Improvement in Gait Performance after Training Based on Declarative Memory Cues in Patients with Parkinsons Disease: A Randomized Clinical Trial
Received: 31-Oct-2015 / Accepted Date: 25-Nov-2015 / Published Date: 05-Dec-2015 DOI: 10.4172/2165-7025.1000277
Abstract
Background: Deficits in automatic motor control, a typical feature of Parkinson’s disease (PD), contribute to progressive impairment in gait performance. The use of declarative memory cues in order to promote the engagement of attention and activation of the next movement in gait may minimize the consequences of lack of automatic control.
Objectives: To verify the long-term efficiency of a new strategy based on declarative memory cues to improve the gait performance and independence in activities of daily living (ADL) in patients with PD.
Design: Parallel prospective, single blind, randomized clinical trial.
Setting: Brazil Parkinson Association.
Participants: Forty-four patients with PD in stages 2-3 of disease evolution according to Hoehn and Yahr Classification.
Interventions: The experimental training (ET) consisted of eight gait training sessions, twice a week, using the declarative memory cue strategy (DMCS). The control training (CT) consisted of a similar gait training without DMCS.
Primary outcome measure: Gait performance in terms of speed and stride length.
Secondary outcome measure: Independence in ADL according to Section II of the Unified Parkinson’s Disease Rating Scale.
Randomization: Participants were randomized into a control group (CG), which performed the CT, and an experimental group (EG), which performed the ET, through blinded drawing of names.
Statistical analysis: Gait performance and independence in ADL before, 2 and 60 days after the end of training were compared for CG and EG using RM-ANOVA.
Results: RM-ANOVA revealed a significant improvement of the gait performance in terms of speed and stride length and independence in ADL, remained until 2 months after the end of training, exclusively for the EG after ET.
Conclusion: Gait training associated to declarative memory cues promotes significant long-term improvements in gait performance and can be considered a new useful strategy to compensate the deficiency in automatic motor control of gait in PD patients.
Keywords: Physiotherapy; Automatic motor control; Cognition
361473Introduction
Among the motor alterations in PD patients, gait is particularly affected and characterized by several hypokinetic features such as decrease of the stride length and off-ground elevation of the feet, leading to short stepping, shuffling and consequently reduction in gait speed [1,2]. The search for novel strategies to attenuate gait disturbances in PD has posed a challenge to physiotherapists. Current strategies are based on external cues [3-7], attention engagement [8-13] and both combined [14], which facilitate gait by minimizing the deficit in automatic control associated to PD. In spite of the significant short-term benefits, these strategies on gait performance, evidences on the long-term results and their effects on gait in daily living remain limited [15-19].
One possibility which remains unexplored is based on the use of declarative memory resources to minimize deficits in automatic control on the gait. It is well established that the implicit memory system depends on the striatal network, and PD patients have been shown to be impaired on automatic motor tasks relying on the well-functioning of this system [20]. Basal ganglia are responsible for generating internal signals required by the transition process from one movement component to the next, within an automatic sequence of movements. These internal signals enable an action plan to be prepared, retrieved and implemented from memory [21]. Due to the decreased dopamine levels associated with PD, internal signal generation is reduced, leading to impairments in automatic sequential movements [20,22-24]. This deficiency is correlated with clinical disability and functional outcome [25]. Considering that, although patients with PD tend to present several cognitive alterations, their declarative memory remains partially intact [26-28], we put forward the hypothesis that it should be possible to train patients to guide their actions by following declarative information (cues) concerning the previously memorized sequence of movements. During walking, this information (cues) would be retrieved from declarative memory, facilitating the start and execution of the next step and functioning as a compensatory strategy in order to minimize the lack in the automatic control of gait. In fact, previous studies have confirmed that patients with PD have similar performance in comparison to elderly individuals in sequential movement when they can use advanced information to guide the sequence [29-33].
We differentiate this declarative memory cue strategy (DMCS) from the external cue strategy since, after being memorized, the cues are internally generated from neural structures related to the declarative memory system, remaining available even before the gait initiation.
Furthermore, we differentiate DMCS from attentional and cognitive strategies because, despite the involvement of attentional control and the cognitive functions in both, the key point for DMCS consists of the usage of the declarative memory cues for activating the next component of the sequence allowing a forward motor control. In contrast, attentional and cognitive strategies, according to the more recent physiotherapy guideline for PD [34], involve general instructions to improve the gait performance as: “thinking about taking big steps, lifting knees high up, making wide turns, choosing a point of reference to walk towards”. In other words, the main principle of DMCS consists of using the declarative memory to compensate the deficiency in implicit control on the gait. Evidences from a recent study sustain that PD patients have deficiency in implicit learning with preserved declarative learning capacity and, most importantly, indicate the possibility of compensation from declarative memory for implicit deficiency. It suggest a cooperative interaction between the two systems [35]. In DMCS, the declarative memory is used as compensatory strategy facilitating the chunking of movement involved in the gait. In fact, complex movements such as gait, rely on a combination of the grouping of single movements into sequences, leading to coordinated actions [36]. Such groupings, consolidated into chunks, optimize the automatic execution of movements. This process involves the nigrostriatal dopaminergic system in animal [37,38] and human [39,40]. Thus, the deficiency to control the movements automatically and the excessive dependency on voluntarily controlled cognitive processes consequent of the reduction of dopamine level may be associated with the lack in the movement chunking [40,41].
