Research Article Open Access
In Vitro Effects of Nicotine, Cigarette Smoke Condensate, and Porphyromonas gingivalis on Monocyte Chemoattractant Protein-1 Expression from Cultured Human Gingival Fibroblasts
Eman Allam1,2, Mia Recupito1, Hend Mohamed3 and L. Jack Windsor1*
1Department of Oral Biology, Indiana University School of Dentistry, USA
2Oral and Dental Research Division, National Research Centre, Cairo, Egypt
3Department of Oral Pathology, Faculty of Oral and Dental Medicine, Cairo University, Egypt
- Corresponding Author:
- L. Jack Windsor, PhD
Department of Oral Biology
Indiana University School of Dentistry
1121 West Michigan Street, DS 271
Indianapolis, IN, USA 46202
Tel: 317-274-1448
Fax: 317-278-1411
E-mail: ljwindso@iu.edu
Received Date: January 27, 2015; Accepted Date: March 28, 2015; Published Date: April 02, 2015
Citation: Eman Allam, Mia Recupito, Hend Mohamed, L. Jack Windsor (2015) In Vitro Effects of Nicotine, Cigarette Smoke Condensate, and Porphyromonas gingivalis on Monocyte Chemoattractant Protein-1 Expression from Cultured Human Gingival Fibroblasts. J Interdiscipl Med Dent Sci 3:171. doi: 10.4172/2376-032X.1000171
Copyright: © 2015 Allam, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at JBR Journal of Interdisciplinary Medicine and Dental Science
Abstract
Background: Monocyte chemoattractant protein-1 (MCP-1) is an inducible protein that attracts monocytes to areas of injury and infection. Studies have shown that it is produced by gingival cells in periodontal diseases and that its incidence increases with the severity of the disease. The aim of the present study was to investigate the effects of nicotine, cigarette smoke condensate (CSC), and Porphyromonas gingivalis (P. gingivalis) on MCP-1 expression from human gingival fibroblasts (HGFs) in vitro.
Methods: HGFs were exposed for 72 h to 250 µg/mL of nicotine, 100 µg/mL of CSC, 10% P. gingivalis supernatant, P. gingivalis supernatant with nicotine, or P. gingivalis supernatant with CSC. A control group comprised HGFs without any treatment. The conditioned media was then collected for MCP-1 analysis by enzyme-linked immunosorbent assay (ELISA).
Results: There were significant differences in MCP-1 level between the P. gingivalis (p=0.0432) and P. gingivalis with CSC (p=0.0037) groups when compared to the control group.
Conclusions: P. gingivalis stimulates an inflammatory response in periodontal tissues by increasing MCP-1, which helps attract host cells to combat the bacterial infection. Tobacco usage can mask the inflammatory responses normally seen in periodontal diseases by reducing the levels of MCP-1, thus allowing the bacteria to grow somewhat undetected. This could be one factor that explains why smoking is a major contributing factor to the initiation, development, and progression of periodontal diseases.
Keywords
Smoking; Periodontal disease; Monocyte chemoattractant protein-1; P gingivalis
Introduction
Periodontal diseases, among many other prevalent chronic diseases, have been constantly linked to smoking and tobacco use. The correlation between smoking and periodontal tissue destruction results mainly from the smoking-associated impairment of normal immunological surveillance or defensive mechanisms. Periodontal diseases are initiated by bacterial colonization that activates tissue mechanisms resulting in a series of inflammatory and immunological changes leading to connective tissue and bone destruction. Porphyromonas gingivalis (P. gingivalis) is one of the principal pathogens responsible for the development of adult periodontitis. Several reports have provided evidence to implicate P. gingivalis in the local destruction of periodontitis [1-4].
Monocyte functions are considered determinants to the periodontal breakdown in periodontal diseases. Monocyte chemoattractant protein-1 (MCP-1) is an inducible protein that has chemotactic activity for lymphocytes and monocytes, and is considered a major signal for the chemotaxis of mononuclear leukocytes [5,6]. MCP-1 is secreted by various cell types such as leukocytes, fibroblasts, kerotinocytes and endothelial cells in response to different endogenous and exogenous stimuli [7]. Overexpression of MCP-1 in the periodontal tissues of patients with periodontitis has been reported in the literature [8,9]. In addition, the association between MCP-1 and host responses was suggested to play a role in aggressive periodontitis [10]. All these studies suggest that the MCP-1 levels may be an important factor that affects the progression and the severity of the periodontal diseases. The aim of the present study was to investigate if nicotine, cigarette smoke condensate (CSC), and/or P. gingivalis would alter MCP-1 expression from human gingival fibroblasts in vitro.
