Impact of an Antimicrobial Stewardship Initiative on Ertapenem Use and Carbapenem Susceptibilities at Four Community Hospitals
Received: 17-Oct-2017 / Accepted Date: 25-Oct-2017 / Published Date: 30-Oct-2017 DOI: 10.4172/2332-0877.1000341
Abstract
Background: After observed widespread use of ertapenem at four community hospitals, antimicrobial stewardship initiatives (ASIs) were undertaken to minimize ertapenem use. Our objective was to evaluate the impact of ASIs on antibiotic utilization and Pseudomonas aeruginosa and Enterobacteriaceae susceptibility patterns.
Methods: This was a retrospective pre-post implementation study conducted at four Methodist Healthcare System hospitals in San Antonio, Texas from July 2013 to July 2015. A multilevel ASI to reduce ertapenem utilization was implemented in July 2014. The ASI comprised of a clinical decision support notification for prescribers, order set modification, formal provider education, and retrospective/prospective audit. Monthly ertapenem use was expressed in days of therapy (DOT)/1,000 adjusted patient days (APD). The rates of carbapenem nonsusceptible P. aeruginosa, Escherichia coli and Klebsiella pneumoniae were calculated monthly. A segmented regression analysis for interrupted time series was used to evaluate ertapenem usage pre- and post-intervention.
Results: Overall ertapenem utilization decreased across the study period (18.3 DOT/1,000 APDs in July 2013 vs. 5.1 in July 2015). The mean ertapenem DOT/1,000 APDs declined approximately 60% from the pre vs. post intervention period (17.6 vs. 7.0, p<0.001). Rates of group-2 carbapenem nonsusceptible P. aeruginosa isolates decreased in the post intervention period (4.9 per 10,000 APDs vs. 3.7 per 10,000 APDs; p=0.03), while carbapenem nonsusceptible E. coli and K. pneumoniae or remained stable across the study period.
Conclusion: This multilevel ASI aimed at minimizing ertapenem utilization resulted in substantial declined use. Susceptibilities of P. aeruginosa and Enterobacteriaceae isolates to group 2 carbapenems remained stable in the post-intervention period.
Keywords: Antimicrobial stewardship; Carbapenems; Ecology; Ertapenem; Carbapenem resistance
Abbreviations
APD: Adjusted Patient Days; ASI: Antimicrobial Stewardship Initiatives; CIRE: Carbapenem-Intermediate or–Resistant Enterobacteriaceae; CRE: Carbapenem Resistant Enterobacteriaceae; DOT: Days of Therapy; ESBL: Extended Spectrum Beta-lactamase; MUE: a Medication use Evaluation; MHS: Methodist Healthcare System; MIC: Minimum Inhibitory Concentration.
Introduction
Antimicrobial resistance is one of the largest healthcare threats in the United States, with roughly 2,000,000 illnesses and 23,000 deaths due to resistant pathogens each year [1]. In 2011, U.S. antibiotic resistance resulted in $20 billion in excess healthcare expenditures, $35 billion in societal costs, and 8 million excess hospital days [2]. In addition to facing increased costs and duration of hospitalization, patients with resistant infections may undergo treatment with more toxic agents, including those with limited efficacy data to support optimal outcomes. While the number of resistant organisms continues to grow, the number of antimicrobial agents in development has declined, bolstering the argument for more responsible antimicrobial usage and infection prevention strategies.
Although antibiotics are among the most frequently prescribed drugs, therapy is not optimized in as much as 50% of the time, increasing selective pressure and bacterial resistance development [1]. Antimicrobial stewardship programs, which often track antibiotic prescribing trends and assess for optimal use, have demonstrated the ability to improve patient safety, reverse antimicrobial resistance rates, and decrease healthcare expenditures [3-17]. With the urgent threat from carbapenem-resistant organisms, the utilization of carbapenems and other broad-spectrum antibiotics has drawn attention [18]. Ertapenem is an appropriate target for drug use review and stewardship initiatives, given its broad spectrum activity, multiple approved indications, potential impact on collateral resistance, and economic cost.
