Pierce) and by densitometry scanning with Densimeter and software Quantity One (Biorad). The total protein concentration was also determined by a BCA protein assay (Thermal, Pierce).
Transmission electron microscope (TEM)
The 200 mesh, carbon-coated copper grids were soaked in a drop of the purified particle suspension for 5 min, stained with a drop of 1% of uranyl acetate, pH 6.8 for 5 min and air-dried overnight. The grids were viewed under the TEM (FEI Tecnai G2 20 TWIN), and images were photographed.
SDS-PAGE and Western blotting
Proteins of purified CVA16 particles or infected-cell lysates were separated in a 12.5% SDS-PAGE gel and transferred onto NC membranes. The membranes were blocked overnight at 4°C in 1% BSA in PBS containing 0.05% Tween-20 (PBST). Each membrane strip was incubated for 2 hr with individual mouse serum.
A horseradish peroxidase-conjugated goat anti-mouse secondary antibody (Boster, China) was added at a dilution of 1:2000 in PBSTBSA and incubated for 30 min. The membranes were washed three times with PBST after each incubation step. The protein bands were visualized by adding DAB-substrate solution.
Enzyme-linked-immunosorbent-assay (ELISA)
Microplates (YunPeng, China) were coated with 200 ng/well of purified FP or EP in 100 μl of 0.1 M carbonate-bicarbonate buffer (pH 9.6) at 4°C overnight. The plates were washed 5 times with PBST after each incubation step. Each well was blocked with 150 μl PBST-BSA at 37°C for 1 h. Then 100 μl of serial twofold dilutions of mouse serum in BSA-PBST were incubated at 37°C for 1 hr.
Each well was incubated with 100 μl of horseradish peroxidaseconjugated goat anti-mouse IgG (Boster, China) diluted with PBSTBSA to 1:10,000. The plates were incubated at 37°C for 1 hr. Then 100 μl of the substrate solution (Sigma) was added to each well and incubated at 37°C for 30 min. Subsequently, 50 μl of 1M H2SO4 were added to each well, and absorbance at 450 nm wavelengths was measured in a microplate reader (Multiskan MK3, Thermo).
Neutralizing antibody assay
Mouse sera were inactivated at 56°C for 30 min, and serial 2-fold dilutions in DMEM were made. Viruses were diluted in DMEM so that 50 μl of the virus suspension contained 100 TCID50. Equal volumes (50 μl) of serum dilution and virus suspension were mixed and added to each well in 8 duplicates for each dilution.
After incubation at 37°C for 2 h, 1 × 104 RD or Vero cells in 100 μl of DMEM per well were added. Cells were fixed and stained 7 days later. The virus back-titration was performed and titers were in a range of 32 to 320 TCID 50/50 μl. The neutralizing titer was calculated using the Reed-Muench method [38] and expressed as the reciprocal of the highest serum dilution at which CPE in 50% of the wells was completely inhibited.
Immunogenicity and efficacy studies in mice
The purified FP and EP were treated or untreated with commercially-supplied formaldehyde at dilution of 1:2,000 at 37°C for 48 hr. Formaldehyde was removed by ultracentrifugation of particles as described above and residual infectivity were tested in Vero cells. Each antigen were emulsified in 1 mg/ml of Al(OH)3 or 0.2 mg/ml of monophosphoryl lipid A (MPL-A). Groups of ten 6-8 week-old female Balb/C mice were immunized intramuscularly with 0.1 ml of the adjuvant-antigen at 1.5 and 4.5 μg/dose and boosted twice with the same dose at 2 week intervals.
Five mice in each group were sacrificed and the sera were collected on days 10, 21 and 35 after priming. Seven days after the second boosting, spleen lymphocytes were collected for analysis of T-cellmediated immune responses. For challenge experiments, five mice in each group were mated with male mice on the day of first boosting.
Seven days post-second-boosting, groups of 7-16 one-day-old pups were challenged intraperitoneally with known LD50 doses of BIII and P5 strains of CVA16, respectively. The mice were observed daily for 14 days for clinical signs, limb paralyses, eye irritation, loss of balance and death.
IFN-γ Enzyme-linked immunospot (ELISPOT) Assay
The mouse IFN-γ ELISPOT kit (Dakewei Biotech) was used to determine the number of IFN-γ expressing cells in the single-cell suspension following the manufacturer’s instruction. Lymphocytes from spleen of three mice in each group were prepared using EZ-Sep Mouse Lymphocyte Separation Medium following the manufacturer’s recommendation.
Cells were diluted to 2 × 106/ml with Lympho-Spot serum-free medium for rodent containing stimulus, 2 μg/well of phytohemagglutinin or 0.5 μg/well of inactivated FPs) and 2 × 105 cells were added per well. IFN-γ spot-forming cells (SFCs) were enumerated using an ELISPOT Reader (Biosys Bioreader 4000). The number of spots in triplicate wells with medium only was subtracted from the number of spots in test wells. The mean number of antigenspecific IFN-γ SFCs per million cells for each group in triplicate wells was calculated.