Materials and Methods
Cell cultures and treatment
Human gingival fibroblasts (HGFs) were cultured from clinically healthy gingival tissues removed from patients undergoing crown-lengthening surgery as described previously 11 with Institutional Review Board approval. The cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% bovine growth serum, 4 mM L-Glutamine (HyClone), antibiotics (100 U/ml of penicillin, 100 U/ml of streptomycin and 0.25 µg/ml of fungi zone). Cultures were maintained at 37°C in a humidified atmosphere of 5% CO2 and 95% air. Confluent cells were detached with 0.25% trypsin and aliquots of separated cells were sub-cultured. Cells between the third and eighth passages were used.
Nicotine and CSC were purchased from Sigma (St. Louis, MO) and Murty Pharmaceuticals (Lexington, KY), respectively. P. gingivalis ATCC 33277 supernatant was obtained as a generous gift from Dr. John Ruby (University of Alabama at Birmingham, Birmingham, AL). The P. gingivalis were cultured in supplemented brain heart infusion growth medium. The collected supernatant was filtered twice through 0.2 µm membranes and then stored at -20°C until used.
The HGFs were re-suspended in serum-free DMEM, seeded as single colonies (100,000 cells per well) in the six-well plates, and exposed to 250 µg/mL of nicotine, 100 µg/mL of total particulate matter of CSC, 10% P. gingivalis supernatant, P. gingivalis supernatant with nicotine, or P. gingivalis supernatant with CSC diluted in serum-free media. Cell viability and sublethal cytotoxic effects on the cells were determined according to previous studies [11,12]. A control group comprised HGFs without treatment. After 72 hours, the conditioned media were collected for MCP-1 analysis.
Enzyme-Linked Immunosorbent Assay (ELISA)
Quantikine Human CCL2/MCP-1 Immunoassay kit (R&D Systems, Minneapolis, MN) was used to determine the concentration of MCP-1 in the conditioned media. Standard ELISA assays were performed. In brief, duplicate 5 µl aliquots of each sample were placed in 96-well microtiter plates pre-coated with rabbit anti-MCP-1 antiserum, incubated overnight, and blocked with bovine serum albumin (BSA). Biotinylated rabbit MCP-1 antibody was then added and the plates were developed with diluted avidin-horseradish peroxidase (HRP) followed by peroxidase substrate and hydrogen peroxide. The color development was stopped by the addition of H2SO4 and the absorbance was measured on a BioTek-ELISA reader (BioTek, Winooski, VT) at 450 nm and 540 nm. Readings at 540 nm were subtracted from the readings at 450 nm to correct for optical imperfections in the plate according to the manufacturer’s protocol. Final concentrations were determined from standard curves.
Statistical analysis
Statistical analysis was conducted by using the Statistical Analysis System (SAS version 9.3, SAS Institute Inc., Cary, NC). Summarized data were presented as mean ± standard deviation (SD) and the statistical significance was calculated by one-way analysis of variance (ANOVA) and t test. A p valueËÂ?0.05 was considered statistically significant.
Results
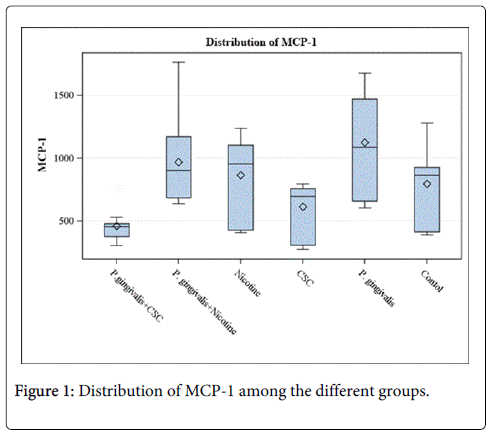
Results from analysis of the data are presented in Table 1 and Figure 1. Compared to the control group, higher levels of MCP-1 were detected from cells treated with P. gingivalis, nicotine, and P. gingivalis with nicotine. Lower levels of MCP-1 were detected from cells treated with CSC and P. gingivalis with CSC compared to the control. Significant differences were detected between the control group and the P. gingivalis (p=0.0432), and P. gingivalis with CSC (p=0.0037).
| Group | MCP-1 Mean ± SD | p-value |
|---|---|---|
| Control | 797.4 ± 313 | |
| P. gingivalis | 1125.9 ± 395.8 | 0.0432* |
| CSC | 612 ± 209.8 | 0.1197 |
| Nicotine | 865.3 ± 312.7 | 0.6166 |
| P. gingivalis + nicotine | 971.5 ± 337.3 | 0.2239 |
| P. gingivalis + CSC | 461.2 ± 130.9 | 0.0037* |
Table 1: MCP-1 expression from HGFs. *statistically significant at the 0.05 level compared to the control.