Ertapenem is a 1β-methyl carbapenem approved for complicated intra-abdominal infections (cIAI), prophylaxis of surgical site infection in elective colorectal surgery, amongst other indications [19-21]. This agent has a broad-spectrum coverage, including activity against extended spectrum beta-lactamase (ESBL) and AmpC producing bacterial pathogens, with evidence supporting carbapenems as superior agents [19-21]. However, it is generally accepted that widespread use of any antibiotic will eventually lead to the development of resistance, supporting the reservation of broad-spectrum, last-line agents [22]. In vitro studies have shown that ertapenem may select for resistant Pseudomonal isolates and lead to cross-resistance to imipenem and meropenem, although this has never been proven in vivo [23-26]. Carbapenem use has been shown to increase the risk of carbapenem-resistant Klebsiella pneumoniae infections in inpatients, further illustrating the need for prudent utilization [27,28].
Many institutions have reported improvement in carbapenem susceptibilities after implementing ASIs to restrict carbapenems as a class; however, the impact of ASIs to decrease ertapenem is not known. After observed widespread prescribing of ertapenem at four community hospitals, antimicrobial stewardship initiatives (ASIs) were undertaken to minimize its use. Our objective was to evaluate the impact of ASIs on antimicrobial use and Pseudomonas aeruginosa and Enterobacteriaceae susceptibility patterns.
Methods
Study setting
This study was conducted in four community hospitals in the Methodist Healthcare System (MHS) in San Antonio, TX. The hospitals consisted of Methodist Hospital (845 beds), Methodist Specialty and Transplant Hospital (267 beds), Methodist Metropolitan Hospital (388 beds), and Northeast Methodist Hospital (169 beds).
Implementation of ASI
In late 2013, a medication use evaluation (MUE) was completed across the Methodist Healthcare System (MHS) to review the use of ertapenem. Results of this MUE showed extensive use of ertapenem in a variety of patient populations from a wide range of prescribers [29]. In January 2014 (Phase I of ASIs), a robust educational initiative was implemented across the four MHS hospitals to minimize ertapenem use and provide awareness of alternatives. Several groups were targeted for the education including hospitalists, emergency medicine physicians, intensivists, multiple surgery groups, and pharmacists.
In June 2014, additional measures to minimize indiscriminate use of ertapenem were implemented including targeted education and removal of ertapenem from all MHS order sets. Physicians who frequently prescribed ertapenem were targeted for further education. An automated alert was developed within the electronic medical record that would notify the prescribing physicians of appropriate indications for ertapenem use. The ertapenem alert was implemented in July 2014 (Phase II of ASI). Targeted education continued throughout the study period.
Study design
This was a retrospective pre-post implementation study from July 2013 to July 2015. A multilevel ASI to reduce ertapenem utilization was implemented over 6 months January-July 2014. The post-ASI phase I period included 19 months (January 2014 to July 2015) from the start of ASI with a pre-ASI period of 6 months (July 2013 to December 2013). The post-ASI phase II period included 12 months (August 2014 to July 2015) with a pre-ASI period of 7 months (January 2014 to July 2014).
Drug utilization data and analysis
Drug consumption data was collected via central mining software (MEDITECH; Westwood, MA). Ertapenem consumption was measured as number of days of therapy (DOT) per 1,000 patient days [30]. To identify other temporal changes to other antimicrobials during the post-implementation phase, consumption data for the following antibiotics were evaluated: ampicillin/sulbactam, aztreonam, cefepime, ceftriaxone, fluoroquinolones, aminoglycosides, and carbapenems. Only antibiotics intended for systemic use were evaluated; antibiotics prescribed as an ophthalmic solution, ointment, irrigation, or enema were not included. DOT were aggregated monthly for all antibiotics at each facility and across all sites.
Microbiological data and analysis
All isolates were obtained from clinical cultures from January 2014 to July 2015. Only the first isolate per patient per month was included in the analysis. Antimicrobial susceptibility testing was conducted using the VITEK2 system (bioMerieux, Durham, NC). Antimicrobial minimum inhibitory concentrations (MICs) were interpreted according to the Clinical and Laboratory Standards Institute document M100-S14 (2014) [31]. Monthly E. coli and K. pneumoniae susceptibilities to carbapenems were collected to indicate the prevalence of carbapenem-non susceptible Enterobacteriaceae. Carbapenem non-susceptibility in E. coli and K. pneumoniae was defined as isolates for which the imipenem and meropenem MIC was >2 μg/mL or ertapenem MIC was >1 μg/mL [32]. Susceptibilities for P. aeruginosa to meropenem and imipenem were collected. Nonsusceptible rates were measured as the proportion of isolates non-susceptible to carbapenems and incidence density on a monthly basis. The incidence density was calculated as the number of non-susceptible isolates per 10,000 patient days.
