Research Article Open Access
Impact of Dermatologists’ Perceptions about Antibiotic Resistance on Antibiotic Prescribing for Acne
Suzanne MA Abdelmalek1*, Juma Alowaissi1, Salim A Hamadi1 and Laith Akkash21Department of Pharmacology and Biomedical Sciences, Faculty of Pharmacy and Medical Sciences, Petra University, Amman, Jordan
2Director, Akkash Comprehensive Skin Clinic, Amman, Jordan
- *Corresponding Author:
- Suzanne MA Abdelmalek
Department of Pharmacology and Biomedical Sciences
Faculty of Pharmacy and Medical Sciences
Petra University, Amman
Jordan
Tel: 00962795511248
E-mail: sabdelmalek@uop.edu.jo
Received date: Sep 06, 2016; Accepted date: Sep 19, 2016; Published date: Sep 23, 2016
Citation: Abdelmalek SMA, Alowaissi J, Hamadi SA, Akkash L (2016) Impact of Dermatologists├ó┬?┬? Perceptions about Antibiotic Resistance on Antibiotic Prescribing for Acne . J Infect Dis Ther 4: 294. doi:10.4172/2332-0877.1000294
Copyright: © 2016 Abdelmalek SMA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Infectious Diseases & Therapy
Abstract
Background/objective: The use of antibiotics in the treatment of acne has been debatable especially when the abuse of antibiotics is strongly linked to emerging antibiotic resistant strains of bacteria. The aim of this study is to assess dermatologists’ knowledge about antimicrobial drug resistance and find out if their trends of antibiotic prescribing for acne would contribute to spread antibiotic resistance. Methods: A cross sectional survey was conducted on 51 dermatologists in the city of Amman, Jordan. The study sample was categorized to dermatologists from the private and public sectors and those who work in academia and those who don’t. The questionnaire collected demographical characteristics of dermatologists, patterns of antibiotic and acne drugs prescribing and causes of microbial antibiotic resistance and therapeutic failure. Answers were compared to the European guidelines for acne treatment 2012. Results: Total Percentage of deviation from the European standard guidelines was 56.94%. This percentage was almost the same for the four groups of dermatologists. Patterns of antibiotic prescribing varied between dermatologists. 43% of the sample acknowledged the occurrence of antibiotic resistant P. acnes in Jordan. 45.5% of dermatologists were unaware of the relation between antibiotics prescribing and the spread of antibiotic resistance. A large percentage of dermatologists (71%) attributed therapeutic failure to patient noncompliance. Opinions of dermatologists differed about factors resulting in antibiotic resistance. Conclusion: Ambiguous knowledge about antibiotic resistance among dermatologists affects their antibiotic prescribing patterns and highlights the need for awareness campaigns. High percentage of deviation from the European guidelines in treatment of acne stresses the need for national standardized therapeutic guidelines.
Keywords
Antibiotics prescribing; Acne; Dermatologists; Antibiotic resistance; Jordan
Abbreviations:
Pr-Ac: Dermatologists in Private Sector and work in Academia; Pu-Ac: Dermatologists in Public Sector and Work in Academia; Pr- NoA: Dermatologists in Private Sector and donâ┬?┬?t Work in Academia; Pu- NoA: Dermatologists in Public Sector and donâ┬?┬?t Work in Academia
Introduction
Acne is a common skin condition that is most prevalent in adolescents. The pathophysiology of acne is a result of complicated processes within the pilosebaceous unit. It includes excessive sebum production followed by epithelial hyper keratinization that furnishes for colonization and overgrowth of Propionibacterium acnes (P. acnes ) and inflammation [1,2]. Clinically, Acne vulgaris presents with different lesions of variable degrees of severity, including comedones (the primary lesions of acne), papules, pustules, nodules and cysts. Consequently, several treatment options exists that vary between topical agents (like keratolytics, peeling agents, and topical antibiotics), and systemic agents (like antibiotics, systemic retinoid and hormones). Antibiotics, both topical and oral remain a therapeutic mainstay for the management of inflammatory Acne vulgaris [3]; however, the increasingly emerging resistance to commonly prescribed antibiotics is negatively affecting therapeutic success. High levels of resistance have been correlated with high outpatient use of antibiotics for acne [4]. A clear association has been established long time ago between poor therapeutic response in acne and antibiotic-resistant Propionibacteria [5], and between the influence of antibiotic prescribing practices and the rate of antibiotic resistance [6]. In spite of that, some dermatologists still believe that the problem of antibiotic resistance is relevant primarily to pathogenic bacteria in hospitals and antibiotics used towards them and not applicable to use of antibiotics for outpatients like in the case of acne [1]. It is predicted that dermatologists will soon be faced with patients suffering from inflamed acne caused by community acquired resistant P. acnes [7]. Several parties play a significant role in the antibiotic resistance process: patient, pharmacist, physician and bacteria. Proper diagnoses followed by the proper use of the correct regimen as well as awareness of the antibiotic resistance dispute are factors that are vitally controlled by the treating dermatologist. Antibiotic stewardship programs have been worldwide thought of and applied to restrict antibiotic resistance. Such programs include provisions of advice and guidelines on appropriate antibiotic treatment for specific infections.