Therefore, the aim of this study was to verify the immediate and long-term effects of the DMCS on gait performance and independence in activities of daily living (ADL), in patients with PD. To isolate the effects of the declarative memory cues, we compared the results obtained by DMCS with results obtained by identical gait training without the support of the declarative memory cues.
We hypothesized that DMCS could compensate the consequences of the deficiency in automatic control, improving the gait performance and consequently, improving the independence in ADL due to generalization of improvements to gait-related ADLs. This hypothesis was based on the supposition that declarative memory cues could compensate the deficiency in implicit memory system promoting the engagement of attention and activation of the next movement in a predictable sequence. Considering that automatic motor control depends on the optimal movement chunking, this strategy could be efficient for initiating and maintaining an efficient movement sequence in the gait of PD patients, minimizing the consequences of deficiencies in automatic control.
Methods
Trial design
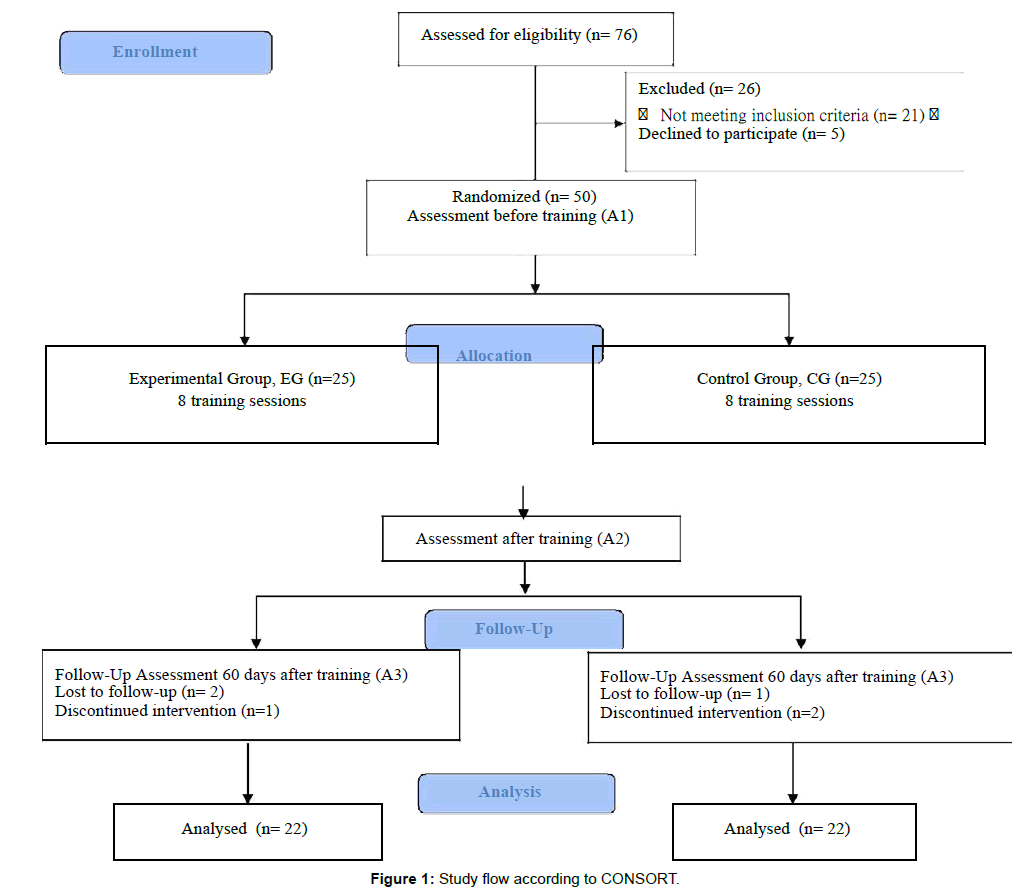
This was a parallel, prospective, single-blind, randomized clinical trial. The study was performed in accordance with the Consolidated Standards of Reporting Trials (CONSORT) guidelines [42]. Figure 1 presents a diagrammatic representation of the study and flow of participants based on the CONSORT flowchart. This study was approved by the Local Ethics and Research Committee (number 058/15), and registered on clinicaltrials.gov (identifier: NTC02600728).
Participants
Inclusion criteria: Eligible participants were patients with a diagnosis of idiopathic Parkinson’s disease according to the UK Brain Bank criteria [43], in stage 2-3 of the disease evolution according to the Hoehn and Yahr classification [44], aged 65 to 80 years, treated with levodopa or its synergists; capable to ambulate independently indoors without aid; referring to 5 to 15 years of education.
Exclusion criteria: There were excluded patients with other neurological (excluding PD), orthopedic or cardiopulmonary problems, visual and auditory deficiency, dementia [assessed by the Mini Mental State Examination (MMSE), cut-off 23] [45] or depression [according to the Geriatric Depression Scale (GDS-15), cut-off 6] [46]. Patients receiving physical therapy training were also excluded from study.
Settings and locations: Forty-four patients with Idiopathic Parkinson Disease, who had as complaint the gait alterations, were recruited from PD associations.