Statistical analysis
Data were summarized by means and compared by Student’s t-test for pre and post intervention groups. Segmented regression analysis for interrupted time series was used to determine the significance of the differences in levels and slopes of ertapenem utilization over time after implementation of the two intervention phases in July 2014. Estimations were made of the change in ertapenem utilization and isolation of carbapenem non-susceptible E. coli, K. pneumoniae, and P. aeruginosa following phase II, the difference between the pre- and post-intervention slopes of the outcome, and the 12 month intervention effect after the intervention. Significance was determined at the 0.05 level, and SPSS 23.0 (IBM Corp, Armonk, NY, USA) was used for all statistical analyses. The Appendix contains detailed description of the model.
Results
Impact of ASIs on ertapenem utilization
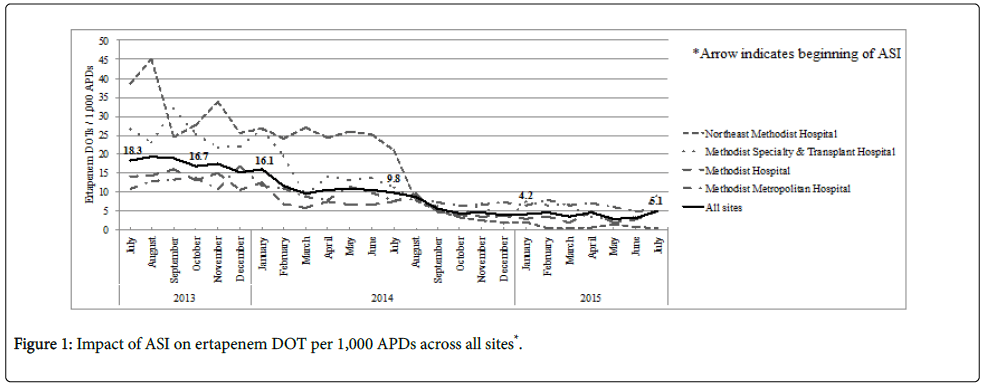
Overall ertapenem use declined from 18.3 to 5.1 DOT/1,000 APDs between July 2013 and July 2015. This represented a reduction from 841 doses administered during the first month and 253 doses in the last month of the study period. Among the four participating facilities, Northeast Methodist Hospital experienced the highest reduction in utilization, with a decline from 38.6 to 0.4 DOT/1,000 APDs across the study period. This reduction was followed by Methodist Specialty & Transplant Hospital, Methodist Hospital, and Methodist Metropolitan Hospital with decreased utilization from 26.6 to 9.2, 14.2 to 5.0, and 10.7 to 4.9 DOT/1,000 APDs, respectively.
The mean ertapenem DOT/1,000 APDs declined by approximately 68% compared to the pre-ASI phase I (14.2 vs. 4.6, p<0.001). The implementation of the ASIs was associated with a decline in ertapenem utilization from an already downward trend (Figure 1). While there was an immediate relative level effect (-7%), the rate of decline leveled off in ertapenem utilization by 12 months post-intervention (p=0.04).
Overall antibiotic use
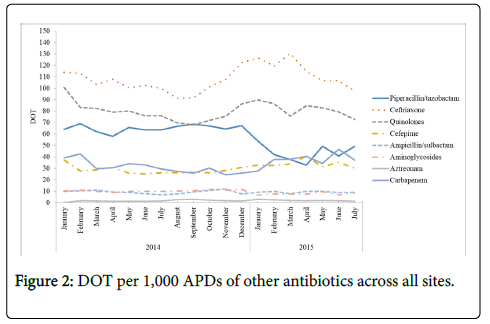
Between January 2014 and July 2015, overall antibiotic use decreased from 385 to 317 DOT/1,000 APDs (-17.8%). As shown in Figure 2, ceftriaxone maintained the highest utilization, but also decreased from 114.0 to 97.5 DOT/1,000 APDs (-14.5%) across the study period. This was followed by fluoroquinolones (declined from 109.9 to 83.7 (-23.8%) DOT/1,000 APDs) and piperacillin/tazobactam (declined from 64.1 to 49.3 (-23.1%) DOT/1,000 APDs) across the study period, respectively. Carbapenems as a class, aminoglycosides, ampicillin/sulbactam, and aztreonam utilization remained stable between January 2014 and July 2015.