Results
Isolation, plaque-purification and characterization of CVA16-P4-L73
The virus was chosen from 20 plaque-purified clones as a vaccine candidate based on (1) its high similarity in the VP1 sequences with the most recently circulating CVA16 isolates in mainland China and Taiwan [30,39]; (2) high yields in Vero cells; (3) high ratio of FP to EP.
Sequence (GenBank accession number, KF924762) comparison showed that it belongs to CVA16 cluster B2b (Supplementary Figure 1).
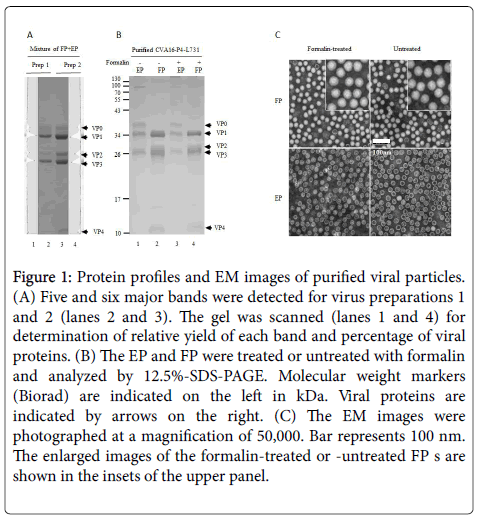
Proteins of the mixed FP and EP of virus preparations were separated by SDS-PAGE, scanned by densitometry, and relative yields of individual proteins were calculated (Figure 1A).
Figure 1: Protein profiles and EM images of purified viral particles. (A) Five and six major bands were detected for virus preparations 1 and 2 (lanes 2 and 3). The gel was scanned (lanes 1 and 4) for determination of relative yield of each band and percentage of viral proteins. (B) The EP and FP were treated or untreated with formalin and analyzed by 12.5%-SDS-PAGE. Molecular weight markers (Biorad) are indicated on the left in kDa. Viral proteins are indicated by arrows on the right. (C) The EM images were photographed at a magnification of 50,000. Bar represents 100 nm. The enlarged images of the formalin-treated or -untreated FP s are shown in the insets of the upper panel.
Based on the molar ratio of VP0 (representing EP) and VP2 (representing FP), the FP and EP ratios were determined. The purities of FP and EP preparations (Figure 1B) were more than 99%. The EP consisted of VP0, VP1 and VP3, representing the empty procapsids.
The FP contained VP1, VP2, VP3 and VP4 as well traces of VP0. The FP contained less VP2 than calculated if the amounts of VP0 were fully cleaved, suggesting that some of the FP were not matured and infectious. Thus, the FPs are a mixture of provirions and mature, infectious particles [40,41].
When formalin-treated and untreated particles were compared by transmission EM (Figure 1C), defined shapes were observed for untreated FP and EP.
The formalin-treated FP appeared to have a less-dense outline than the untreated FP as also observed by Liu [33]. This image may have resulted from formalin cross-linking of viral proteins.
Immunogenicity of inactivated and non-inactivated FP and EP
Vaccine adjuvants are fundamental to stimulate an intense, durable and fast immune response in the presence of low doses and of inactivated antigens. Therefore, Al(OH)3 and MPL-A were coadministered with viral particles to investigate the roles played by them in enhancing humoral and T cell-mediated immune responses.
The FP and EP were formalin-treated and untreated for a fair comparison of immunogenicity of the four particle preparations.
The total level of anti-CVA16 IgG in each group at day 7 postsecond- boosting was determined by a semi-quantitative ELISA.
As shown in Table 1, titers were detected for all mouse groups immunized with antigens in a dose dependent manner. Higher titers were detected in the EP-coated assays than in the FP-coated assays.
| Treatment | aNT | % of protection (ratio) | OD450b | ||
|---|---|---|---|---|---|
| Group | Titer | BIII strain | P5 strain | FP-coated | EP-coated |
| PBS | <8 | 0 (0/14) | 0(0/7) | -0.002 | -0.009 |
| Alum | <8 | 0 (0/12) | 0(0/8) | 0.001 | -0.005 |
| Alum-L731/4.5µg-FP/livec | 841 | 40 (4/10) | 71.4(5/7) | 0.395 | 0.472 |
| Alum-L731/1.5µg-FP/live | 8,192 | 100.0 (9/9) | 64.3 (9/14) | 0.162 | 0.397 |
| Alum-L731/4.5µg-EP/live | 1,263 | 57.1 (8/14) | 46.2 (7/13) | 1.576 | 2.196 |
| Alum-L731/1.5µg-EP/live | 2,048 | 70.0 (7/10) | 100.0 (6/6) | 1.018 | 1.489 |
| Alum-L731/4.5µg-FP/killedd | 1,325 | 91.7 (11/12) | 100.0 (7/7) | 0.948 | 1.335 |
| Alum-L731/1.5µg-FP/killed | 4,291 | 71.4 (10/14) | 83.3(5/6) | 0.426 | 1.036 |
| Alum-L731/4.5µg-EP/killed | 796 | 100.0 (9/9) | 91.7 (11/12) | 0.51 | 0.983 |
| Alum-L731/1.5µg-EP/killed | 192 | 100.0 (7/7) | 70.0 (7/10) | 0.309 | 0.826 |
| Alum-L731/4.5µg-FP+EP/killed | 1,792 | 63.6 (7/11) | 87.5 (14/16) | 1.012 | 1.31 |
| Alum-L731/1.5µg-FP+EP/killed | 1,177 | 50.0 (4/8) | 100.0 (7/7) | 0.311 | 0.493 |
| MPL-A | <8 | 0(0/8) | 0(0/14) | 0.121 | 0.266 |
| MPL-A-L731/4.5µg-FP/killed | 1,191 | 45.4 (5/11) | 100.0 (8/8) | 0.331 | 0.603 |
| MPL-A-L731/1.5µg-FP/killed | 1,092 | 80.0 (4/5) | 85.7(6/7) | 0.143 | 0.179 |
| Naïve | <8 | 0(0/15) | 0(0/13) | -0.002 | -0.011 |
aAverage neutralizing titers of two experiments, using 100TCID50/well
bSera were diluted at 1:10000 and tested in 3 duplicate wells. The wells were coated with FPs or EPs at 0.2 μg/well
c,dNot treated or treated with formalin
Table 1: Neutralizing titers of antisera of immunized mice and protective efficacies of vaccine formulations in the pup mouse challenge model. Antisera were collected from groups 7 and 8 as indicated in Table 1 Formalin-treated L731/FPs was used for immunization of mice.