Intervention
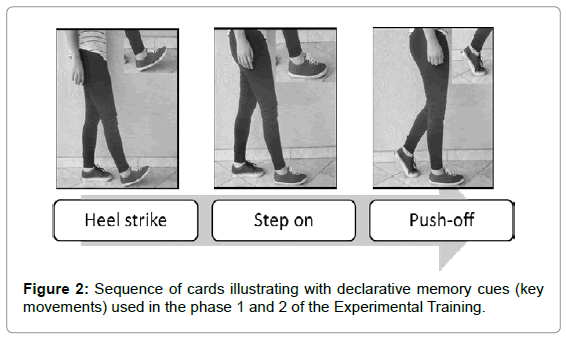
First, we defined which declarative memory cues were more efficient to guide the gait. In fact, the main challenge in developing the DMCS was to identify the most efficient cues to be memorized. These cues must be specific and short to facilitate the memorization and retrieval, yet sufficiently meaningful to guarantee the engagement of the next movement in sequence, facilitating the movement chunking. Considering the biomechanical and motor control aspects of gait in patients with PD, we tested several sequences of cues in order to find the most feasible. At the end of a pilot study, we selected (1) the foot movements as the best focal attentional point and (2) a sequence of 3 cues summarizing the three important phases of gait as the most feasible among the tested sequences. The three declarative memory cues were (Figure 2): HEEL STRIKE, associated to first heel contacts on the walking surface; STEP ON, associated to loading response when the foot comes in full contact with the floor, and body weight is fully transferred onto the stance limb; PUSH-OFF, associated to time at which the foot is removed from the walking surface [47].
Subsequently, we defined the procedures involved in the experimental and control training. Afterward, participants were randomly distributed between two groups according to type of training. The experimental group fulfilled the gait training based on DMCS while the control group fulfilled the gait training without the support of any kind of cues or cognitive strategies. To verify retention after the training, participants were assessed and re-assessed 2 and 60 days after the end of training, as a follow-up.
Both trainings consisted of 8 individual training sessions, twice a week, for four weeks. After the training sessions, no instruction was offered to patients for training at home.
Experimental training
The ET consisted of 3 phases, the first one (Phase 1) was done only in the first session of training, and the other two (Phase 2 and 3) were repeated at each of the 8 sessions.
Phase 1: Initially, in order to better understand the strategy, patients received a short and simple explanation about the deficiency in automatic movement resulting from PD. Following the explanation, the patients memorized a sequence of declarative cues (Figure 2).
The patients would then move on to the next stage only after having successfully memorized the cue sequence.
Phase 2: Patients organized a sequence of cues using cards illustrating the subcomponent movements (key movement) involved in taking steps. The sole purpose of this approach was to further consolidate the memorization of cues. The patients would then move on to stage 3 only upon completion of 5 consecutive successful attempts.
Phase 3: Gait motor training guided by the cues. In this stage, the patients had to train using declarative cues as a gait performance support through 8 sets following the instruction “Walk in your ordinary speed. Use the key movements to guide your steps saying each of them while you do them”. Each set was performed following different four trajectories with 80 meters of extension. Markers on the ground delimited the straight and crooked trajectories. The declarative cues had to be evoked verbally by the patients themselves, during gait, triggering the corresponding movement. Whenever patients proved unable to use the cues properly, e.g. they were not able to coordinate the retrieval of cues together with the respective movement, they returned to phase 2.
Control training
The CT consisted of 3 phases, the first one (Phase 1) was done only in the first session of training, and the other two (Phase 2 and 3) were repeated at each of the 8 sessions.
Phase 1: Patients received a short and simple explanation about the deficiency in automatic movement resulting from PD.
Phase 2: Patients received a general verbal attentional instruction of “pay attention to your steps and try to walk as well as you can”, before starting the walk.
Phase 3: Motor training of gait, where the patient had to perform 8 sets, following the instruction “Walk in your ordinary speed, paying attention to your steps” in the identical four trajectories of ET. Additional instructions or cues were not provided to patients by the physiotherapist.
Outcome measures and test procedure
T?he three assessments were performed in individual sessions by an independent blinded examiner, before (A1) and two (A2) and sixty days (A3) after the end of training.
All patients were tested at between 40 and 120 minutes after their last L-dopa dose, whereby each patient was tested at the same time of the day.
Primary outcome: The primary outcome was the gait performance in terms of speed and step length. Patients were asked to walk in a straight trajectory of 20 meters following the sole instruction “upon the go signal, walk as fast as possible to the line and stop”. The speed was calculated based on the time to walk 20 meters timed using a digital chronometer. The step length was calculated based on the number of steps measured using a pedometer.
Secondary outcome: The secondary outcome was independence in activities of ADL, assessed by Section II of the Unified Parkinson Disease Rating Scale (UPDRS-II). This section includes 12 questions (items 5 to 16) on patient’s performance in ADL. Among these questions, two of them investigate gait performance [frequency of falls due to freezing (14); ability to walk (15)], with scores ranging from zero (normal) to 4. The application followed the procedure recommended by Goetz et al. [44]: (1) Reading to the patient the introductory statement for each item of the UPDRS-II. (2) After the introduction, the interviewer asked the patient: “With all these considerations in mind, do you have any problems in this activity?” (3) If the initial answer was “No” (likely rating is “Code 0”), the rater probed for “Code 1” to verify that this response is not more appropriate. (4) If the initial answer was “Yes,” the interviewer probed for the moderate option, using “Code 2” as an anchor. (5) Depending on subsequent answers to this probed regarding “Code 2,” the interviewer should move up or down the scale (to more or less severe options) to find the most appropriate item response. (6) When the best item response code was determined, the interviewer verified this by reviewing those response codes immediately above and the patient should confirm that these other response codes were not appropriate [48].