Carbapenem nonsusceptibility
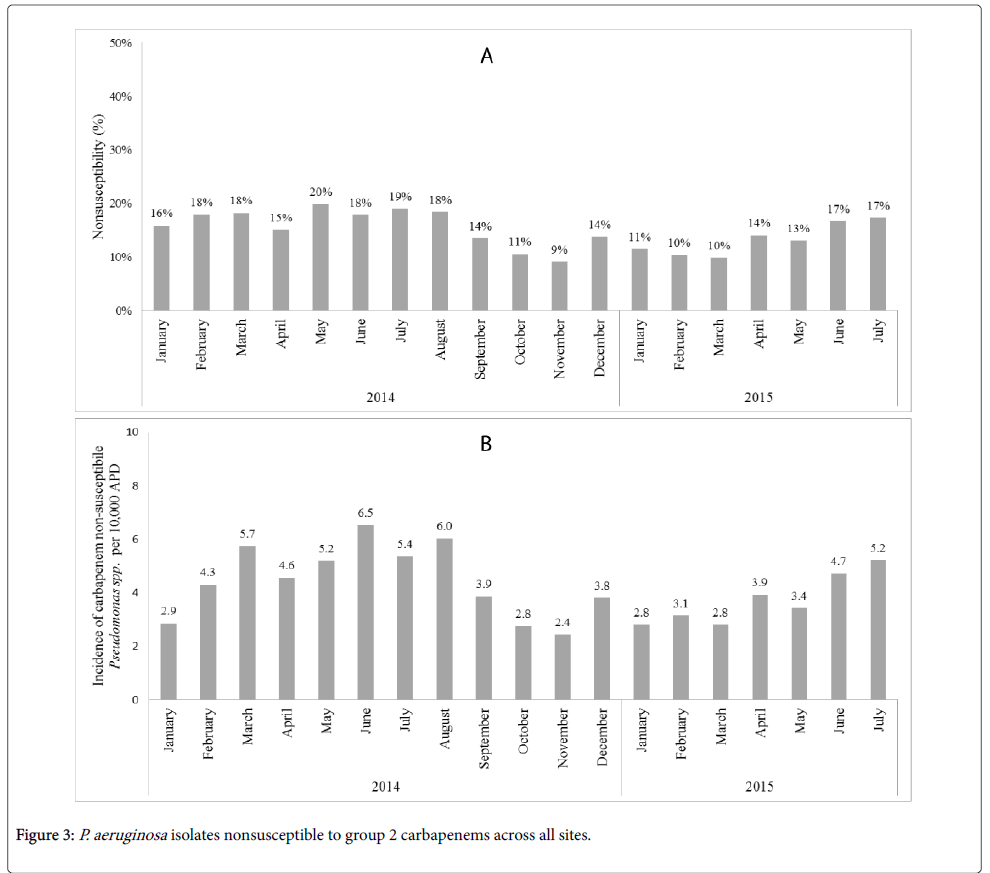
The percentage of P. aeruginosa isolates nonsusceptible to a group-2 carbapenem remained stable across the post-intervention period, with 16% nonsusceptible in January 2014 and 17% nonsusceptible in July 2015 (Figure 3A).
Figure 3B shows the incidence of carbapenem non-susceptible Pseudomonas spp. per 10,000 APDs. In the post-ASI phase II, the average incidence of carbapenem non-susceptible Pseudomonas spp. per 10,000 APDs significantly decreased compared to the pre-ASI phase II (4.9 vs. 3.7; p=0.03). However, no significant trend of carbapenem non-susceptible Pseudomonas spp. was observed in the regression model (p=0.81).