No antibodies were detected using the sera from naive, PBS- and PBS-adjuvants-injected mice. If not treated with formalin, the EP induced higher antibody response than the FP. Interestingly, if treated the FP stimulated higher antibody response than the EP. Furthermore, if treated both, the FP and FP+EP mixtures, induced stronger antibody responses than the EP did. When the FP were formulated with MPL-A, the antibody response was weaker than those with Al(OH)3.
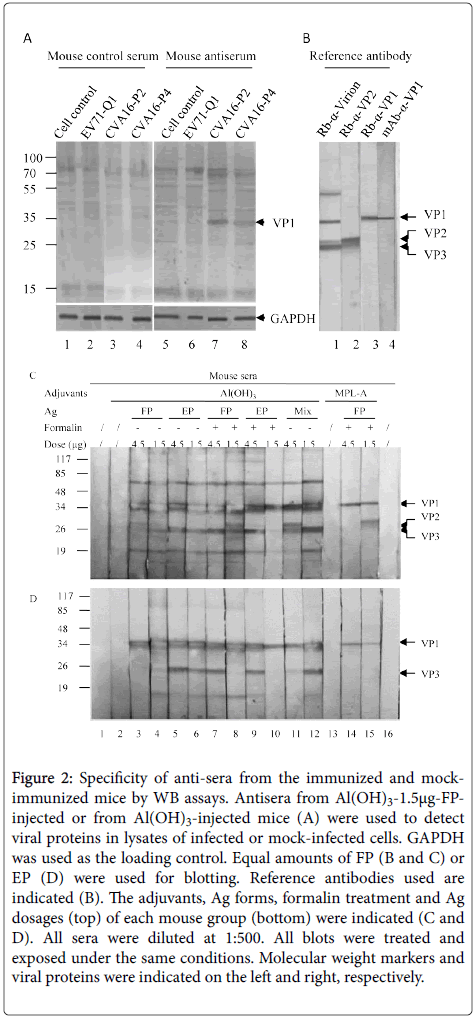
Specificity of antibodies was determined by WB assays (Figure 2). As shown in Figure 2A, antiserum against CVA16 vaccine strain raised from immunized mouse only recognized the VP1 (Figure 2A, lanes 7 and 8) in the lysates of cells infected by CVA16-P2 and CVA16-P4 but not by EV71-Q1 or mock infected cells (Figure 2A, lanes 5 and 6).
Figure 2: Specificity of anti-sera from the immunized and mockimmunized mice by WB assays. Antisera from Al(OH)3-1.5μg-FPinjected or from Al(OH)3-injected mice (A) were used to detect viral proteins in lysates of infected or mock-infected cells. GAPDH was used as the loading control. Equal amounts of FP (B and C) or EP (D) were used for blotting. Reference antibodies used are indicated (B). The adjuvants, Ag forms, formalin treatment and Ag dosages (top) of each mouse group (bottom) were indicated (C and D). All sera were diluted at 1:500. All blots were treated and exposed under the same conditions. Molecular weight markers and viral proteins were indicated on the left and right, respectively
The antisesun recognized other structural proteins weekly under these conditions. The antiserum from control mouse did not recognize the VP1 in these same lysates (Figure 2A, lanes 1-4).
In order to demonstrate the specificity of the VP1 detected in Figure 2A, viral proteins of purified CVA16 particles were detected with rabbit anti-virion, anti-VP1, anti-VP2 and mouse monoclonal antibody against VP1(Figure 2B).
When the FP was blotted (Figure 2C), sera from the FP-immunized mice recognized the VP1, VP2 and VP3 while those of the EPimmunized mice recognized the VP1 and VP3 but not the VP2. When the EP was blotted (Figure 2D), antisera of both FP- and EPimmunized mice recognized the VP1 and VP3 but not the VP0. The results showed that the antigenicity of the VP0 in the EP was poor.
