Integrated Effect of Rhizobium and Azotobacter Cultures on the Leguminous Crop Black Gram (Vigna mungo)
Received: 14-May-2017 / Accepted Date: 29-May-2017 / Published Date: 05-Jun-2017 DOI: 10.4172/2329-8863.1000289
Abstract
A pot experiment was performed to evaluate the integrated effect of Rhizobium and Azotobacter sp. on the plant growth, nodule appearance, no of leaf, shoot length, root length, chlorophyll contents and carbohydrate content in black gram during 2016 growing period at the Department of Microbiology, Dr. Ram Manohar Lohia Avadh University Faizabad, UP, India. Different treatments viz., T1: Control (Sterile soil+Seeds without culture treatment), T2: Sterile Soil and Seeds both are treated with Azotobacter sp., T3: Sterile Soil and Seeds both are treated with Rhizobium sp., T4: Sterile Soil and Seeds both are treated with mixed culture of Azotobacter sp. and Rhizobium sp., T5: Sterile Soil+Seeds treated with Azotobacter sp., T6: Sterile Soil + Seeds treated with Rhizobium sp., T7: Sterile Soil+Seeds treated with mixed culture of Azotobacter sp. and Rhizobium sp. All experiments were carried out in triplicate set. The T4 treatment showed maximum shoot length (51.6 cm), root length (17.3 cm), fresh and dry shoot biomass (12.99 and 3.21 g), fresh and dry root biomass (3.54 and 0.99 g), no. of leafs (20.4), root nodules per plant (18.2) and chlorophyll content (1.3 mg/g) and reducing (867.4 μg/g) and non-reducing sugar (1905.5 μg/g) content per plant biomass respectively. The Azotobacter and Rhizobium sp. have friendly associations and they have different physiology and habitat. Therefore, they help plant growth promotion by them own system. Therefore, such combination can be recommended for field application for sustainable agriculture. Excessive application of chemical fertilizers causes environmental and economic problems; hence the use of PGPR and Rhizobium bacteria can be acceptable due to cut contribution expenditure, increase in grain yield and environmental friendly.
Keywords: Azotobacter; Black gram; Co-culture; Rhizobium; Biofertilizers; Vigna mungo ; Germination
406192Introduction
Black gram is one of the important pulse crops in India. It is also generally grown in other tropical/subtropical countries. Black gram is extremely nutritious due to having higher protein contents (24-26%) along with higher content of potassium, phosphorus, calcium, sodium and vitamins (retinoic acid, thiamine, riboflavin) [1]. It has several therapeutic properties, like curing diabetes, sexual dysfunction, nervous, hair, and digestive system disorders and rheumatic afflictions [2]. Black gram seeds have shown anti-anthrogenic activity in guinea pigs.
Chemical fertilizers are frequently used to achieve maximum crop production in agricultural field. These cost effective chemicals, however, when used roughly, have resulted in loss of soil fertility and consequently, the crop production [3]. Due to these reasons, focus in recent times has been shifted towards the use of cost-competitive biological resources such as Plant Growth Promoting Rhizobacteria that colonize the roots of plants following inoculation onto seeds and that enhance plant growth [4]. The PGPR used as biofertilizers have ability to mobilizing significant nutrients in the soil from unavailable to available form for most vegetation and important for crops [1,5,6]. Therefore, its use is an eco-friendly approach to reduce the utilization of chemical fertilizers, enhance soil fertility and increase crop production by their biological activity in the rhizosphere. Several bacteria like Pseudomonas, Azospirillum, Azotobacter, Klebsiella, Enterobacter, Alcaligenes, Arthrobacter, Burkholderia, Rhizobium, Flavobacteriaum, Bacillus and Serratia, phosphobacteria and VAM fungi have been used as biofertilizers supplement of nitrogen and phosphorus fertilizers for improved crop production [7-16].