The UPDRS has been considered by the Movement Disorders Society to be the gold standard assessment for patients with Parkinson’s disease, and it is the most widely used instrument for its clinical trials [34,49,50].
Sample size calculation
The sample size calculation was based on the gait performance according to 20 meters walking test (20MW). According to a pilot study with PD patients with similar features to the PDs in this study, the sample size calculation showed that 36 patients (18 in each group) would be sufficient for a power greater than 90% (α =0.05).
Randomisation and blinding: Randomisation was achieved through a computer-generated random-sequence Table 1. The subject’s randomisation was carried out by a researcher who is not involved in enrolling the participants, in assigning them to their groups, or in performing follow-up measurements. This researcher kept the allocation concealed and prepared sealed envelopes, which were opened individually only when each participant started their training. Subjects were distributed between two groups. Only the researcher responsible for conducting the training knew how the participants were distributed. The researcher responsible for evaluations was not aware of the allocations at any time during the data collection.
| Control Group (n=22) | Experimental Group (n=22) | All (n=44) | p-value | |
|---|---|---|---|---|
| Age (years) | 70.31 (4.11) | 70.45 (6.38) | 70.38 (5.34) | >0.05 |
| Gender (male) | 13 | 13 | 26 | >0.05 |
| Hoehn&Yahr stage | 2(12); 3(10) | 2(12); 3(10) | 2(24); 3(20) | - |
| PD duration (years) | 6.5 (1.97) | 6.63 (2.57) | 6.56 (2.28) | >0.05 |
Table 1: Computer-generated random-sequence.
Statistical methods
Demographic and clinical characteristics of the patients in the CG and the EG were compared using the unpaired t-test.
The Kolmogorov–Smirnov and Levene tests were used to assess normality and homogeneity of variance of gait speed, number of steps and scores in UPDRS-II.
We performed three comparisons using 2 X 3 repeated-measure ANOVAs (type of training X assessments) with repeated measure on the second factor. The first comparison focused on the effect of training type (ET, CT) and assessment (A1, A2 and A3) on gait speed. The second examined the effect of training type (ET, CT) and assessment (A1, A2 and A3) on number of steps. The third focused on the effect of training type (ET, CT) and assessment (A1, A2 and A3) on score in UPDRS-II.
In order to investigate the percentage change of the variables after training, we calculated the percentage improvement in [GSPI (gait speed percentage improvement)], step length [SLPI (step length percentage improvement)], and ADL [ADLPI (ADLs percentage improvement)] for A1 X A2 and A1 X A3. The Pearson Correlation Coefficient (r) was used to verify the correlation between GSPI X ADLPI and SLPI X ADLPI.
The effect sizes (ES) were calculated for all ANOVAs (alpha = .05). A Tukey HSD post-hoc test was performed whenever required. Statistica software version 13.0 (StatSoft, USA) was used for all analyzes. P-values smaller than 0.05 were considered statistically significant.
Results
Demographic and clinical characteristics of patients in the two groups at baseline are presented in Table 1. Forty-four patients presenting mean disease duration of 6.5 years (SD 2.28), mean age of 70.38 years (SD 5.34), comprising 18 women and 26 men, 24 had stage 2, and 20 stage 3, disease evolution according to the Hoehn and Yahr classification. There were no significant differences between the two groups (unpaired t-test; p > 0.05). All participants completed the training without any adverse effects.
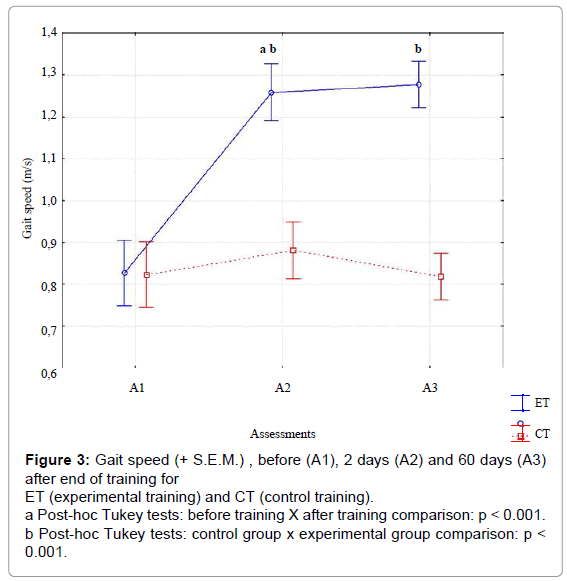
For gait speed (Figure 3), significant effects were observed for training type [F(1,58)= 40.23, p<.01, ES=.90], and assessments [F(2,116)= 142.31, p<.01, ES=.90] and their interaction [F( 2,116)= 113.29, p<.001, ES=.95]. The interaction demonstrated that gait speed increased for ET, but not for CT. Post-hoc intra-group comparisons using the Tukey HSD test showed significant improvement between A1xA2, and A1xA3 for ET, but not for CT. Inter-group comparison showed non-significant differences in gait speed in A1, but significant differences in A2 and A3 between EG and CG.