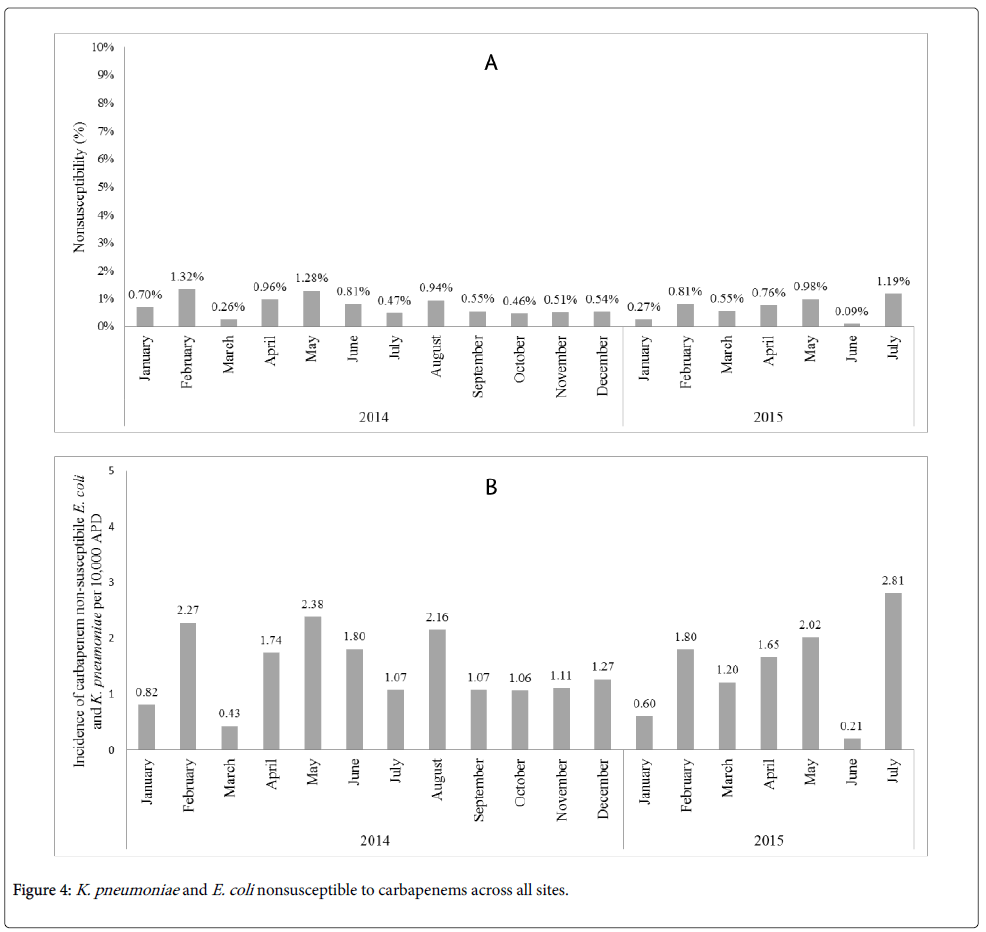
The percentage of E. coli and K. pneumoniae isolates nonsusceptible to a carbapenem remained stable across the post-intervention period, with 0.7% nonsusceptible in January 2014 and 1.19% nonsusceptible in July 2015, as shown in Figure 4A. The incidence of carbapenem non-susceptible E. coli and K. pneumoniae per 10,000 APDs, underwent slight fluctuations over the post-intervention period (Figure 4B). No associations on the nonsusceptibility rates of E. coli and K. pneumoniae per 10,000 APDs were observed related to the ASI implementation.
Discussion
Targeted ASI and restriction of broad-spectrum antimicrobial agents have emerged as important initiatives at many institutions due to the rise of multidrug-resistant Enterobacteriaceae across the United States [33]. We described the short-term ecological results of restriction of ertapenem usage amongst multiple hospitals, specifically focused on E. coli, K. pneumoniae, and Pseudomonas spp. As a result of targeted education, removal of ertapenem from commonly used order sets across four community hospitals and an electronic alert for prescribers, ertapenem usage decreased substantially over the span of the study period of two years.