This study intends to assess dermatologistsâ┬?┬? knowledge about antimicrobial drug resistance and find out if their trends of antibiotic prescribing for acne would contribute to spread of antibiotic resistance.
Materials and Methods
Methods A randomized cross sectional study was carried out. A structured questionnaire was prepared after reviewing the literature for similar studies. Both closed and open ended questions were used according to the type of information required. The questionnaire was divided into four main sections. The first section collected demographic data and general information on dermatologists and their patients; questions asked about dermatologists gender, years of experience, their work setup as practicing in private or public sector and whether they worked in academia or not and the number of acne patients seen per week. This part also questioned the age of patients that consulted for acne and whether those patients were mainly males, females, pregnant or a mixture of all three. The second section questioned general treatment of Acne. All types of acne medications were listed and dermatologists were asked to choose the least and the most effective on patients in Jordan. The same list was given to dermatologists to choose the medication that he/she uses according to the severity of acne condition. The European guidelines classification of acne was adopted and acne conditions were classified as mild-moderate, moderate, moderate-sever and severe. Opinion of dermatologists about reasons of therapeutic failure in acne was questioned through offering three choices: development of antibiotic resistance, patientâ┬?┬?s noncompliance and any other suggested reasons. Antibiotic prescribing and knowledge of antibiotic resistance were evaluated in the third section of the questionnaire. Dermatologists were asked to select the most commonly prescribed antibiotic for acne from an offered list of antibiotics. Effectiveness of antibiotic treatment as well as duration of use for topical and systemic antibiotics and whether antibiotics are prescribed alone or in combination with other drugs were also questioned. Dermatologistsâ┬?┬? knowledge about antibiotic resistance was evaluated through questions that inquired about its commonness in Jordan and factors that contributed to its appearance. The final section was left for the dermatologist to add any extra thoughts.
Study sample
Questionnaires were handed to 80 Jordanian board certified dermatologists. The study sample was categorized into 4 groups: (1) dermatologists who work in private sector and academia (Pr-Ac) (n=15), (2) dermatologists who work in public sector and in academia (Pu-Ac) (n=14), (3) dermatologists who work in private sector but do not work in academia (Pr-NoA) (n=13), and (4) those who work in public sector and do not work in academia (Pu- NoA) (n= 9). All dermatologists were given a specific date and time for the survey visit and there was no prior notification on the purpose and the subject of the study. The allocated duration time that was given for the completion of the survey was 30 minutes, which insured sufficient time to answer all questions. The investigator was not allowed to answer questions or add comments regarding any issue that could change the opinion of the participant. All participants signed an informed consent prior to filling the questionnaire. Confidentiality and anonymity of information was assured.
Analysis
Data collected were coded and entered into Microsoft Excel 2007. Descriptive analysis (mean and standard deviation) was used to interpret the quantitative data. While qualitative data were reported as frequencies and percentages. Percentage deviation of answers from the European guidelines in the treatment of Acne vulgaris [8] was determined using equation 1.1, where the majority of answers to a question were taken and the other answers where neglected. This was then compared with the correct answer in the guidelines considering the standard to be 100%. Analysis was also made to categorize deviation according to the four different groups of dermatologists.