Figure 3: Gait speed (+ S.E.M.) , before (A1), 2 days (A2) and 60 days (A3) after end of training for ET (experimental training) and CT (control training).
a Post-hoc Tukey tests: before training X after training comparison: p < 0.001.
b Post-hoc Tukey tests: control group x experimental group comparison: p < 0.001.
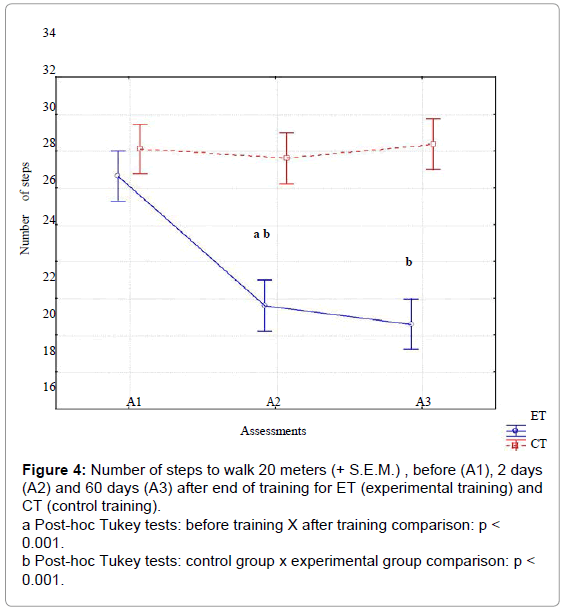
For step length (Figure 4), significant effects were observed for training type [F(1,58)= 47.66, p<.01, ES=.90], and assessments [F(2,116)= 181.10, p<.001, ES=.95] and their interaction [F(2,116)=177.24, p<.001, ES=.99]. The interaction demonstrated that step length increased for ET, but not for CT. Post-hoc intra-group comparisons using the Tukey HSD test showed significant improvement between A1 X A2, and A1xA3 for ET, but not for CT. Inter-group comparison showed nonsignificant differences in step length in A1, but significant differences in A2, and A3 between EG and CG.
Figure 4: Number of steps to walk 20 meters (+ S.E.M.) , before (A1), 2 days (A2) and 60 days (A3) after end of training for ET (experimental training) and CT (control training).
a Post-hoc Tukey tests: before training X after training comparison: p < 0.001.
b Post-hoc Tukey tests: control group x experimental group comparison: p < 0.001.
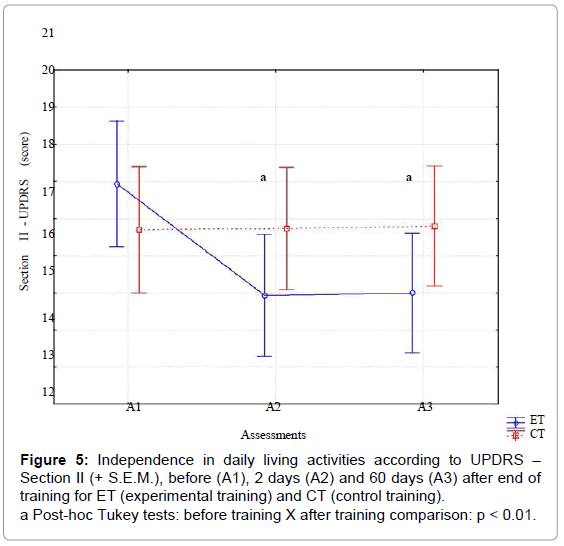
For independence in ADL (Figure 5), significant effects were observed for assessments [F (2,116) = 358.35, p<.01, ES=.80] and their interaction with training type [F (2,116) =118.35, p<.01, ES=.85]. The interaction demonstrated that punctuation decrease for ET, but not for CT. Post-hoc intra-group comparisons using the Tukey HSD test showed significant improvement between A1 X A2, and A1 X A2 for ET, but not for CT.
There was a significant correlation between the improvements in the gait parameters and independence in ADL in A2 (SLPI X ADLPI, R=.46; GSPI X ADLPI, R=.70) and A3 (SLPI X ADLPI, R=.49; GSPI X ADLPI, R=.75), indicated that the effects of DMCS were generalized to gait-related ADLs.
To summarize, there was a significant improvement in gait speed, step length and independence in ADL after ET, which remained 60 days after training.
Discussion
The present study aimed at investigating the effects of the gait training based on declarative memory cue strategy on gait performance in patients with PD.
Two key findings emerged from this study. The first of these was that DMCS was effective for improving gait speed and step length in patients with PD and, most importantly, the training effects remained after 60 days without any additional training. Few studies have shown long-term results after cue training. Some studies reported retention of the gait improvements after 4 weeks without training [4,10]. The most complete studies that investigated the largest number of patients showed that the effects of the intervention on the gait in absence of cues reduced significantly after 3 and 6 weeks without training [17,18].
Several factors may have contributed to maintenance of the gait improvement in the current study: (1) the support of the declarative memory system, (2) easy management of cues and, (3) the detailed explanation on the deficiency in automatic control provided to patients before the training in order to emphasize the need of implementation of the new strategy to minimize the gait disturbance resulting from PD. In comparison with previous studies, these factors may have facilitated the continuous use of the declarative memory cues by patients after the training, increasing the retention of the training effects [51-53].