Rhizobium bio-fertilizer approx fix 50-200 kg of N/ha/season and increase the crop yield about 10-15% agriculture field [17]. Biofertilizers comprised mostly the nitrogen fixing, phosphate solubilizing and plant growth-promoting microorganisms [18]. The main agents of biofertilizers are Azotobacter , Azospirillum , blue green algae, Azolla, P-solubilizing microorganisms and mycorrhizae [19]. However, apart from providing N to plants, Azotobacter promotes plant growth directly by secreting considerable amounts of biologically active substances like B vitamins, nicotinic acid, pantothenic acid, biotin, gibberellic acid, Indole-3 Acetic Acid (IAA) and cytokinin [20-22] and ammonia [23] or indirectly by protecting the plant from diseases [24]. Hence, a trial was performed to study the effect of bio-fertilizer on plant growth, yield and linked protein content changes in black gram. In this paper, we have observed and discussed the combined effect of Rhizobium and Azotobacter on growth promotion of black gram. In this aspect, a stain of Rhizobium and Azotobcter was isolated from methi plants and wheat rhizosphere respectively.
Materials and Methods
Isolation, screening and identification of Rhizobia and Azotobacter sp .
For the isolation of Rhizobia , healthy plants root nodules from methi was used. All selected root nodules were washed with water and then immerse the nodules in HgCl2 (0.1%) or H2O2 (3-5%) for five minutes to surface sterilization. After that, nodules were washed in sterile water for 3-4 times. All nodules sticking to the root system were removed and surface sterilized by treating with 70% alcohol for 1 minute, after which they were treated by chloramine-T solution (1%) for 3 minutes and washed thoroughly by with sterile water. Now, nodules were crushed in 1000 μl of water with a sterile rod and make a suspension of Rhizobia with sterile water. Then suspension of Rhizobium (0.1 ml) was spread on the yeast extract agar medium plate which contain (g/l): Yeast extract: 1.0, K2HPO4: 0.5, K2SO4.7H2O: 0.2, NaCl: 0.1, Mannitol: 10.0, Agar: 20 and 2.5 ml congo red solution (1%) with pH 6.9. The inoculated plates were incubated for 5-6 days at 26°C for proper growth. After that, culture was maintained on the same medium and stored at 4°C in the refrigerator. The culture was reculturing after every 15 days.
For isolation of Azotobacter , one gram soil sample (Wheat rhizospheric soil) was added to 9 ml of sterile water, shake well by vortex allowing standing for 30 minutes. Then 1 ml of sample suspension was transferred to 9 ml sterile distilled water. Through this method, samples were serially diluted up to 105 dilutions. After that, 1 ml of serial diluted samples (from 103 to 105 fraction) was used in a sterilized Petri Plates containing Ashby’s medium and then incubated at 28 ± 2°C temperature for 2-3 days for proper growth. The isolates were purified through streak plate technique.
Preparation of inoculum of Rhizobium and Azotobacter sp .
One full loop of pure Rhizobium and Azotobacter culture was inoculated in 100 ml Nutrient broth for 24 h for preparation of inoculum, which was used for soil and seed treatment. The Soil sample (2 Kg) and seed sample (8 seeds) was treated with pure culture of Rhizobium and Azotobacter with 25 and 10 ml inoculum.
Soil collection and sterilization for enrichment by Rhizobium and Azotobacter
Soil samples were collected in sterile polybags (each bag contain 2 Kg Soil) from different garden of Avadh University Campus and then autoclaved at 15 lbs for 15 min. After that, sterile soil samples were supplemented with different combinations of Rhizobium , Azotobacter and mixed culture of Rhizobium and Azotobacter sp . Sterile soil sample without any culture treatment was work as control. Then treated and untreated soil samples were ready for soil sowing.
Seed preparation and sowing in treated and untreated soil
For seed preparation, the seeds of Black gram were collected and treated with 10 ml of Rhizobium and Azotobacter culture for 30 min, then sowing it into the treated and untreated soil samples and left it for its proper growth.
Optimization of different parameters for maximum plant growth promotion
Germination studies: Seeds were allowed to germinate in treated and non-treated soil sample in polybags under field conditions.
Site of experiment: The plants were maintained under natural condition in the garden, Dr. Ram Manohar Lohia Avadh University, Faizabad, UP, India in 2016. The weather was temperate and slightly wet at the time of starting the experiment. The temperatures ranged between 35°C to 38.5°C (maximum) and 25.6°C to 30.7°C (minimum), respectively during the experimental period.
Experimental plan and design (Treatments)
T1: Control (Sterile soil+Seeds without culture treatment).
