Implications of Lipid Profile Dosages in Fasting and Postprandial Status
Received: 14-Mar-2018 / Accepted Date: 09-Apr-2018 / Published Date: 16-Apr-2018
Abstract
Introduction: To improve patient adherence to lipid tests, many laboratories around the world perform these tests without the need for a 12 h fast at random times during the day.
Methods: The study consisted of 51 volunteers and venous blood was collected in a 12 h fast and after a meal the next day. The volunteer returns to the laboratory after having his usual breakfast to be collected blood 2, 3 and 4 h after that meal. The following tests will be performed: cholesterol, triglycerides, C-LDL, C-HDL and VLDL with the Enzyme/Colorimetric method on Beckman-Coulter®AU5800 equipment and Beckman-Coulter reagent. In addition to the dosage, C-LDL was calculated by Friedewald Equation.
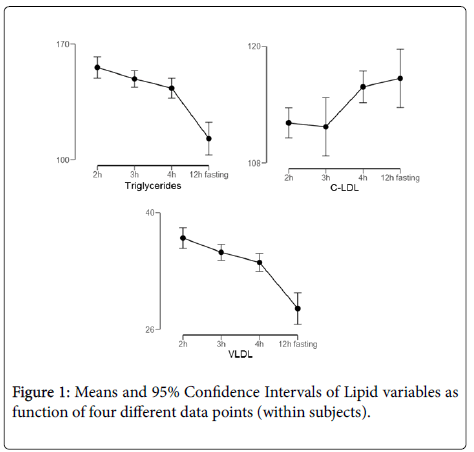
Results: In the comparison of the fasting lipid profile versus 2, 3 and 4 h after the meal, it was observed that there was no significant difference for the parameters of TC and C-HDL and for the calculated C-LDL, with average TC (p=0.237), C-HDL (p=0.130) and for C-LDL (p=0.089). However, for the dosed C-LDL, TG and VLDL showed significant differences with the respective mean concentrations and standard deviation for each hour after 2 h C-LDL (112.1 ± 33.6 mg/dL, p=0.008), 3 h (111.7 ± 35.0 mg/dL, p=0.019) and 4 h (115.0 ± 34.9 mg/dL, p=0.017) for TG 2 h (156.0 ± 86.4 mg/dL, p=0.000), 3 h (148.5 ± 92.0 mg/dL, p=0.000) and 4 h (143.4 ± 93.0 mg/dL, p=0.000) and for VLDL calculated: 2, 3 and 4 h (35.9 ± 53.5 mg/dL, p=0.000, 35.2 ± 53.6 mg/dL, p=0.001, and 34.0 ± 53.6 mg/dL, p=0.000).
Conclusion: Our data confirmed that the meal did not influence the TC, C-HDL and C-LDL calculate data, but for TG, VLDL and C-LDL doses a significant difference was observed at post-meal concentrations. Although disturbing the C-LDL for methodological reasons, did not affect the clinic. Analyzing our data, we observed that the best blood collection time could be between 2 and 3 h after the meal, where the degree of lipemia would have less influence in most individuals.
Keywords: Lipid profile; Venous blood; Cardiology
Introduction
The technological evolution of clinical laboratories currently involves the automation of almost all of a clinical analysis service, which, together with a quality control of excellence, directly infers the reliability of the results obtained. It should be noted that medicine is constantly evolving and cannot rule out new changes, since based on scientific studies in Laboratory Medicine, such as in the areas of Cardiology, Clinical Analysis, among others that have officially stood against the obligation of fasting for 12 hours (12 h) for cholesterol and triglyceride tests [1].
A 12 h fast is a big problem for people who need to get tested, especially children and the elderly. A recent study by the European Heart Journal published that assessment of lipid profile parameters at 12 h or after meal does not clinically affect patient outcomes.
To improve patient adherence to lipid tests, many labs around the world perform these tests without the need for a 12 h fasting with blood collection at random times during the day [2]. We know that the exams collected immediately after the meal have the effect of postprandial lipemia, and that in many of the laboratory methods still generate interference by lipemic turbidity. Fasting will still be recommended in specific situations, for example, when the patient has a high blood triglyceride concentration (above 440 mg/dL, the reference value being up to 150 mg/dL fasting, unlike the not fasting). In general, however, laboratories should perform blood collection independent of fasting time [3].
Measurement of lipids in the non-fasting condition is a simple approach to evaluate lipids, however, it does not allow a complete functional evaluation of postprandial lipid clearance and possible abnormalities. A glycemic-like method to evaluate lipid parameters at fixed time points after eating a high-fat meal, i.e. an oral fat tolerance test (OFTT), to examine the efficiency of lipid metabolism. This test is not performed routinely in the clinic, mainly due to the lack of standardized methodology and reference values for the interpretation of the results. However, postprandial lipid responses to fat-containing meals have been examined in research contexts in humans in the last decades [4-6].