The extra larger bands might represent the cross-linking of viral proteins as the formalin-treated FP and EF were used in these experiments. The neutralizing titers of sera were determined (Table 1).
It was observed that the higher titers (groups 3 to 8), or the roughly same titers (groups 14 and 15) were reached when low dose of 1.5 μg of particles was used. Dose dependent effects were recorded only in groups 9 to 12. The FP induced higher titers than the EP and the untreated preparations higher titers than the formalin-treated ones.
Dynamics of humoral immune response
The titers of neutralizing antibody after priming and the first and second boosting at two dosages (1.5 and 4.5 μg) of Alum-inactivated- FP were compared (Table 2). The results showed that 1.5 μginactivated FP induced the same level of antibody post-first-boosting and much high level of antibody post-second-boosting than the 4.5 μg dosage.
| Dose/Adjuvant | Neutralizing antibody titers | ||
|---|---|---|---|
| Priming>a | 1stboostingb | 2ndboostingc | |
| 4.5µg/Al(OH)3 | 24 | 786 | 1325 |
| 1.5µg/Al(OH)3 | 16 | 786 | 4291 |
Table 2: Comparison of titers of neutralizing antibodies after each of immunization in Balb/C mice; Sera were collected 10 days postpriminga, 7 days post-firstb and secondc boosting at an interval of 14 days.
Cross-neutralizing activity of mouse anti-sera against other strains
As shown in Table 3A, antisera, pooled from the groups of immunized mice, cross-neutralized other isolates with different VP1 or genome sequences.
| Virus | CVA16-731 | CVA16-P5 | CVA16-P6 |
|---|---|---|---|
| Cluster | B2b | B2b | B2a |
| VP1 Aa difa | / | 1 | 0 |
| NT titer | 1024 | 512 | 407 |
Table 3A: Cross neutralizing activity of anti-L731 antiserum pool against different subgenotypes and genotype; A: Cross neutralizing activity of serum raised with CVA16-P4-L731 against other B2 subgenotypes of CVA16;aAmino acid difference.
Furthermore, the prototype G10 and other subgenotypes were also well neutralized by the mouse antiserum pool or by rabbit hyperimmune antiserum (Table 3B).
| Strain | Titer | ||||
|---|---|---|---|---|---|
| Genotype | Rb-L731a | Ms-L731 | HS-1 | HS-2 | |
| L731 | B2b | >16438 | 1072 | 9333 | 335 |
| LZ101 | B2b | >16438 | 464 | 3715 | 81 |
| LZ112 | B2b | >16438 | 1288 | 9333 | 211 |
| LZ134 | B2b | >16438 | 192 | 333 | <8 |
| G10 | A | >16438 | 1024 | 5370 | 106 |
Table 3B: Cross neutralizing activity of anti-L731 antiserum pool against prototype G10 and other subgenotypes; aRb-731, hyperimmune rabbit antisera; Ms-L731, pooled mouse antisera; HS-1 and HS-2, human sera from CVA16-infected patients. RD cells were used. Titers were average of three experiments.
Also, L731 was neutralized by human reference sera collected from patients infected with currently prevailing CVA16 strains (Table 3B).
The results suggested that the CVA16-P4-L731 vaccine candidate might provide broad-spectrum-protection against infection and disease caused by different genotypes and prevailing isolates of CVA16.
Protection of pups from challenge with homologous and heterologous virus strains
The homologous CVA16-P4 and heterologous CVA16-P5 strains were grown in Vero cells for 11 passages. The CVA16-P4 strain was further adapted to infect new-born mice by passaging in mice for 3 times and designated as BIII.
Infection of Balb/c mice indicated that BIII was able to cause the death of both new-born and 19 day mice within a few days.
The BIII virus obtained from homogenates of brain tissues of mice with acute flaccid paralysis was passaged once in Vero cells before use. The CVA16-P5 was not adapted further in mice and able to infect newborn mice only.
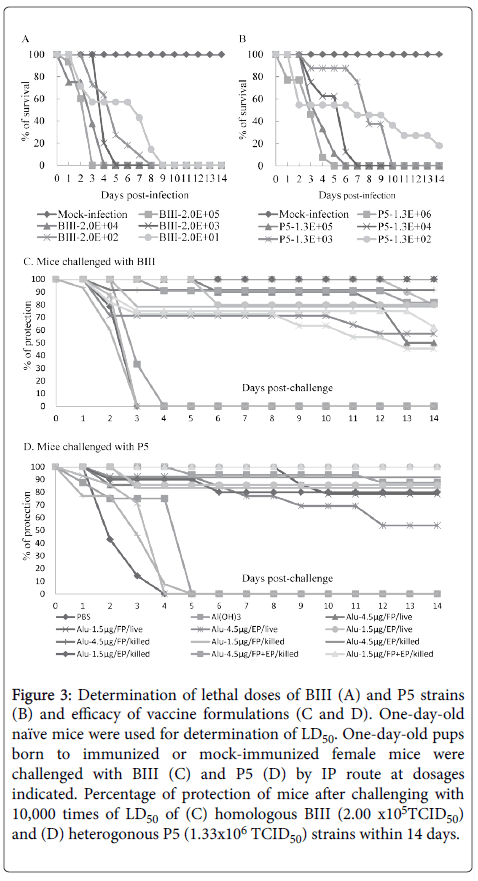
The vaccine strain was derived from plaque-purified CVA16-P4. The LD50 of the BIII and P5 strains were determined first (Figure 3).