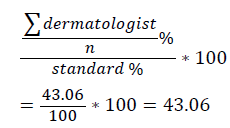
x = 100 - 43.06 = 56.94% → 1.1
Results
Fifty one out of eighty questionnaires were returned, which gave a response rate of 63.75%. Demographic data of dermatologists are shown in Table 1. 84.5% of dermatologists stated that patients who consulted for acne were a mix of males, females and pregnant women. 83% of the dermatologists treated patients suffering from acne at the age group between 11-23 ys.
| Number | |
| Gender | |
| Male   | 43 |
| Female | 8 |
| Experience (years) | |
| =5 | 9 |
| 10 | 5 |
| 15 | 13 |
| 20 | 11 |
| =51 | 13 |
| Age (years) | |
| 20-35 | 9 |
| 36-50 | 19 |
| =51 | 23 |
| Number of cases examined per week | |
| 0-10 | 0 |
| Oct-20 | 18 |
| 20-50 | 28 |
| Â =51 | 5 |
Table 1: Demographics of Dermatologists.
Percentage deviation from European standards
The mean of total answers that deviated from the European evidence-based Guidelines for the treatment of acne was 56.94%. There were no substantial differences in percentage deviation of answers between private and public sector dermatologists. Also, there were no difference between those who practiced in academia and those who did not (Table 2).
| Sector classification | % Deviation |
|---|---|
| Private | 50.4 |
| Public | 49.6 |
| Academia classification | |
| Academic | 49.7 |
| Non Academic | 50.3 |
| Sector and Academia classification | |
| Private Academic | 27 |
| Private Non Academic | 23.4 |
| Public Academic | 22.7 |
| Public Non Academic | 26.9 |
Table 2: Percentage deviation of answers according to category of dermatologists.
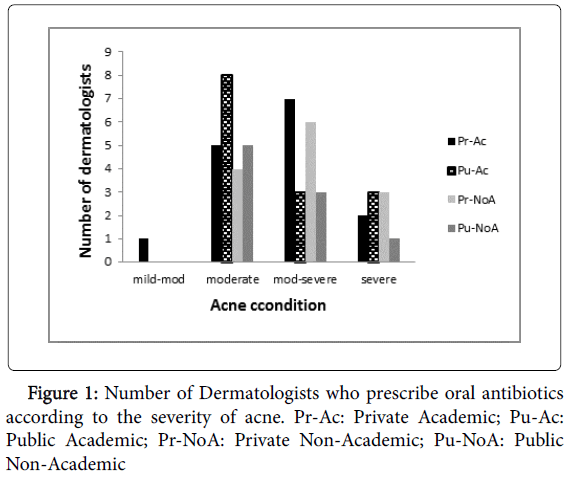
Percentages of answers that adhered to the standards in general treatment of acne are demonstrated in Table 3. The use of oral retinoids to treat severe acne was approved by 81% of dermatologists and was believed to be the best treatment for acne by 91% of them. On the other hand, the use of oral antibiotics in moderate to severe conditions was approved by 36% of the participants, and 44.5% of them approved its use for moderate acne only and not for severe cases (Figure 1).
| Question | Majority answers % |
Standard | Adherence % |
|---|---|---|---|
| When do you use oral retinoids? | Sever 81.5% | Sever | 81.50% |
| When do you use topical retinoids? | Mild-moderate 68% | Mild-moderate | 68% |
| When do you use oral antibiotics? | Moderate 44.5% Mode-severe 36% |
Mode-severe | 36% |
| When do you use topical antibiotics? | Mild-moderate 48.5% | Mild-moderate | 48.50% |
| When do you use oral contraceptives? | Unanswered 76.5% Moderate 15% |
Moderate | 15% |
| When do you use spironolactone? | Unanswered 83% Moderate 17% |
Moderate | 17% |
| When do you use cortisone injections? | Unanswered 76.5%Â Sever 17% |
Sever | 17% |
| When do you use benzoyl peroxide? | Mild-moderate 48% Mild 25.5% |
Mild | 25.50% |
| When do you use salicylic acid? | Mild 47% | Mild | 47% |
| When do you use sulfur products? | Unanswered 49% Mild 39% |
Mild | 39% |
| When do you use Azelaic acid? | Mild 51% | Mild | 51% |
| Oral retinoids have the most or least activity? | Most 91% | Most | 91% |
| Topical retinoids have the most or least activity? | Unanswered 47% Most 41% |
Most | 41% |
| Oral antibiotics have the most or least activity? | Most 75.5% | Most | 75.50% |
| Topical antibiotics have the most or least activity? | Least 36.5% Most 32% |
Most | 32% |
| Oral contraceptives have the most or least activity? | Unanswered 66% Least 29.5% |
Least | 29.50% |
| Spironolactone have the most or least activity? | Unanswered 73% Least 27% |
Least | 27% |
| Cortisone injections have the most or least activity? | Unanswered 64% Least 32% |
Least | 32% |
| Benzoyl peroxide have the most or least activity? | Unanswered 51% Most 29% |
Most | 29% |
| Salicylic acid have the most or least activity? | Unanswered 44.5% Most 14.5% |
Most | 14.50% |
| Sulfur products have the most or least activity? | Unanswered 72.5% Least 27.5% |
Least | 27.50% |
| Azelaic acid have the most or least activity? | Least 52%, Most 13.5% |
Most | 13.50% |
Table 3: Percentage adherence of dermatologists answers to European standards in general treatment of acne.