The second key finding was that the positive effect was transferable to gait-related ADL, considering the improvements in the independence in ADL. These results suggest that, after training, DMCS can be used by patients at home. The tool used to assess the effect on ADL (section II of the UPDRS) has been widely validated and assesses the perception of the patients themselves regarding their performance in ADLs, over the preceding two weeks [49]. The analysis of the longitudinal metric attributes of the UPDRS showed that the independence in ADL is a valid measure for follow-up of PD patients, being more precise than other scales [54]. Additionally, the minimal clinically important change in reference to the status before treatment for the UPDRS-ADL score is two points for Hoehn and Yahr stages 1-2 and three points for Hoehn and Yahr stage 2-3 [55]. Therefore, the mean change found in the current study of 3 points (17.93 ± 4.44 to 14.83 ± 4.13), can be considered clinically important. This represents a further considerable contribution to gait treatment in the light of a systematic review on effects of external cues on gait, which concluded that, despite reliable results in laboratory tests, the evidences of generalization of improvement to gait-related ADLs are limited [56].
Taken together, these findings indicate that the DMCS constitutes an important alternative for treatment of gait dysfunction in PD. One of the mechanisms that might be involved in this strategy could be the attention to movement. Undoubtedly, the increase in attention on gait is an important mechanism activated in this strategy, given the need to retrieve the cues from declarative memory and to manage them during gait. This process most likely depends on working memory and it is well known that this memory module is closely associated with attention. Some studies have indicated that working memory is hampered in PD [57,58] but, even considering that patients in the current study might have had undetected working memory deficits, this would not impair their ability to use the declarative cues. Moreover, it is important to point out that the CT in this study also involved increased attention on gait, and yet positive effects have not been found. Thus, there are two possible alternative explanations behind the differences observed between results obtained from the two strategies: the DMCS allows best engagement of attention, or attention is not the most important factor in improving gait. Further studies are necessary to elucidate possible differences in the demand of attention between the strategies, since this goes beyond the scope of this study. Considering the second possibility, we believe that declarative cues were a key factor impacting the results. After memorization and training, the cues not only engage the patient’s attention to their foot movements, but also facilitate the movement chunking involved in the gait, triggering the next movement into a previously memorized sequence. It may compensate the deficiency in automatic control on gait associated to the lack in the movement chunking [40,41]. This evidence sustains the possibility of compensation from declarative memory for implicit deficiency, suggesting a cooperative interaction between the two systems. Recent findings have confirmed the possibility that the declarative memory system is able to compensate the deficiency in the implicit memory system in individuals with striatal damage (e.g., individuals with PD), minimizing the lack in automatic control [35]. Although the level of gait automaticity after training has not been investigated by the current study, it is reasonable to assume that DMCS might have minimized the deficiency in automatic control of gait due to the facilitation in movement chunking and gait programming, since long-term effects and generalization to gait-related ADL were observed. According to our knowledge, this is the first evidence about the clinical potential of training based on this type of compensatory strategy for improving gait performance. The positive results found may have important implications for the development and modification of interventions, which could improve rehabilitation programs for patients with PD.
Besides the limitation of our study previously outlined, a further limitation is the absence of specific tests to assess declarative and working memory capacity. Although the MMSE evaluated memory and attention, it is not specifically for these memory modules. Additional studies are necessary to investigate a possible correlation between the memory function and effects of the DMCS. Further studies are also necessary to investigate the effects on DMCS on gait performance under dual-task condition in order to investigate the level of attention demanded before and after the intervention.
In conclusion, DMCS leads to significant long-term improvements in gait performance in patients with PD. Consequent improvements in independence in ADL demonstrate the usefulness of this strategy in motor rehabilitation of such patients.
Acknowledgement
Associação Brasil Parkinson.
References
- Sofuwa O, Nieuwboer A, Desloovere K, Willems AM, Chavret F, et al. (2005) Quantitative gait analysis in Parkinson's disease: comparison with a healthy control group. Arch Phys Med Rehabil 86: 1007-1013.
- Rodriguez-Oroz MC, Jahanshahi M, Krack P, Litvan I, Macias R, et al. (2009) Initial clinical manifestations of Parkinson's disease: features and pathophysiological mechanisms. Lancet Neurol 8: 1128-1139.
- Suteerawattananon M, Morris GS, Etnyre BR, Jankovic J, Protas EJ (2004) Effects of visual and auditory cues on gait in individuals with Parkinson's disease. J NeurolSci 219: 63-69.
- Sidaway B, Anderson J, Danielson G, Martin L, Smith G (2006) Effects of long-term gait training using visual cues in an individual with Parkinson disease. PhysTher 86: 186-194.
- Capato TT, Tornai J, Ãvila P, Barbosa ER, et al. (2015) Randomized controlled trial protocol: balance training with rhythmical cues to improve and maintain balance control in Parkinson's disease. BMC Neurol 15: 162.
- Rochester L, Burn DJ, Woods G, Godwin J, Nieuwboer A (2009) Does auditory rhythmical cueing improve gait in people with Parkinson's disease and cognitive impairment? A feasibility study. MovDisord 24: 839-845.