Figure 3: Determination of lethal doses of BIII (A) and P5 strains (B) and efficacy of vaccine formulations (C and D). One-day-old naïve mice were used for determination of LD50. One-day-old pups born to immunized or mock-immunized female mice were challenged with BIII (C) and P5 (D) by IP route at dosages indicated. Percentage of protection of mice after challenging with 10,000 times of LD50 of (C) homologous BIII (2.00 x105TCID50) and (D) heterogonous P5 (1.33x106 TCID50) strains within 14 days.
Inoculation of BIII and P5 induced death of new-born Balb/C in an age- and dose-dependent manner. The BIII caused the death of all mice on day 9 and the P5 caused death in 80% of the mice on day 14 at their highest dilution.
Therefore, the LD50 values of BIII and P5 are less than 2 × 101 and 1.33 × 102 TCID50, respectively.
For challenging experiments, groups of 7 to 16 one-day-old pups born to CVA16-P4-L731-immunized or mock-immunized mice were intraperitonealy inoculated with BIII and P5 challenging strains at more than 10,000 times of LD50, i.e., 2 × 105 and 1.33 × 106 TCID50/ mouse pup respectively.
As shown in Figure 3 and Table 1, for the vaccine groups the percentage of survival ranged between 45.5-100% for BIII and 53.9-100% for P5. In contrast, groups of pups born of naïve, PBS-, Al(OH)3, and MPL-A-injected mice fell ill and died within 3-4 days and 4-5 days post-challenging with BIII and P5, respectively.
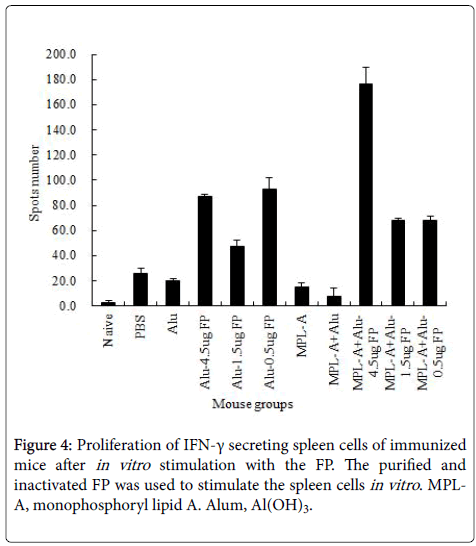
Specific proliferation of IFN-γ secreting T cell
The T cell mediated immune response to the vaccine strain was evaluated. As shown in Figure 4, the FP emulsified with Alum at all doses enhanced the proliferation of INF-γ secreting cells, specifically after in vitro stimulation of spleen cells with the purified vaccine strain.
The FP at a high dose co-administrated with MPL-A-Alum mixture significantly increased this innate immune response compared to administration of FP with alum alone.
Discussion
A bivalent EV71-CVA16 or polyvalent vaccines including other enteroviruses causing HFMD may be needed in a long term perspective of HFMD prevention and in case of the emergence of new virulent viruses with antigenic variations. Bivalent vaccine, or polyvalent vaccine in circumstances of outbreaks caused by CVA6 [12], CVA9 [8,10], CVA10 and CVA12 [15], would increase public confidence and acceptance of HFMD vaccines [16,32].
A major issue for multivalent vaccines is the balancing of immunogenicity for each of the individual viruses. Based on published reports [31,42,43] and our experience, the immunogenicity of CVA16 is much weaker than that of EV71. For EV71, cross-neutralizing activity of antibodies against different genotypes is generally high [30,44]. For CVA16, however, lower cross-neutralizing activity or onesided cross-activity may exist between different genotypes or even within subgenotypes as indicated in this study although a report describes high cross-neutralizing activities [45]. Unlike L731, the LZ134 strain was not well neutralized by the human reference antisera, showing more than 10 times differences in titers of neutralizing antibodies against other strains. The genetic variations among subgenotypes and clusters might result in antigenic difference, making it difficult to select appropriate vaccine candidates. Our data suggest that the neutralizing epitopes determine the cross-neutralizing activity instead of genotypes, subgenotypes or clusters based on the VP1 nucleotide sequences. For example, the identity of L731 (B2b) is 79.0% and 91.8% in nt sequence with the G10 (A) and P6 (B2a) and there are 27 and zero amino acid changes in the VP1, respectively. However, the cross-neutralizing titers are the same or very close to those against itself (Table 3).
The experimental evidence has shown that CVA16-P4-L731 is a vaccine candidate with excellent immunogenicity against itself and other relatively closely-related enterovirus strains. The titers of neutralizing antibodies reached to 8,192 for the non-inactivated and 4,291 for inactivated FP, the highest reported so far, in a neutralization assay with an end point of complete inhibition of CPE in seven days rather than in 3 days [39,46]. The neutralizing titers were much higher than those previously reported [31,43,44].