Previous investigations on the impact of carbapenem restriction have revealed decreased rates of CRE; however, the studies focused primarily on the restriction of group 2 carbapenems, imipenem and meropenem, without measurement of ertapenem [34]. Ertapenem is a carbapenem antibiotic that lacks activity against Pseudomonal isolates, but retains activity against ESBL- and AmpC-producing organisms. It is known that ertapenem’s favorable pharmacokinetics and broad spectrum of activity leads to inappropriate prescribing practices, yet it is unknown whether its restriction results in decreased CRE.
Multiple studies have investigated the impact of ertapenem introduction to the overall hospital ecology, specifically focusing on Pseudomonas spp. [35-37]. In many instances, these investigations reported improved susceptibilities of Enterobacteriaceae and Pseudomonas spp. to other broad-spectrum antimicrobial agents, such as cefepime and piperacillin/tazobactam following the introduction of ertapenem to a hospital formulary. However, none have reported significant changes in carbapenem susceptibility following ertapenem usage alone, with many methodological problems cited in these studies [38-41].
The two-year ASI aimed at decreasing ertapenem utilization in this study revealed a corresponding decrease in the incidence of group-2 carbapenem resistant Pseudomonas spp. in the post-intervention period. Similar to other studies that identified significant decreases in the proportion of carbapenem resistant P. aeruginosa after carbapenem restriction, we observed this decrease with the restriction of ertapenem alone. Previous data regarding ertapenem use and susceptibility to P. aeruginosa have been inconsistent. In a long-term study, despite an increasing use of ertapenem over a 9 year period, no association was found between ertapenem and changes in pseudomonal susceptibility to carbapenems [37]. Other studies have reported an increased susceptibility to imipenem among P. aeruginosa after introduction of ertapenem, presumptively due to decrease in group 2 carbapenems [35,36]. While the present study detected an overall decrease in carbapenem non-susceptible Pseudomonas isolates in the post-intervention period, whether this is a direct impact of ertapenem or through indirect changes in other antimicrobials is unknown. The rates of carbapenem non-susceptible Enterobacteriaceae did not significantly change during the post-implementation period. Together, our findings demonstrate that future initiatives focused on eliminating unnecessary ertapenem usage may not come with untoward ecological consequences. Longer studies are likely needed to identify significant trends.
This study has many limitations. During the study period, multiple national drug shortages affected the supply of many commonly used anti-Pseudomonal antibiotics, such as cefepime and piperacillin/tazobactam. Although this study was performed at four hospitals, there were a limited number of isolates nonsusceptible to carbapenems over the study period, which might have limited the ability to show to identify significant trends in susceptibility patterns. Additionally, the short duration of the study limited the ability to detect any longer-term ecological effects.
In summary, this multilevel ASI aimed at decreasing ertapenem utilization resulted in substantially declined use. Susceptibilities of P. aeruginosa to group 2 carbapenems improved overall while Enterobacteriaceae isolates remained stable in the post-intervention period.
Appendix
Definitions: The coefficient for ‘time’=slope of the regression line pre-intervention; the coefficient for ‘phase’=the change in intercept; the coefficient for ‘interact’=the change in slope pre and post intervention. The coefficient for time is β1, for phase is β2 and for interact is β3. The regression model is: Outcome=constant+β1time+β2phase+β3interact. Pre intervention: Outcome=constant+β1time and post intervention: Outcome=constant+β1time+β2+β3interact=(constant+β2)+(β1+β3) time (as time and interact are the same post intervention). Therefore, the difference in constant (intercept) pre and post intervention is β2 and the difference in slope is β3.
Acknowledgement
We thank the pharmacy department, infection control, microbiology, and clinical laboratory departments at Methodist Healthcare System in San Antonio for providing data for this study.
References
- Centers for Disease Control and Prevention (2015) About antimicrobial resistance.
- National Institute of Allergy and Infectious Diseases (2016) Antimicrobial (drug) resistance quick facts.
- Centers for Disease Control and Prevention (2015) Core elements of hospital antibiotic stewardship programs.
- Echols R, Kowalski SF (1984) The use of antibiotic order forms for antibiotic utilization review: influence on physicians’ prescribing patterns. J Infect Dis 150: 803-807.