- Rochester L, Baker K, Nieuwboer A, Burn D (2011) Targeting dopa-sensitive and dopa-resistant gait dysfunction in Parkinson's disease: selective responses to internal and external cues. MovDisord 26: 430-435.
- Behrman AL, Teitelbaum P, Cauraugh JH (1998) Verbal instructional sets to normalise the temporal and spatial gait variables in Parkinson's disease. J NeurolNeurosurg Psychiatry 65: 580-582.
- Canning CG (2005) The effect of directing attention during walking under dual-task conditions in Parkinson's disease. Parkinsonism RelatDisord 11: 95-99.
- Lehman DA, Toole T, Lofald D, Hirsch MA (2005) Training with verbal instructional cues results in near-term improvement of gait in people with Parkinson disease. J NeurolPhysTher 29: 2-8.
- Farley BG, Koshland GF (2005) Training BIG to move faster: the application of the speed-amplitude relation as a rehabilitation strategy for people with Parkinson's disease. Exp Brain Res 167: 462-467.
- Lowry KA, Carrel AJ, McIlrath JM, Smiley-Oyen AL (2010) Use of harmonic ratios to examine the effect of cueing strategies on gait stability in persons with Parkinson's disease. Arch Phys Med Rehabil 91: 632-638.
- Kelly VE, Shumway-Cook A (2014) The ability of people with Parkinson's disease to modify dual-task performance in response to instructions during simple and complex walking tasks. Exp Brain Res 232: 263-271.
- Lohnes CA, Earhart GM (2011) The impact of attentional, auditory, and combined cues on walking during single and cognitive dual tasks in Parkinson disease. Gait Posture 33: 478-483.
- Morris ME, Iansek R, Matyas TA, Summers JJ (1996) Stride length regulation in Parkinson's disease. Normalization strategies and underlying mechanisms. Brain 119 : 551-568.
- Rubinstein TC, Giladi N, Hausdorff JM (2002) The power of cueing to circumvent dopamine deficits: a review of physical therapy treatment of gait disturbances in Parkinson's disease. MovDisord 17: 1148-1160.
- Nieuwboer A, Kwakkel G, Rochester L, Jones D, van Wegen E, et al. (2007) Cueing training in the home improves gait-related mobility in Parkinson's disease: the RESCUE trial. J NeurolNeurosurg Psychiatry 78: 134-140.
- Rochester L, Nieuwboer A, Baker K, Hetherington V, Willems AM, et al. (2007) Theattentional cost of external rhythmical cues and their impact on gait in Parkinson's disease: effect of cue modality and task complexity. J Neural Transm (Vienna) 114: 1243-1248.
- Lim I, van Wegen E, Jones D, Rochester L, Nieuwboer A, et al. (2010) Does cueing training improve physical activity in patients with Parkinson's disease? Neurorehabil Neural Repair 24: 469-477.
- Siegert RJ, Taylor KD, Weatherall M, Abernethy DA (2006) Is implicit sequence learning impaired in Parkinson's disease? A meta-analysis. Neuropsychology 20: 490-495.
- Goerendt IK, Messa C, Lawrence AD, Grasby PM, Piccini P, et al. (2003) Dopamine release during sequential finger movements in health and Parkinson's disease: a PET study. Brain 126: 312-325.
- Elsinger CL, Harrington DL, Rao SM (2006)From preparation to online control: reappraisal of neural circuitry mediating internally generated and externally guided actions. Neuroimage 31: 1177-1187.
- Smiley-Oyen AL, Lowry KA, Emerson QR (2006) Learning and retention of movement sequences in Parkinson's disease. MovDisord 21: 1078-1087.
- Ruitenberg MF, Duthoo W, Santens P, Notebaert W, Abrahamse EL (2015) Sequential movement skill in Parkinson's disease: a state-of-the-art. Cortex 65: 102-112.
- Price A, Shin JC (2009) The impact of Parkinson's disease on sequence learning: perceptual pattern learning and executive function. Brain Cogn 69: 252-261.
- Muslimovic D, Post B, Speelman JD, Schmand B (2007) Motor procedural learning in Parkinson's disease. Brain 130: 2887-2897.
- Zgaljardic DJ, Borod JC, Foldi NS, Mattis P (2003) A review of the cognitive and behavioral sequelae of Parkinson's disease: relationship to frontostriatal circuitry. CognBehavNeurol 16: 193-210.
- Salmon DP, Filoteo JV (2007) Neuropsychology of cortical versus subcortical dementia syndromes. SeminNeurol 27: 7-21.
- Foster ER, McDaniel MA, Repovs G, Hershey T (2009) Prospective memory in Parkinson disease across laboratory and self-reported everyday performance. Neuropsychology 23: 347-358.
- Georgiou N, Bradshaw JL, Iansek R, Phillips JG, Mattingley JB, et al. (1994) Reduction in external cues and movement sequencing in Parkinson's disease. J NeurolNeurosurg Psychiatry 57: 368-370.
- Jahanshahi M, Jenkins IH, Brown RG, Marsden D, Passingham RE, et al. (1995) Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson’s disease subjects. Brain 118: 913-933.
- Currà A, Berardelli A, Agostino R, Modugno N, Puorger CC, et al. (1997) Performance of sequential arm movements with and without advance knowledge of motor pathways in Parkinson's disease. MovDisord 12: 646-654.