The EP and FP of EV71, and maybe those of CVA16, are different in size, conformation, protein cleavage and density due to packaging of viral RNA [40,47,48]. Our results demonstrated that immunization with FP stimulated higher titers of neutralizing antibodies than with EP in agreement with the report Chou AH, et al. [31]. The reasons for this observation might be as follows: The EP degraded quicker than the FP; some of neutralization specific epitopes were not formed or exposed before the final cleavage of VP0 into the VP2 and VP4; or viral RNA might function as an adjuvant. We found that the FPs were better than the EPs at stimulating high levels of neutralizing antibodies. Western blot assays showed that the EPs did not induce much of the anti-VP2 antibody and none of the mouse sera recognized the unprocessed VP0. If the VP2 of CVA16 carries the neutralization specific epitopes, the non-cleaved VP0 in EPs may not induce antibodies against them.
It has been documented that formalin treatment could partially damage the neutralization specific epitopes of viral vaccines [31]. However, it was also reported that viral proteins purified from formalin-inactivated poliovirus induced neutralizing antibodies but not those from untreated virus, as the treatment protected the proteins from degradation [49]. The results presented here support the use of formalin-inactivated CVA16-EV71 bivalent vaccine against HFMD. Vaccines against HFMD formulated with adjuvants enhancing both B cell- and T cell-mediated immune responses might be more effective [50,51]. This might be achieved by use of a combination of alum and MPL-A adjuvants as shown in this study.
Tremendous observations indicate that interferon (IFN) plays a pivotal role in the antiviral immunity [47] and transcription factors (TFs), such as IRF3 and IRF7, along with NF-κB and AP1, have been found to be essential for the initiation of IFN genes transcription in human [52,53]. In addition to these TFs, which are able to directly bind to the promoters of IFN genes, some epigenetic modifiers also are also involved in these processes. For instance, histone H3K9 modifier G9a has been found to reversibly regulate the expression of IFN genes in humans [44]. As recent observation indicates that G9a is also required for the maintenance of imprinted DNA methylation via interaction with DNMTs [54], the promoters of IFN genes are very likely to be protected from establishment of DNA methylation, a stable epigenetic mark that associated with gene suppression. Therefore, it will be a new direction to investigate the underlying mechanisms that associated with occupying and preventing gain of 5 meC DNA methylation at the promoters of IFN genes.
Funding
This work was supported partially by the Ministry of Science and Technology, PR China [2013ZX09402-302].
Acknowledgement
We thank Jie Wu for support in animal work, Jing Guo, Mi Deng and Gaobo Zhang for helping with some of the experiments, and Prof. Dingxiang Liu, National University of Singapore, and Dr. Ulrich Desselberger, the University of Cambridge, U.K., for support with the analysis of the data and comments on the manuscript.
Conflicts of Interests
The authors declare that they have no conflicts of interests. The information on this work was not presented previously in any meeting and was not submitted for publication elsewhere.
References
- Pallansch MA, Oberste MS, Whitton JL (2013) Enteroviruses: Polioviruses, Coxsackieviruses, Echoviruses, and Newer Enteroviruses. Fields Virology 6: 490-530.
- Knowles NJ, Hovi T, Hyypiae T, Adams MJ, EB Carstens, et al. (2011) Picornaviridae. In: AMQ King,. Classification and nomenclature of Viruses. Ninth Report of the ICTV. Elsevier San Diego pp: 855-880.
- Mao QY, Li N, Yu X, Yao X, Li FX, et al. (2012) Antigenicity, animal protective effect and genetic characteristics of candidate vaccine strains of enterovirus 71. Arch Virol 157: 37-41.
- Bishop NE, Anderson DA (1993) RNA-dependent cleavage of VP0 capsid protein in provirions of hepatitis A virus. Virology 197: 616-623.
- Compton SR, Nelsen B, Kirkegaard K (1990) Temperature-sensitive poliovirus mutant fails to cleave VP0 and accumulates provirions. J Virol 64: 4067-4075.
- Chen L, Mou X, Zhang Q, Li Y, Lin J, et al. (2012) Detection of human enterovirus 71 and coxsackievirus A16 in children with hand, foot and mouth disease in China. Mol Med Rep 5: 1001-1004.
- Mao QY, Wang YP, Yao X, Bian LL, Wu X, et al. (2013) Coxsackievirus A16: Epidemiology, diagnosis, and vaccine. Hum Vaccin Immunother 10: 360-367.
- Cui A, Yu D, Zhu Z, Meng L, Li H, et al. (2010) An outbreak of aseptic meningitis caused by coxsackievirus A9 in Gansu, the People’s Republic of China. Virol J 7: 72.
- Alsop J, Flewett TH, Foster JR (1960) "Hand-foot-and-mouth disease" in Birmingham in 1959. Br Med J 2: 1708-1711.
- Flewett TH, Warin RP, Clarke SK (1963) Hand, foot, and mouth disease associated with coxsackie A5 virus. J Clin Pathol 16: 53-55.
- Hughes RO, Roberts C (1972) Hand, foot, and mouth disease associated with coxsackie A9 virus. Lancet 2: 751-752.