T2: Sterile Soil and Seeds both are treated with Azotobacter sp .
T3: Sterile Soil and Seeds both are treated with Rhizobium sp .
T4: Sterile Soil and Seeds both are treated with mixed culture of Azotobacter sp . and Rhizobium sp .
T5: Sterile Soil+Seeds treated with Azotobacter sp .
T6: Sterile Soil+Seeds treated with Rhizobium sp .
T7: Sterile Soil+Seeds treated with mixed culture of Azotobacter sp . and Rhizobium sp .
Seedlings: In one set of experiment, treated black gram seeds were sown in plastic bag containing sterile soil which treated with Azotobacter sp ., Rhizobium sp ., and mixed culture of Azotobacter sp. and Rhizobium sp ., and in another set of experiment treated black gram seeds were sown in plastic bag containing sterile soil without any culture treatment. In this experiment, sterile soil and seeds without any culture treatment were worked as control as mentioned earlier. Seven experimental replicates were set for all treatment. Black gram seeds were also treated with Azotobacter sp ., Rhizobium sp ., and mixed culture of Azotobacter sp . and Rhizobium sp ., after surface sterilized with sodium hypocholride (0.005%) for 45 min and wash twice with sterile distilled water and then grown into a 5 cm depth in polythene bag under natural condition for two months.
Growth parameters
The growth parameters were calculated on every 15th, 30th, 45th and 60th day of the plant growth in all the treatments.
Shoot length: The plants were uprooted carefully without damaging the root system. The shoot length of the plants was measured from the collar region to the tip of the plant, using a standard scale and values were recorded in centimeters.
Root length: The plants were uprooted carefully without damaging the root system. The root length of the plants was measured from the end region to the shoot of the plant, using a standard scale and values were recorded in centimeters.
Number of leaf: Number of completely opened leaf in all the treatments was calculated manually.
Number of root nodules: Number of completely developed nodules in all the treatments was calculated manually.
Fresh shoot biomass: The shoot part was segregated from the root and was blotted on the Whatman No. 1 filter paper to absorb the water content. The mass (in gram) of the shoot was measured by saltorious.
Fresh root biomass: The plants were carefully uprooted and roots were rinsed with tap water to remove the soil particles. The clean roots were blotted on the Whatman No. 1 paper and the roots were weighed using electrical balance in grams.
Dry shoot biomass: To obtain the fresh shoot biomass of the plants, the shoots were dried in a hot air oven at 60°C for 48 hours to get constant mass. After that, dry weights of the shoots were measured in grams by electrical balance.
Dry root biomass: After measuring the dry root biomass of the plants, it was dried in a hot air oven at 60°C, for 48 hours. After that, dry weights of the roots were calculated in grams by electrical balance.
Physiological parameters
Estimation of chlorophyll content: The chlorophyll content of the plant leaves were estimated in all the treatments on 15th, 30th, 45th and 60th days by Arnon method [25]. The leaves were expurgated from the plants and rinsed with water and then blotted dry. One gram of leaf sample was homogenized with 10 ml pre-chilled 80% acetone and a bit of CaCO3 was supplemented to make easy grinding. After grinding, the extract sample was centrifuged at 10000 rpm for 10 minutes. The supernatant was filtered through Whatman No. 1 filter paper and the apparent supernatant was transferred to 1 cm quartz cuvette. The absorbance was measured using specific absorptions co-efficient for chlorophyll a and b at 645 nm and 663 nm using 80% acetone as blank in UV-Visible spectrophotometer. The following simultaneous equations were setup for measuring chlorophyll concentrations.
Chlorophyll “a”=(0.0127 × OD at 663 nm)-(0.00269 × OD at 645 nm).
Chlorophyll “b”=(0.0229 × OD at 645 nm)-(0.00468 × OD at 663 nm).
Estimation of carbohydrates: Reducing and non-reducing sugars were extracted from shoot and root from all the test treatments were estimated by Highkin and Frankel method [26]. The carbohydrate contents were estimated on 15th, 30th, 45th and 60th days of plant growth. Two hundred milligram of oven dry sample was extracted with 20 ml (80%) boiled ethanol and then centrifuged at 10000 rpm. The supernatant was reduced to a half volume (10 ml) on boiling water bath and cooled at room temperature. After that, 5 ml of saturated neutral lead acetate was added in the supernatant to precipitate proteins. Saturated aqueous disodium phosphate (10 ml) was also added in the supernatant to precipitate excess of lead. Then activated charcoal (300 mg) was added and the supernatants were shaken for 30 min and then filtered. The filtrate supernatant was diluted to 100 ml with distilled water and was used for reducing and non-reducing sugars estimation.