- Siegert RJ, Harper DN, Cameron FB, Abernethy D (2002) Self-initiated versus externally cued reaction times in Parkinson's disease. J ClinExpNeuropsychol 24: 146-153.
- Roy S, Park NW, Roy EA, Almeida QJ (2015) Interaction of memory systems during acquisition of tool knowledge and skills in Parkinson's disease. Neuropsychologia 66: 55-66.
- Seidler RD (2006) Differential effects of age on sequence learning and sensorimotor adaptation. Brain Res Bull 70: 337-346.
- Levesque M, Bedard MA, Courtemanche R, Tremblay PL, Scherzer P, et al. (2007) Raclopride-induced motor consolidation impairment in primates: role of the dopamine type-2 receptor in movement chunking into integrated sequences. Exp Brain Res 182: 499-508.
- Tremblay PL, Bedard MA, Levesque M, Chebli M, Parent M, et al. (2009) Motor sequence learning in primate: role of the D2 receptor in movement chunking during consolidation. Behav Brain Res 198: 231-239.
- Boyd LA, Edwards JD, Siengsukon CS, Vidoni ED, Wessel BD, et al. (2009) Motor sequence chunking is impaired by basal ganglia stroke. Neurobiol Learn Mem 92: 35-44.
- Tremblay PL, Bedard MA, Langlois D, Blanchet PJ, Lemay M, et al. (2010) Movement chunking during sequence learning is a dopamine-dependant process: a study conducted in Parkinson's disease. Exp Brain Res 205: 375-385.
- Verwey WB (2010) Diminished motor skill development in elderly: indications for limited motor chunk use. ActaPsychol (Amst) 134: 206-214.
- Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, et al. (2012) CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg 10: 28-55.
- Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J NeurolNeurosurg Psychiatry 55: 181-184.
- Goetz CG, Poewe W, Rascol O, Sampaio C, Stebbins GT, et al. (2004) Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. MovDisord 19: 1020-1028.
- Stewart R, Kondraske G, Chisty K, Hussan S, Movahid M (2009) Analysis of clock drawing (CD), Mini Mental Status Examination (MMSE) and quantitative subsystem performance (QSP) in Parkinson’s disease (PD) using nonlinear causal resource analysis (NCRA). MovDisord 24: 436-437.
- Almeida OP, Almeida SA (1999) [Reliability of the Brazilian version of the Geriatric Depression Scale (GDS) short form]. ArqNeuropsiquiatr 57: 421-426.
- Hansen AH, Childress DS, Meier MR (2002) A simple method for determination of gait events. J Biomech 35: 135-138.
- Goetz CG, LeWitt PA, Weidenman M (2003) Standardized training tools for the UPDRS activities of daily living scale: newly available teaching program. MovDisord 18: 1455-1458.
- Movement Disorder Society Task Force on Rating Scales for Parkinson's Disease (2003) The Unified Parkinson's Disease Rating Scale (UPDRS): status and recommendations. MovDisord 18: 738-750.
- Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, et al. (2008) Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. MovDisord 23: 2129-2170.
- Verschueren SM, Swinnen SP, Dom R, De Weerdt W (1997) Interlimb coordination in patients with Parkinson's disease: motor learning deficits and the importance of augmented information feedback. Exp Brain Res 113: 497-508.
- Rochester L, Baker K, Hetherington V, Jones D, Willems AM, et al. (2010) Evidence for motor learning in Parkinson's disease: acquisition, automaticity and retention of cued gait performance after training with external rhythmical cues. Brain Res 1319: 103-111.
- Lesch MF, Chang CC, Chang WR (2015) Prospective gait changes as a function of shifting perceptions of slipperiness: effects of visual and somatosensory cues. Ergonomics.
- Martinez-Martin P, Prieto L, Forjaz MJ (2006) Longitudinal metric properties of disability rating scales for Parkinson's disease. Value Health 9: 386-393.
- Schrag A, Sampaio C, Counsell N, Poewe W (2006) Minimal clinically important change on the unified Parkinson's disease rating scale. MovDisord 21: 1200-1207.
- Lim I, van Wegen E, de Goede C, Deutekom M, Nieuwboer A, et al. (2005) Effects of external rhythmical cueing on gait in patients with Parkinson's disease: a systematic review. ClinRehabil 19: 695-713.
- Levin BE, Katzen HL (2005) Early cognitive changes and nondementing behavioral abnormalities in Parkinson's disease. AdvNeurol 96: 84-94.
- Filoteo JV, Maddox WT, Ing AD, Song DD (2007) Characterizing rule-based category learning deficits in patients with Parkinson's disease. Neuropsychologia 45: 305-320.
Citation: Piemonte MEP, Okamoto E, Cardoso CAR, Tatiana de Paula Oliveira, MS, Miranda CS, et al. (2015) A Comparison between Task Oriented and Client- Centred Task-Oriented Approaches to Improve Upper Limb Functioning in People with Sub-Acute Stroke. J Nov Physiother 5: 277 DOI: 10.4172/2165-7025.1000277
Copyright: © 2015 Piemonte MEP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 11168
- [From(publication date): 12-2015 - Aug 29, 2025]
- Breakdown by view type
- HTML page views: 10208
- PDF downloads: 960