- He YQ, Chen L, Xu WB, Yang H, Wang HZ, et al. (2013) Emergence, circulation, and spatiotemporal phylogenetic analysis of coxsackievirus A6- and coxsackievirus A10-associated hand, foot, and mouth disease infections from 2008 to 2012 in Shenzhen, China. J Clin Microbiol 51: 3560-3566.
- Lu QB, Zhang XA, Wo Y, Xu HM, Li XJ, et al. (2012) Circulation of coxsackievirus A10 and A6 in hand-foot-mouth disease in China, 2009-2011. PLoS One 7: e52073.
- Tian H, Zhang Y, Sun Q, Zhu S, Li X, et al. (2014) Prevalence of multiple enteroviruses associated with hand, foot, and mouth disease in shijiazhuang city, Hebei province, china: outbreaks of coxsackieviruses A10 and B3. PLoS One 9: e84233.
- Yang F, Zhang T, Hu Y, Wang X, Du J, et al. (2011) Survey of enterovirus infections from hand, foot and mouth disease outbreak in China, 2009. Virol J 8: 508.
- Lee MS, Tseng FC, Wang JR, Chi CY, Chong P, et al. (2012) Challenges to licensure of enterovirus 71 vaccines. PLoS Negl Trop Dis 6: e1737.
- Wang CY, Li LF, Wu MH, Lee CY, Huang LM (2004) Fatal coxsackievirus A16 infection. Pediatr Infect Dis J 23: 275-276.
- Legay F, Lévêque N, Gacouin A, Tattevin P, Bouet J, et al. (2007) Fatal Coxsackievirus A16 pneumonitis in adult. Emerg Infect Dis 13: 1084-1086.
- Xing WJ, Liao QH, Viboud C, Zhang J, Sun J, et al. (2014) Hand, foot, and mouth disease in China, 2008-12: an epidemiological study. Lancet Infect Dis 14: 308-318.
- Li L, He Y, Yang H, Zhu J, Xu X, et al. (2005) Genetic characteristics of human enterovirus 71 and coxsakievirus A16 circulating from 1999 to 2004 in Shenzhen, People’s Republic of China. J Clin Microbiol 43: 3835-3839.
- Zhao K, Han X, Wang G, Hu W, Zhang W, et al. (2011) Circulating Coxsackievirus A16 identified as recombinant type A human enterovirus, China. Emerg Infect Dis 17: 1537-1540.
- Liang ZL, Mao QY, Gao F, Wang J (2013) Progress on the research and development of human enterovirus 71 (EV71) vaccines. Front Med 7: 111-121.
- Li C, Wang JC, Taylor MW, Zlotnick A (2012) In vitro assembly of an empty picornavirus capsid follows a dodecahedral path. J Virol 86: 13062-13069.
- Li RC, Liu LD, Mo ZJ, Wang X, Xia J, et al. (2014) An inactivated enterovirus 71 vaccine in healthy children. N Engl J Med 370: 829-837.
- Zhu FC, Liang ZL, Li XL, Ge HM, Meng FY, et al. (2013) Immunogenicity and safety of an enterovirus 71 vaccine in healthy Chinese children and infants: a randomised, double-blind, placebo-controlled phase 2 clinical trial. Lancet 381: 1037-1045.
- Zhu FC, Meng FY, Li JX, Li XL, Mao QY, et al. (2013) Efficacy, safety, and immunology of an inactivated alum-adjuvant enterovirus 71 vaccine in children in China: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 381: 2024-2032.
- Li YP, Liang ZL, Xia JL, Wu JY, Wang L, et al. (2014) Immunogenicity, safety, and immune persistence of a novel inactivated human enterovirus 71 vaccine: A Phase II, randomized, double-blind, placebo-controlled trial. J Infect Dis 209: 46-55.
- Zhu FC, Xu WB, Xia JL, Liang Z, Liu Y, et al. (2014) Efficacy, Safety, and immunogenicity of an enterovirus 71 Vaccine in China. N Engl J Med 370: 818-828.
- McMinn PC (2014) Enterovirus vaccines for an emerging cause of brain-stem encaphilitis. N Engl J Med 370: 792-794.
- Chang JY, Chang CP, Tsai HH, Lee CD, Lian WC, et al. (2012) Selection and characterization of vaccine strain for Enterovirus 71 vaccine development. Vaccine 30: 703-711.
- Chou AH, Liu CC, Chang JY, Jiang R, Hsieh YC, et al. (2013) Formalin-inactivated EV71 vaccine candidate induced cross-neutralizing antibody against subgenotypes B1, B4, B5 and C4A in adult volunteers. PLoS One 8: e79783.
- Chong P, Guo MS, Lin FH, Hsiao KN, Weng SY, et al. (2012) Immunological and biochemical characterization of coxsackie virus A16 viral particles. PLoS One 7: e49973.
- Chong P, Hsieh SY, Liu CC, Chou AH, Chang JY, et al. (2012) Production of EV71 vaccine candidates. Hum Vaccin Immunother 8: 1775-1783.