75.5% of the participants adhered to guidelines in considering oral antibiotics to have the most activity in acne treatment. And only 36% of participants prescribed it to the correct condition (Table 3).
High percentage of unanswered questions was observed. Low percentage of adherence for answers about the use of other medications such as benzoyl peroxide, salicylic acids, Sulphur products, azelaic acid, topical retinoid, oral contraceptives, and Spironolactone, cortisone injections was detected (Table 3).
Therapeutic failure
Therapeutic failure in acne treatment was attributed by 71% of participants to patientsâ┬?┬? noncompliance and by 23% to both antibiotic resistance and patient noncompliance while only 6% related it to antibiotic resistance alone.
Pattern of antibiotic prescribing
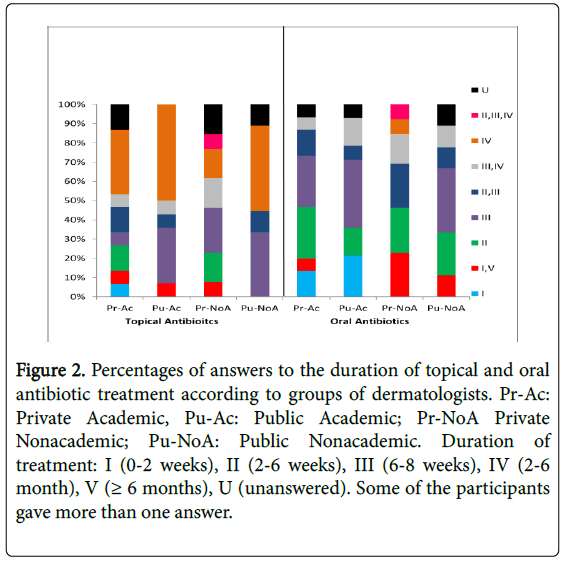
96% of dermatologists chose doxycycline as the antibiotic of choice for treating acne which represents a 96% adherence to the standards (Table 4). However, variations appeared in the duration of the course. Only 17% of answers adhered to the guidelines in prescribing topical antibiotics for 2-6 weeks and these belonged to Private sector dermatologists. None of the public sector dermatologists prescribed topical antibiotics for this duration (Figure 2). Almost 40% of the answers showed that dermatologists were prescribing those antibiotics for 2-6 months and around 30% for 6-8 weeks (Table 3). Discrepancy between categories of dermatologists in the duration of antibiotic use was observed (Figure 2). On the other hand, 60.5% of dermatologists adhered to standards in prescribing oral antibiotics for 2-6 weeks that might be extended to 3 months if the patient was unresponsive.