Evaluating the metabolism of postprandial lipids provides indications of an individual's ability to process dietary lipids from digestion and absorption of lipids through lipoprotein secretion and clearance [7,8].
As can be observed, the determination of the lipid profile in fasting or without fasting can bring us more information, which goes beyond the identification of dyslipidemias, as well as in the classification and elucidation of lipid clearance mechanisms in humans [9,10].
In this context, before adopting non-fasting to perform dosages of the lipid profile in our laboratory, the authors considered it prudent to do a study to determine the best time interval after the meal that we could recommend in our laboratory, considering the variability of lipid absorption of each individual [11].
The authors of this study aimed to validate within our conditions, equipment and methodologies, so that we can introduce these changes in the pre-analytical phase of our laboratory routine. It was also part of our objectives to determine the mean concentration range of each parameter of the lipid profile studied at 2, 3 and 4 h after the meal.
Methods
Subjects
Fifty-one individuals from the community were recruited to perform laboratory tests of basic lipid profile, with and without fasting. Individuals signed the EHIC and received guidance on the study. Venous blood was collected with a 12-h fast and the next day the volunteers returned to the laboratory after having their usual breakfast, to be collected venous blood samples at 2, 3 and 4 h postprandial to be performed and lipid profile of each time.
Laboratory procedures
The following tests were performed: cholesterol, triglycerides, CLDL, C-HDL and VLDL with the Enzymatic/Colorimetric method on Beckman-Coulter® AU5800 equipment and the Beckman-Coulter reagent. In addition to the dosage, LDL-C was calculated by the Friedewald equation (LDL-C=Total Col-(HDL-C+VLDL).
Statistical analysis
For the analyses a Repeated Measures GLM was used with time point (2 h, 3 h, 4 h and 12 h fasting) as a fixed effect. The Sphericity assumption was tested using Mauchly test and in case of nonconformity with this assumption, a Greenhouse correction was calculated. The Tukey posthoc test was used for univariate results and the graphs with confidence intervals (95%) was presented to describe the significant differences among time points with Cohen d reported as a measurement of effect size. For all analyses the significance level was adopted as 5% (p<.05).
Results
Table 1 shows the general characteristics of the sample studied with their respective mean and standard deviation values of each parameter of the basic lipid profile.
| 2 h | 3 h | 4 h | 12 h fasting | ||
|---|---|---|---|---|---|
| Cholesterol | Mean | 189.8 | 185.6 | 193.5 | 184.8 |
| SD | 41.95 | 46.49 | 42.06 | 45.02 | |
| N | 50 | 50 | 50 | 50 | |
| Triglycerides* | Mean | 155.9 | 148.9 | 143.4 | 112.8 |
| SD | 86.41 | 92.06 | 93.05 | 69.00 | |
| N | 51 | 51 | 51 | 51 | |
| C-LDL* | Mean | 112.1 | 111.7 | 115.8 | 116.7 |
| SD | 33.62 | 35.08 | 34.07 | 33.27 | |
| N | 50 | 50 | 50 | 50 | |
| C-HDL | Mean | 55.94 | 56.47 | 57.35 | 56.76 |
| SD | 14.93 | 15.01 | 14.90 | 14.99 | |
| N | 51 | 51 | 51 | 51 | |
| VLDL* | Mean | 36.94 | 35.24 | 34.02 | 28.49 |
| SD | 53.53 | 53.61 | 53.65 | 53.75 | |
| N | 51 | 51 | 51 | 51 | |
| *Variables that presented significant effect of time collection using Repeated Measures GLM | |||||
Table 1: Descriptive data from lipid information as a function of time points (2, 3, 4 and 12 h fasting).
In the comparison of the fasting lipid profile versus 2, 3 and 4 h after the meal, it was observed that there was no significant difference for the parameters of TC and C-HDL and for the calculated C-LDL, with mean indices for TG (p=0.237), C-HDL (p=0.130) and for VLDL (p=0.089). However, for the dosed C-LDL, TG and VLDL showed significant differences with the respective mean concentrations and standard deviation for each hour after 2 h C-LDL (112.1 ± 33.6 mg/dL, p=0.008), 3 h (111.7 ± 35, 0 mg/dL, p=0.019) and 4 h (115.0 ± 34.9 mg/dL, p=0.017) for TG 2 h (156.0 ± 86, 4 mg/dL, p=0.000), 3 h (148.5 ± 92.0 mg/dL, p=0.000) and 4 h (143.4 ± 93.0 mg/dL, p=0.000) and for VLDL calculated: 2, 3 and 4 h (35.9 ± 53.5 mg/dL, p=0.000, 35.2 ± 53.6 mg/dL, p=0.001, and 34.0 ± 53.6 mg/dL, p=0.000) (Figure 1).