Discussion
Periodontal diseases affect approximately 15%–35% of the adult population of the United States. It is considered a multifactorial disorder that may result in significant tissue damage and tooth loss eventually. 13 smokers are reported to be at increased risk for periodontal diseases [3,4,13]. Since most of the literature indicate that the microbial flora in the smoker oral cavity is similar to that found in non-smokers, it was concluded that the increased risk for periodontal diseases is not associated with an altered microbial profile, but rather to some changes in the host responses to these microbial pathogens.
There is evidence that HGFs derived from diseased sites in smokers produce higher levels of inflammatory cytokines. P. gingivalis has been identified as one of the major bacteria that contribute to the development of periodontal disease and the breakdown of periodontal tissues [14]. The present study investigated the effects of nicotine and CSC exposure either alone or combined with P. gingivalis on MCP-1 expression from HGFs in vitro. The aim was to test the hypothesis that smoking increases the risk of developing periodontal diseases by altering MCP-1 expression.
The results showed that levels of MCP-1 in the cell supernatants increased by cells treated with nicotine, P. gingivalis, and P. gingivalis with nicotine, whereas it decreased with CSC and P. gingivalis with CSC. These differences were significant between the control group and the P. gingivalis (p=0.0432), and P. gingivalis with CSC (p=0.0037). These results support the hypothesis that both smoking and P. gingivalis infection affects the levels of MCP-1 produced by HGFs and thus may contribute to inflammatory process and the development of periodontal diseases. The significantly increased level of MCP-1 expression from HGFs infected with P. gingivalis indicates the virulence property of this pathogen that was previously reported to induce increased expression of other inflammatory cytokines at the periodontal tissues [15-17].
The results of this study are in accordance with previous reports. A study by Pradeep et al. [18] examined the gingival crevicular fluid (GCF) levels of MCP-1 in periodontal health and disease and indicated that MCP-1 concentrations increased in periodontal disease compared to health and correlated positively with the severity of disease. Tonetti et al. [19] reported marked expression of MCP-1 in gingival biopsies and in the inflammatory infiltrate from diseased periodontal sites. Kurtis et al. [10] also reported higher GCF levels of MCP-1 in patients with aggressive periodontitis or chronic periodontitis compared to controls.
The results also demonstrated that CSC caused down-regulation of MCP-1, which means less monocytes recruitment and thus weaker defensive mechanisms that leads to enhanced disease. So it could be concluded that tobacco usage can mask the inflammatory responses normally seen in periodontal diseases by reducing the levels of MCP-1, thus allowing the bacteria to grow somewhat undetected and permitting more rapid tissue destruction. CSC affected MCP-1 levels differently than nicotine probably because it is comprised of over 4000 chemicals that are collected in their particulate phase from the cigarette smoke when tobacco is burned. It is not clear, at this time, which of these chemicals is responsible for reducing the MCP-1 levels.
Conclusion
The results of the current study provided insights into the effects of nicotine, CSC, and P. gingivalis on the expression of MCP-1 from HGFs. It was observed that HGFs increased the expression of MCP-1 when exposed to P. gingivalis and the opposite when exposed to CSC which may help to explain why smoking is a major contributing factor that plays a role in the initiation, development, and progression of periodontal diseases by allowing for more bacterial growth. Further studies are needed to investigate the exact mechanisms that smoking ingredients disrupt the immune responses of the periodontal tissues and alter cytokines expression.
Acknowledgements
This study was funded by the Indiana University Purdue University Indianapolis Multidisciplinary Undergraduate Research Institute and the Tobacco Cessation and Biobehavioral Group.
References
- Griffen AL, Becker MR, Lyons SR, Moeschberger ML, Leys EJ (1998) Prevalence of Porphyromonas gingivalis and Periodontal Health Status. Journal of clinical microbiology 36: 3239-3242.
- Chang YC, Tsai CH, Yang SH, Liu CM, Chou MY (2003) Introduction of cyclooxygenase-2 mRNA and protein expression in human gingival fibroblasts stimulated with nicotine. Journal of periodontal research 38: 496-501.
- Hilgers KK, Kinane DF (2004) Smoking, periodontal disease and the role of the dental profession. International journal of dental hygiene 2: 56-63.