| Question | Majority % |
Standard | Adherence % |
|---|---|---|---|
| When prescribing an antibiotic which of the following would you use? Erythromycin, Tetracycline , Doxycycline or Minocycline, Clindamycin Combination of two antibiotics |
doxycycline 96% | doxycycline | 96% |
| How long do you recommend Topical Antibiotics? | 2-6 weeks 17% |
2-6 weeks | 17% |
| How long do you recommend Oral Antibiotics? | 2-6 month or 2-6 weeks 60.50% |
2-6 weeks, if unresponsive 2-6months | 60.50% |
| How effective are antibiotics in the treatment of acne? Effective Effective in combination with a drug from another group. |
Effective in combination 57.5%, Effective 42.50% |
Effective in combination | 57% |
| If answer (effective in combination) in previous question, why? | |||
| A) Fear of antibiotic resistance | U 44.5% | B | 23.50% |
| B) Pathophysiology of acne | B 23.5% | ||
| C) Other â┬?¦â┬?¦â┬?¦â┬?¦â┬?¦â┬?¦â┬?¦â┬?¦â┬?¦â┬?¦ | |||
| U) unanswered | |||
| Do you find prescribing antibiotics always effective? =25% of cases 50% of cases 75% of cases 100% of cases |
75% of cases 62% |
75% of cases | 62% |
Table 4: Percentage adherence of dermatologists answers to European standards in antibiotic treatment.
Figure 2: Percentages of answers to the duration of topical and oral antibiotic treatment according to groups of dermatologists. Pr-Ac: Private Academic, Pu-Ac: Public Academic; Pr-NoA Private Nonacademic; Pu-NoA: Public Nonacademic. Duration of treatment: I (0-2 weeks), II (2-6 weeks), III (6-8 weeks), IV (2-6 month), V (= 6 months), U (unanswered). Some of the participants gave more than one answer.
The use of antibiotics alone was believed by 42.5% of dermatologists (50% of which are dermatologists in academia) to be effective in treating acne. The rest of the participants (57.5%) believed that the use of antibiotics in combination with other drugs is more effective (Table 3). Perception of dermatologists to the causes of combining other drugs with antibiotics also varied. 23% of participants thought (53% of dermatologists in academia and 6% of non-academia) that other drugs are prescribed with antibiotics to avoid antibiotic resistance and 44% of the sample left the question unanswered. The rest of the participants correctly knew that combination of drugs is used to control the multiple pathological factors involved in acne (Table 3).
Dermatologistsâ┬?┬? perception of antibiotic resistance
Only 22 dermatologists (43%) of the 51 sample acknowledged the occurrence of antibiotic resistant strains of P. acnes in Jordan and these varied between private and public sector with the majority being from the private sector. Interestingly though, only five of these 22 actually prescribed the correct antibiotic for the correct condition and for the right duration. Factors contributing to antibiotic resistance were divided into dermatologist related and patient related. A considerable percentage of dermatologists believed that this problem was patient related. For example 52% believed that it was due to patient demands of antibiotics and 60% believed that unrestricted sales of antibiotics is an important cause while 65% thought that patient noncompliance to the use of antibiotics is an important factor. On the other hand, responses to dermatologist related factors varied as follows: 47% inappropriate empiric choice of antibiotics, 57.5% inappropriate duration of the course, 30% prescribing broad spectrum antibiotics and 38% absence of clear guidelines (Table 5).
| Causes | Private sector | Public sector | ||
|---|---|---|---|---|
| Degree of importance | very | moderate | very | moderate |
| Wide spread use of antibiotics | 18 | 8 | 13 | 8 |
| Inappropriate empiric choices | 12 | 11 | 12 | 3 |
| Inappropriate duration of the course | 14 | 14 | 15 | 7 |
| Microbial mutation | 3 | 2 | 2 | 8 |
| Lack of guidelines | 8 | 14 | 11 | 9 |
| Use of broad spectrum antibiotics | 7 | 14 | 8 | 8 |
| Patient demands of antibiotics | 18 | 6 | 9 | 6 |
| Patient noncompliance | 18 | 9 | 15 | 6 |
Table 5: Dermatologists perceptions of the important factors leading to emergence of antibiotic resistance. Participants were given a list of choices for the factors that might lead to antibiotic resistance and were asked to rate their belief as moderately important or very important. Participants were allowed to give more than one choice. Numbers of dermatologists are reported in the table.