- Liu CC, Guo MS, Lin FH, Hsiao KN, Chang KH, et al. (2011) Purification and characterization of enterovirus 71 viral particles produced from Vero cells grown in a serum-free microcarrier bioreactor system. PLoS One 6: e20005.
- Li J, Huo X, Dai Y, Yang Z, Lei Y, et al. (2012) Evidences for intertypic and intratypic recombinant events in EV71 of hand, foot and mouth disease during an epidemic in Hubei Province, China, 2011. Virus Res 169: 195-202.
- Robinson CR, Doane FW, Rhodes AJ (1958) Report of an outbreak of febrile illness with pharyngeal lesions and exanthem: Toronto, summer 1957; isolation of group A coxsackie virus. Can Med Assoc J 79: 615-621.
- Li YP, Liang ZL, Gao Q, Huang LR, Mao QY, et al. (2012) Safety and immunogenicity of a novel human enterovirus 71 (EV71) vaccine: a randomized, placebo-controlled, double-blind, Phase I clinical trial. Vaccine 30: 3295-3303.
- Chou AH, Liu CC, Chang JY, Lien SP, Guo MS, et al. (2012) Immunological evaluation and comparison of different EV71 vaccine candidates. Clin Dev Immunol pp:1-8.
- Reed LG, Muench H (1938) A simple method to estimating fifty percent endpoints. Am J Hyg 27: 493-497.
- Liu Q, Yan K, Feng Y, Huang X, Ku Z, et al. (2012) A virus-like particle vaccine for coxsackievirus A16 potently elicits neutralizing antibodies that protect mice against lethal challenge. Vaccine 30: 6642-6648.
- Cifuente JO, Lee H, Yoder JD, Shingler KL, Carnegie MS, et al. (2013) Structures of the procapsid and mature virion of enterovirus 71 strain 1095. J Virol 87: 7637-7645.
- Qi AW,Guo SZ,Wen PR, Ping YB, Chao ZHY, et al. (2014) The immunogenicity and protection effect of the BPL-inactivated CA16 vaccine in different animal systems. Hum Vaccin Immunother10: 628-639.
- Yang E,Cheng C,Zhang Y,Wang J, Che Y, et al. (2014) Comparative study of the immunogenicity in mice and monkeys of an inactivated CA16 vaccine made from a human diploid cell line. Hum Vaccin Immunother 10: 1266-1273.
- Xie Z, Pu J, Huang H, Liu Z, Dong C, et al. (2013) Immunogenicity and cross-reactivity of four coxsackievirus group A type 16 strains. Chinese J Biologicals 26: 1366-1370.
- Cai Y, Ku Z, Liu Q, Leng Q, Huang Z (2014) A combination vaccine comprising of inactivated enterovirus 71 and coxsackievirus A16 elicits a balanced protective immunity against both viruses. Vaccine 32: 2406-2412.
- Wang X, Peng W, Ren J, Hu Z, Xu J, et al. (2012) A sensor-adaptor mechanism for enterovirus uncoating from structures of EV71. Nat Struct Mol Biol 19: 424-429.
- Samuel CE(2001) Antiviral actions of interferons. Clin Microbiol Rev 14: 778-809.
- Van Wezel AL, van der Marel P, Hazendonk TG, Boer-Bak V, Henneke MA (1983) Antigenicity and immunogenicity of poliovirus capsid proteins. Dev Biol Stand 55: 209-215.
- Ahlers JD, Belyakov IM (2010) Memories that last forever: strategies for optimizing vaccine T-cell memory. Blood 115: 1678-1689.
- Kaech SM, Wherry EJ, Ahmed R (2002) Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol 2: 251-262.
- Agalioti T, Lomvardas S, Parekh B, Yie J, Maniatis T, et al. (2000) Ordered recruitment of chromatin modifying and general transcription factors to the IFN-beta promoter. Cell 103: 667-678.
- Panne D, Maniatis T, Harrison SC (2007) An atomic model of the interferon-beta enhanceosome. Cell 129: 1111-1123.
- Fang TC, Schaefer U, Mecklenbrauker I, Stienen A, Dewell S, et al. (2012) Histone H3 lysine 9 di-methylation as an epigenetic signature of the interferon response. JEM 209: 661-669.
- Zhang T, Termanis A, Özkan B, Bao XX, Culley J, et al. (2016) G9a/GLP Complex Maintains Imprinted DNA Methylation in Embryonic Stem Cells. Cell Rep 15: 77-85.
Relevant Topics
- Bacteriostatic antibiotics
- Cell signaling and activation
- Chemokines
- Class I MHC molecules
- Class II MHC molecule
- Colitis Antibiotics
- Immune response
- Immunochemistry
- Immunogenicity of biopharmaceuticals
- Immunogenomics
- Immunoglobulins
- Immunoglycomics
- Immunomodulatory xenobiotics
- Immunopharmacology
- Immunoproteomics
- Immunosenescence
- Immunotolerance
- Molecular Immunology
- Non classical MHC class I molecules
Recommended Journals
Article Tools
Article Usage
- Total views: 13680
- [From(publication date):
June-2017 - Aug 18, 2025] - Breakdown by view type
- HTML page views : 12694
- PDF downloads : 986