- Pestotnik SL, Classen DC, Evans RS, Burke JP (1996) Implementing antibiotic practice guidelines through computer-assisted decision support: clinical and financial outcomes. Ann Intern Med 124: 884-890.
- White AC, Atmar RL, Wilson J, Cate TR, Stager CE, et al. (1997) Effects of requiring prior authorization for selected antimicrobials: expenditures, susceptibilities, and clinical outcomes. Clin Infect Dis 25: 230-239.
- Evans RS, Pestotnik SL, Classen DC, Clemmer TP, Weaver LK, et al. (1998) A computer-assisted management program for antibiotics and other antiinfective agents. N Engl J Med 338: 232-238.
- Gums JG, Wancey RW, Hamilton CA, Kubilis PS (1999) A randomized, prospective study measuring outcomes after antibiotic therapy intervention by a multidisciplinary consult team. Pharmacotherapy 19: 1369-1377.
- Bassetti M, DiBiagio A, Rebesco B, Cenderello G, Amalfitano ME, et al. (2000) Impact of an antimicrobial formulary and restriction policy in the largest hospital in Italy. Int J Antimicrob Agents 16: 295-299.
- Gentry CA, Greenfield RA, Slater LN, Wack M, Huycke MM (2000) Outcomes of an antimicrobial control program in a teaching hospital. Am J Health-Syst Pharm 57: 268-274.
- Vlahovic-Palcevski VM, Morovic M, Palcevski G (2000) Antibiotic utilization at the university hospital after introducing an antibiotic policy. Eur J Clin Pharmacol 56: 97-101.
- Barenfanger J, Short MA, Groesch AA (2001) Improved antimicrobial interventions have benefits. J Clin Microbiol 39: 2823-2828.
- Lemmen SW, Becker G, Frank U, Daschner FD (2001) Influence of an infectious disease consulting service on quality and costs of antibiotic prescriptions in a university hospital. Scand J Infect Dis 33: 219-221.
- Cannon JP, Silverman RM (2003) A pharmacist-driven antimicrobial approval program at a Veterans Affairs hospital. Am J Health-Syst Pharm 60: 1358-1362.
- Martin C, Ofotokun I, Rapp R, Empey K, Armitstead J, et al. (2005) Results of an antimicrobial control program at a university hospital. Am J Health-Syst Pharm 62: 732-738.
- Nowak MA, Nelson RE, Breidenbach JL, Thompson PA, Carson PJ (2012) Clinical and economic outcomes of a prospective antimicrobial stewardship program. Am J Health-Syst Pharm 69: 1500-1508.
- Centers for Disease Control and Prevention (2015) Core elements of hospital antibiotic stewardship programs.
- Centers for Disease Control and Prevention (2013) Antibiotic resistance threats in the United States.
- Keating GM, Perry CM (2005) Ertapenem: a review of its use in the treatment of bacterial infections. Drugs 65: 2151-2178.
- Collins VL, Marchaim D, Pogue JM (2012) Efficacy of ertapenem for treatment of bloodstream infections caused by extended-spectrum-beta-lactamase-producing Enterobacteriaceae. Antimicrob Ag Chemother 56: 2173-2177.
- Livermore DM, Oakton KJ, Carter MW, Warner M (2001) Activity of ertapenem (MK-0826) versus Enterobacteriaceae with potent beta-lactamases. Antimicrob Ag Chemother 45: 2831-2837.
- Sexton DJ (2006) Carbapenems for surgical prophylaxis? N Engl J Med 355: 2693-2695.
- Livermore DM, Muchtaq S, Warner M (2005) Selectivity of ertapenem for Pseudomonas aeruginosa mutants cross-resistant to other carbapenems. J Antimicrob Chemother 55: 306-311.
- Costa SS, Mourato C, Viveiros M (2013) Rapid selection of carbapenem-resistant Pseudomonas aeruginosa by clinical concentrations of ertapenem. Int J Antimicrob Ag 41: 488-498.
- Falagas ME, Tansarli GS, Kapaskelis A, Vardakas KZ (2013) Ertapenem use and antimicrobial resistance to group 2 carbapenems in Gram-negative infections: a systematic review. Expert Rev Anti Infect Ther 11: 69.