Discussion
Acne sufferers in Jordan share the same gender and age group with other acne sufferers in other parts of the world [2]. Thus dermatologists in Jordan as other dermatologists worldwide are expected to share the same difficulties in acne treatment, such as therapeutic failure, medication side effects and emergence of antibiotic resistant P. acnes . Since our analysis reveals that high percentage of dermatologistsâ┬?┬? answers deviated from the European guidelines, it seems that neither practicing in public sector (or private sector) nor being in academia affects dermatologistsâ┬?┬? knowledge about acne medication or their prescribing patterns of antibiotics. Absence of evidence based guidelines for acne treatment in Jordan may explain the high percentage deviation of answers, and may also explain discrepancies between dermatologists in use of different acne medications. Such discrepancies reflect a general attitude of personalized way of treatment that relies on the dermatologistâ┬?┬?s knowledge and preference.
Therapeutic failure in acne treatment is common and has been attributed mostly to the development of antibiotic resistant P. acnes [9,10]. The small number of dermatologists that appreciated existence of resistance to antibiotics in P. acnes indicates either those dermatologists are unaware of the existence of antibiotic resistant P. acnes. Therefore, they donâ┬?┬?t relate it to therapeutic failure (which is evident from the high percentage (71%) of dermatologists who believe that therapeutic failure is due to patient noncompliance), or that they are not aware of the prevalence of antibiotic resistant P. acnes in Jordan. In this regard, and up to the best of our knowledge, there have been no studies carried out to explore this issue in Jordan. Antibiotic resistance is defined as the microbial acquisition of a mobile genetic material that will provide the microbe with mechanisms of resistance towards certain antibiotics [11]. P. acnes has been shown to exchange antibiotic resistance genes from and to microbial flora on the skin of the same person [12], and is also found to spread primarily through person-to-person contact [6,7]. Prolonged use of antibiotics has been documented to select for antibiotic resistant resident skin flora [3,8,14,15]; also premature stopping of antibiotic treatment selects for bacteria having variable degrees of resistance [13]. And since oral antibiotics are the mainstay of treatment for moderate to severe inflammatory acne [16], it becomes crucial that the type of antibiotic prescribed and the duration of use be critically chosen in order to control resistance spread. In the current study, although = 50% of dermatologists agree that the inappropriate duration of the course is a factor in the development of antibiotic resistance, the percentage of dermatologists that actually applied this information is negligible. Variation in the indicated duration of use is primarily the responsibility of the prescribing physician; however, noncompliance of patients can also contribute to that. Whoever is the cause of duration variation, the end result is the ineffectiveness of the antibiotic treatment regimen [8], as well as possibility for development of microbial resistance in commensal flora at all body sites [16].
The majority of dermatologists agree on doxycycline as the antibiotic of choice; however, an alarming confusion in the pattern of prescribing it is noticed, for example, over-treatment of mild acne, as well as, under-treatment of moderateâ┬?┬?severe persistent acne (in contrast to the European standards). Furthermore, an almost similar percentage of dermatologists bared knowledge deficiency (whether public or private, in academia or not) about the role of combination therapy with other medications in the control of acne as well as antibiotic resistance [3,16,17].
Despite the observed discrepancies in prescribing antibiotics to acne in this study, a considerable percentage of dermatologists believed that factors that lead to emergence of antibiotic resistance are mainly patient related and partially dermatologist related. Antibiotic resistance in acne is a worldwide problem that is well documented and is considered a â┬?┬?real phenomenonâ┬?┬Ł [18]. Dermatologists and health care workers in general, have a huge responsibility in controlling its spread. Antibiotic stewardship involves many acts starting with acknowledging the problem. Thus, raising the awareness of dermatologists as well as the health care workers and the patients is an important step towards controlling spread of antibiotic resistance.
The current study relied on the European guidelines as a reference; however, other guidelines are easily accessible [19], which explain variation in antibiotics prescribing patterns. Thus the development of national guidelines will certainly unify the source of information for dermatologists.
Conclusion
Information about antibiotic resistance in general was not clear to participants. Dermatologistsâ┬?┬? vague knowledge about proper prescribing of antibiotics in relation to case severity, duration and dosage frequency, and susceptibility to resistance, could contribute to the emergence of antibiotic resistant strains. Such results restate the strong need for large scale antibiotic resistance awareness campaigns for dermatologists, pharmacists and patients, as well as the need for a practical, user friendly Jordanian guidelines for the treatment of acne vulgaris.