All variables presented significance according with a GLM Repeated Measures. The 95% Confidence Intervals among time points that not intersect, report a significant difference (p<.05). Triglycerides presents significance (p=0.002, partial n2=0.03) with 12 h fasting time different form all other time points (pooled Cohen d=-0.78). C-LDL presents significance (p=0.042, partial n2=0.02) with 2 h-3 h different form 4 h (Cohen d=0.5) and 12 h (Cohen d=0.38). VLDL presents significance (p=.001, partial n2=0.08) with 12 h fasting difference with all other time points (Pooled Cohen d=1.01).
Discussion
Several factors affect the TG response to a fat-containing meal, including the amount of fat consumed, the consumption of alcohol before or during the meal, fiber content, contents of other macronutrients and physical activity [12,13]. Another important factor to consider is the methodological limitations for the dosages of this profile with regard to serum prandial lipemias [14]. A paradigm shift for postprandial measurement, as opposed to fasting lipids, has occurred in the last decades. Some countries have already adopted lipid tests without the need for a 12-h fast in a random blood sample.
Factors such as gender, body mass index (BMI), age, are also of great importance for these dosages. However, it was not considered in this small study because the initial objective was to analyze the laboratory methodological behavior of these determinations in the laboratorial reality in the two conditions (fasting and postprandial) [15].
In this study, the authors demonstrated that although some significant differences were found, they did not present a clinical impact on the classification of dyslipidemias, when the test was performed with the usual meal of the individuals [16,17].
Evaluation of the functional postprandial lipid profile with a standardized meal is the preferred methodology to ensure optimal comparability between test subjects. However, the methodology of the oral fat tolerance test (OFTT) continues to be widely used but not standardized. Researches that use these methodologies standardize their own meal [1,4].
In this scenario further studies are needed to develop standard procedures that can distinguish between healthy and at-risk populations, including population-specific meal sizes, nutrient composition, blood sampling time points and markers to measure [18-20].
Another analysis that was performed was the comparison of the LDL-C calculated with the LDL-C dosed for each time in both conditions and statistically significant differences were found with low clinical impact, which is in agreement with many studies in the literature [21,22].
In addition, robust reference values, which are critical for interpreting postprandial parameters, continue to be precisely established. However, these should be specific to each methodological condition used. Lipid profile can be made in some differentiated conditions: fasting lipid profile of 12 h, lipid profile after individual home meal and lipid peril after OFTT. For each type of profile has to have a reference value that best suits the applicability of the tests [6,11,16].
The authors also analyzed that the best time for blood collection would be between 2 and 3 h after the meal, when the maximum peak of triglycerides reached these times. Recent studies have clearly demonstrated the importance of intestinal lipid dysfunction in the pathogenesis of insulin-resistant and diabetic conditions. The translation of new important findings from basic research studies to the clinic is essential to improve the clinical evaluation of postprandial dyslipidemia, increasingly recognized as a major contributor to the development of atherosclerosis and cardiovascular diseases [23].
In this study, the authors did not consider clinical factors that could influence lipid profile results both in fasting and after meal. It should be noted that there are studies that show that metabolic syndrome, inflammation and obesity have a significant influence on the lipid parameters in this different conditions (fasting and postprandial) [24,25].
Another question in this study was that 80% of the participants were women and therefore the authors did not analyze the differences by gender in this casuistic. However, there are studies that show that women have differences in lipid parameters when compared to men related to adiposity levels.
Further studies are also warranted to elucidate mechanisms of postprandial dyslipidemia associated with insulin resistant conditions. A more complete understanding of the underlying pathobiology will allow the subsequent development of standardized methodologies and biomarkers profiles to be used in clinical practice for early and accurate identification of people at risk for cardiovascular disease.
Conclusion
Our findings reinforce studies in the literature that point out that the lipid profile test can be performed with blood sampling at random times without previous fasting. The technological evolution does not allow states of serum lipemia to not suffer significant interferences in the results. The assistance methodologies used presented a good performance with the prandial condition maintaining the not significant variation between the fasted state and the after meal.
The assessment of post-meal lipids was reasonable in many clinical settings, since the prediction of cardiovascular disease risk is similar to fasting condition even using different cutoff points for the different conditions: fasting and non-fasting. The C-LDL parameter calculated or dosed did not present differences with clinical impact and could be used in the different conditions.
Financial Support
This work supported by Associacao Fundo de Incentivo a Pesquisa– Afip (Sao Paulo, Brazil).
Acknowledgements
The authors wish to thank all the members of the improve group for the invitation to submitted in relevant new.
References
- Dyce E (2018) Non-fasting versus fasting cholesterol measurement. Nurse Pract 43: 16-20.