- Nicolau DP, Carmeli Y, Crank CW, Goff DA, Graber CJ, et al. (2012) Carbapenem stewardship: does ertapenem affect Pseudomonas susceptibility to other carbapenems? A review of evidence. Int J Antimicrob Agents 39: 11-15.
- Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP, et al. (2008) Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol 29: 1099-1106.
- Hussein K, Sprecher H, Mashiach T, Oren I, Kassis I, et al. (2009) Carbapenem resistance among Klebsiella pneumoniae isolates: risk factors, molecular characteristics, and susceptibility patterns. Infect Control Hosp Epidemiol 30: 666-671.
- Delgado A, Cowley MC, Gawrys GW, Duhon BM, Koeller JM (2016) Evaluating the use of ertapenem (Invanz®) in a hospital system-epidemiologic and financial implications. Res Rev J Hosp Clin Pharm 2: 35-39.
- Polk RE, Fox C, Mahoney A, Letcavage J, MacDougall C (2007) Measurement of adult antibacterial drug use in 130 US Hospitals: comparison of defined daily dose and days of therapy. Clin Infect Dis 44: 664-670.
- Clinical and Laboratory Standards Institute (2014) M100-S24. Performance standards for antimicrobial susceptibility testing: 24th informational supplement. Wayne: Clinical and Laboratory Standards Institute.
- Dudley MN, Ambrose PG, Bhavnani SM, Craig WA, Ferraro MJ, et al. (2013) Background and rationale for revised clinical and laboratory standards institute interpretive criteria (Breakpoints) for Enterobacteriaceae and Pseudomonas aeruginosa: I. Cephalosporins and Aztreonam. Clin Infect Dis 56: 1301-1309.
- Gupta N, Limbago BM, Patel JB, Kallen AJ (2011) Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin Infect Dis 53: 60-67.
- Pakyz AL, Oinonen M, Polk RE (2009) Relationship of carbapenem restriction in 22 university teaching hospitals to carbapenem use and carbapenem-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother 53: 1983-1986.
- Goldstein EJC, Citron DM, Peraino V, Elgourt T, Meibohm AR, et al. (2009) Introduction of ertapenem into a hospital formulary: effect on antimicrobial usage and improved in vitro susceptibility of Pseudomonas aeruginosa. Antimicrob Agents Chemother 53: 5122-5126.
- Graber CJ, Hutchings C, Dong F (2012) Changes in antibiotic usage and susceptibility in nosocomial Enterobacteriaceae and Pseudomonas isolates following the introduction of ertapenem to hospital formulary. Epidemiol Infect 140: 115-125.
- Eagye KJ, Nicolau DP (2010) Absence of association between use of ertapenem and change in antipseudomonal carbapenem susceptibility rates in 25 hospitals. Infect Control Hosp Epidemiol 31: 485-490.
- Graber CJ, Hutchings C, Dong F (2012) Changes in antibiotic usage and susceptibility in nosocomial Enterobacteriaceae and Pseudomonas isolates following the introduction of ertapenem to hospital formulary. Epidemiol Infect 140: 115-125.
- Lima AL, Oliveira PR, Paula AP, Dal-Paz K, Almeida JN Jr, et al. (2011) Carbapenem stewardship: positive impact on hospital ecology. Braz J Infect Dis 15: 1-5.
- dos Santos RP, Jacoby T, Goldani LZ (2010) Antimicrobial stewardship lessons: do Pseudomonas-sparing agents, such as ertapenem, effectively improve bacterial resistance? Antimicrob Agents Chemother 54: 3076-3077.
- Eagye KJ, Nicolau DP (2011) Change in antipseudomonal carbapenem susceptibility in 25 hospitals across 9 years is not associated with the use of ertapenem. J Antimicrob Chemother 66: 1392-1395.
Citation: Delgado A, Gawrys GW, Duhon BM, Lee GC (2017) Impact of an Antimicrobial Stewardship Initiative on Ertapenem Use and Carbapenem Susceptibilities at Four Community Hospitals. J Infect Dis Ther 5:341. DOI: 10.4172/2332-0877.1000341
Copyright: © 2017 Delgado A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6795
- [From(publication date): 0-2017 - Dec 09, 2025]
- Breakdown by view type
- HTML page views: 5734
- PDF downloads: 1061