References
- Thiboutot D, Gollnick H, Bettoli V, Dréno B, Kang S, et al. (2009) New insights into the management of acne: an update from the Global Alliance to Improve Outcomes in Acne group. J Am AcadDermatol 60: S1-S50.
- Knutsen-Larson S, Dawson AL, Dunnick CA, Dellavalle RP (2012) Acne vulgaris: pathogenesis, treatment, and needs assessment. DermatolClin 30: 99-106.
- Andriessen A, Lynde CW (2014) Antibiotic resistance: shifting the paradigm in topical acne treatment. J Drugs Dermatol 13: 1358-1364.
- Oprica C, Nord CE (2005) European surveillance study on the antibiotic susceptibility of Propionibacterium acnes. Clin Microbial Infec 11: 204-213.
- Cooper AJ (1998) Systematic review of Propionibacterium acnes resistance to systemic antibiotics. Med J Aust 169: 259-261.
- Ross JI, Snelling AM, Eady EA, Cove JH, Cunliffe WJ, et al. (2001) Phenotypic and genotypic characterization of antibiotic-resistant Propinoibacterium acnes isolated from the acne patients attending dermatology clinics in Europe, the USA, Japan and Australia. Brit J Dermatol 144: 339-346.
- Ross JI, Snelling AM, Carnegie E, Coates P, Cunliffe WJ, et al. (2003) Antibiotic-resistant acne: lessons from Europe. Brit J Dermatol 148: 467-478.
- Nast A, Dréno B, Bettoli V, Degitz K, Erdmann R, et al. (2012) European Evidence-based (S3) Guidelines for the Treatment of Acne. J EurAcadDermatol 26: 1-29.
- Leyden JJ, Del Rosso JQ, Webster GF (2007) Clinical considerations in the treatment of acne vulgaris and other inflammatory skin disorders: focus on antibiotic resistance. Cutis 79: 9-25.
- Kinney MA, Yentzer BA, Fleischer Jr AB, Feldman SR (2010) Trends in the treatment of acne vulgaris: are measures being taken to avoid antimicrobial resistance? J Drugs Dermatol 9: 519-524.
- Davies J, Davies D (2010) Origins and evolution of antibiotic resistance. Microbial MolBiol R 74: 417-433.
- Patel M, Bowe MP, Heughebaert C, Shalita AR (2010) The development of antimicrobial resistance due to the antibiotic treatment of acne vulgaris: a review. J Drugs Dermatol 9: 655-664.
- Eady EA (1998) Bacterial resistance in acne. Dermatol 196: 59-66.
- Eady EA, Cove JH (1990) Topical antibiotic therapy: current status and future prospects. Drug ExpClin Res 16: 432-433.
- Worret WI, Fluhr JW (2006) Acne therapy with topical benzol peroxide, antibiotics and azelaic acid. J DtschDermatolGes 4: 293-300.
- Dréno B, Bettoli V, Ochsendorf F, Layton A, Mobacken H, et al. (2004) European recommendations on the use of oral antibiotics for acne. Eur J Dermatol 14: 391-9.
- Worthington RJ, Melander C (2013) Combination approches to combat multidrug resistant bacteria. Trends Biotechnol 31: 177-184.
- Tzellos T, Zampeli V, Makrantonaki E, Zouboulis CC (2011) Treating Acne with antibiotic-resistant bacterial colonization. Expert Opin Pharmaco12: 1233-1247.
- Strauss JS, Krowchuk DP, Leyden JJ, Lucky AW, Shalita AR, et al. (2007) Guidelines of care for acne vulgaris management. J Am AcadDermatol 56: 651-663.
Relevant Topics
- Advanced Therapies
- Chicken Pox
- Ciprofloxacin
- Colon Infection
- Conjunctivitis
- Herpes Virus
- HIV and AIDS Research
- Human Papilloma Virus
- Infection
- Infection in Blood
- Infections Prevention
- Infectious Diseases in Children
- Influenza
- Liver Diseases
- Respiratory Tract Infections
- T Cell Lymphomatic Virus
- Treatment for Infectious Diseases
- Viral Encephalitis
- Yeast Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 12034
- [From(publication date):
October-2016 - Aug 25, 2025] - Breakdown by view type
- HTML page views : 10963
- PDF downloads : 1071