- Tymkiw KD, Thunell DH, Johnson GK, Joly S, Burnell KK, et al. (2011) Influence of smoking on gingival crevicular fluid cytokines in severe chronic periodontitis. Journal of clinical periodontology 38: 219-228.
- Colotta F, Borré A, Wang JM, Tattanelli MO, Maddalena F, et al. (1992) Expression of a monocyte chemotactic cytokine by human mononuclear phagocytes. The Journal of Immunology 148: 760-765.
- Sakallioglu EE, Ayas B, Lütfioglu M, Keles GÇ, Açikgöz G, et al. (2008) Gingival levels of monocyte chemoattractant protein-1 (MCP-1) in diabetes mellitus and periodontitis: an experimental study in rats. Clinical Oral Investigations 12: 83-89.
- Yu X, Graves DT (1995) Fibroblasts, Mononuclear Phagocytes, and Endothelial Cells Express Monocyte Chemoattractant Protein-1 (MCP-1) in Inflamed Human Gingiva. J Periodontol 66: 80-88
- Hanazawa S, Kawata Y, Takeshita A, Kumada H, Okithu M, et al. (1993) Expression of monocyte chemoattractant protein 1 (MCP-1) in adult periodontal disease: increased monocyte chemotactic activity in crevicular fluids and induction of MCP-1 expression in gingival tissues. Infect Immun 61: 5219-5224.
- Seymour GJ, Gemmell E (2001) Cytokines in periodontal disease: where to from here?. Acta Odontol Scand 59: 167-173.
- Kurtis B, Tüter G, Serdar M, Akdemir P, Uygur C, et al. (2005) Gingival crevicular fluid levels of monocyte chemoattractant protein-1 and tumor necrosis factor-alpha in patients with chronic and aggressive periodontitis. J Periodontol 76: 1849-1855.
- Zhou J, Windsor LJ (2006) Porphyromonas gingivalis affects host collagen degradation by affecting expression, activation, and inhibition of matrix metalloproteinases. J Periodont Res 41: 47–54.
- Zhang W, Fang M, Song F, Windsor LJ (2011) Effects of cigarette smoke condensate and nicotine on human gingival fibroblast-mediated collagen degradation. J Periodontol 82: 1071-1079.
- Delima AJ, Karatzas S, Amar S,Graves DT (2002) Inflammation and tissue loss caused by periodontal pathogens is reduced by interleukin-1 antagonists. J Infect Dis 186: 511-516.
- Ojima M, Hanioka T (2010) Destructive effects of smoking on molecular and genetic factors of periodontal disease. Tob Induc Dis 8: 4.
- Sandros J, Papapanou PN, Nannmark U, Dahlen G (1994) Porphyromonas gingivalis invades human pocket epithelium in vitro. J Periodontal Res 29: 62-69.
- Van Winkelhoff AJ, Loos BG, Van Der Reijden WA, Van Der Velden U (2002) Porphyromonas gingivalis, Bacteroides forsythus and other putative periodontal pathogens in subjects with and without periodontal destruction. J Clin Periodontol 29: 1023-1028.
- Scheres N, Laine ML, De Vries TJ, Everts V, Van Winkelhoff AJ (2010) Gingival and periodontal ligament fibroblasts differ in their inflammatory response to viable Porphyromonas gingivalis. J Periodontal Res 45: 262-270.
- Pradeep AR, Daisy H, Hadge P, Garg G, Thorat M (2009) Correlation of gingival crevicular fluid interleukin-18 and monocyte chemoattractant protein-1 levels in periodontal health and disease. J Periodontol 80: 1454-1461.
- Tonetti MS, Imboden MA, Gerber L, Lang NP, Laissue J, et al. (1994) Localized expression of m RNA for phagocytic specific chemotactic cytokines in human periodontal infections. Infect Immun 62: 4005–4014.
Relevant Topics
- Cementogenesis
- Coronal Fractures
- Dental Debonding
- Dental Fear
- Dental Implant
- Dental Malocclusion
- Dental Pulp Capping
- Dental Radiography
- Dental Science
- Dental Surgery
- Dental Trauma
- Dentistry
- Emergency Dental Care
- Forensic Dentistry
- Laser Dentistry
- Leukoplakia
- Occlusion
- Oral Cancer
- Oral Precancer
- Osseointegration
- Pulpotomy
- Tooth Replantation
Recommended Journals
Article Tools
Article Usage
- Total views: 14727
- [From(publication date):
April-2015 - Aug 30, 2025] - Breakdown by view type
- HTML page views : 10087
- PDF downloads : 4640