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) (2002) Third report of the National Cholesterol Education Program (NCEP) Expert panel on detection, evaluation and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation 106: 3143-3421.
- Cooper GR, Myers GL, Smith J, Schlant RC (1992) Blood lipid measurements: variations and practical utility. JAMA 267: 1652-1660.
- Maranhão RC, Feres MC, Martins MT, Mesquita CH, Toffoletto O, et al. (1996) Plasma kinetics of a chylomicron-like emulsion in patients with coronary artery disease. Atherosclerosis 126: 15-25.
- Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, et al. (2007) Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA 298: 309-316.
- Ridker PM (2008) Fasting versus nonfasting triglycerides and prediction of cardiovascular risk: do we need to revisit the oral triglyceride tolerance test? Clin Chem 54: 11-13.
- Narayana S (1996) Pre and post analytical errors in lipid determination. Indian J Clin Biochem 11: 12-16.
- Young DS (1979) Biological variability. In: Brown SS, Mitchell FL, Young DS (editors.) Chemical Diagnosis of Disease. Elsevier, New York. Pp: 1-113.
- Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A (2007) Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease and death in men and women. JAMA 298: 299-308.
- Narayanan S (1993) Physiological variables in blood sampling. Mitt Klin Chem 24: 130-134.
- Nawaz H, Comerford BP, Njike VY, Dhond AJ, Plavec M, et al. (2006) Repeated serum lipid measurements during the peri-hospitalization period. Am J Cardiol 98: 1379-1382.
- Nordestgaard BG, Langsted A, Freiberg JJ (2009) Nonfasting hyperlipidemia and cardiovascular disease. Curr Drug Targets 10: 54-61.
- Mora S, Rifai N, Burring JE, Ridker PM (2008) Fasting compared with nonfasting lipids and Apolipoproeins for predicting incident cardiovascular events. Circulation 118: 993-1001.
- Pedro-Botet J, RodrÃguez-Padial L, Brotons C, Esteban-Salán M, GarcÃa-LerÃn A, et al. (2018) Homogenization of the lipid profile values. Clin Investig Arterioscler 30: 36-48.
- Scuteri A, Najjar SS, Orru' M, Usala G, Piras MG, et al. (2010) The central arterial burden of the metabolic syndrome is similar in men and women: the Sardinia Study. Eur Heart J 31: 602-613.
- Joven J, Villabona C, Vilella E (1990) Abnormalities of lipoprotein metabolism in patients with nephrotic syndrome. N Engl J Med 323: 579-584.
- Ryder REJ, Hayes TM, Mulligan JP, Kingswood JC, Williams S, et al. (1984) How soon after myocardial infarction should plasma lipid values be assessed? Br Med J 289: 1651-1653.
- Alvarez C, Ramos A (1986) Lipids, lipoproteins and apolipoproteins in serum during infection. Clin Chem 32: 142-145.Â
- Nigam PK, Narain VS, Hasan M (2004) Serum lipid profile in patients with acute myocardial infarction. Indian J Clin Biochem 19: 67-70.
- Campose H, Khoo C, Sacks FM (2005) Diurnal and acute pattern of postprandial apolipoprotein B-48 in VLDL, IDL and LDL from normolipidemic human. Atherosclerosis 181: 345-351.
- Mora S, Rifai N, Buring JE, Ridker PM (2009) Comparison of LDL cholesterol concentration by Friedewald calculation and direct measurement in relation to cardiovascular events in 27,331 women. Clin Chem 55: 888-894.
- Sahu S, Chawla R, Uppal B (2005) Comparison of two methods of estimation of low density lipoprotein cholesterol, the direct versus Friedewald estimation. Indian J Clin Biochem 20: 54-61.
- Backer G, Ambrosioni E, Borch-Johnson K, Brotons C (2003) European guidelines on cardiovascular disease and prevention in clinical  practice. Atherosclerosis 171: 145-155.
- Scuteri A, Orru' M, Morrell CH, Tarasov K, Schlessinger D, et al. (2012) Associations of large artery structure and function with adiposity: effects of age, gender, and hypertension. The SardiNIA Study. Atherosclerosis 221: 189-197.
- Scuteri A, Orru M, Morrell C, Piras MG, Taub D, et al. (2011) Independent and additive effects of cytokine patterns and the metabolic syndrome on arterial aging in the Sardinia Study. Atherosclerosis 215: 459-464.
Citation: Feres MC, Perez BB, Thorrecilha JTM, Bini R, Raphael NA, et al. (2018) Implications of Lipid Profile Dosages in Fasting and Postprandial Status. Atheroscler Open Access 3: 121.
Copyright: © 2018 Feres MC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 5508
- [From(publication date): 0-2018 - Dec 16, 2025]
- Breakdown by view type
- HTML page views: 4522
- PDF downloads: 986