Results
Isolation, screening and identification of Rhizobium sp. and Azotobacter sp .
Azotobacter sp . and Rhizobium sp . were isolated on Ashby’s and Yeast extract agar medium at 28°C from wheat sphere soil and methi plant. The appearance of Azotobacter is small and smooth white colony on Ashby’s medium plate. Morphologically, it is rod shape and Gram-negative under microscopic study. The colonies of the isolate were smooth, convex, glistening and opaque on media (Figure 1). Biochemical tests revealed that are isolate P-12 showed positive results for Catalase, Oxidase and Starch hydrolysis, Indole, Motility tests and Citrate utilization. The isolate was positive for H2S production, urea hydrolysis, and produced acid from glucose, maltose, fructose, Trehalose, and raffinose.
Deep Glucose Agar test confirmed that the isolate was obligate aerobes. Halo-tolerance ability is a significant feature of Azotobacter for salinity affected areas. If it is possible to apply halo-tolerant Azotobacter as biofertilizer, the crops productivity will definitely increase. It is found that our isolate was salt resistant Azotobacter (10% NaCl) (Table 1a).
| Morphologic characters | Result | Biochemical characters | Result |
|---|---|---|---|
| Gram reaction | - | Catalase | + |
| Shape | Rod | Oxidase | + |
| Motility Test | + | Carbohydrate utilization | |
| Pigment | White | Glucose | A |
| Spore formation | - | Fructose | A |
| Colony Appearance | Smooth, convex, opaque | Maltose | A |
| Different NaCl concentration (%) | Raffinose | A | |
| 2% | + | Trehalose | A |
| 4% | + | Starch | + |
| 6% | + | Urease test | + |
| 8% | + | Nitrate reductase | + |
| 10% | + | H2S production | + |
| Growth in different temperatures | Amylase | + | |
| 10 | + | Voges-Proskauer | - |
| 20 | + | Indole | + |
| 30 | + | Lysine decarboxylase | - |
| 40 | + | Arginine dihydrolase | - |
| 50 | + | Culture | Azotobacter sp. |
Table 1(a): Morphological and Biochemical Characterization of Azotobacter sp.
The Rhizobium isolates were white pigmented, circular, elevated, semi translucent, mucilaginous with entire margin on yeast extract mannitol agar and Gram-negative rods, arranged singly corresponding to the general character of genus Rhizobium . The bacterial colony was circular, convex and semi- translucent on YEM agar. All bacteria were capsulated but did not form any spore (Table 1b). Rhizobium gives negative result with indole and TSI test and positive result with MR-VP and catalase test. The cultural and biochemical properties exhibited by the bacteria isolated from legumes are is accordance to the cultural description of Rhizobium sp given in Bergey’s manual [27]. When subjected to versatility in utilizing different carbon sources as energy the isolate utilized lactose, glucose, sucrose and starch, respectively (Table 1b). This shows more likeness of isolates towards lactose than other carbon sources.
| Morphologic characters | Result | Biochemical characters | Result |
|---|---|---|---|
| Gram reaction | - | Catalase | + |
| Shape | Rod | Oxidase | + |
| Motility Test | + | Carbohydrate Fermentation | |
| Pigment | Redish | Glucose | + |
| Spore formation | - | Fructose | + |
| Colony Appearance | Smooth, convex, opaque | Maltose | + |
| Different NaCl concentration (%) | Raffinose | + | |
| 2% | + | Trehalose | + |
| 4% | + | Sucrose | + |
| 6% | + | Urease test | + |
| 8% | - | Nitrate reductase | - |
| 10% | - | H2S production | - |
| Growth in different temperatures | Amylase | - | |
| 10 | + | Voges-Proskauer | + |
| 20 | + | Indole | + |
| 30 | + | Gelatin hydrolysis | - |
| 40 | + | Arginine dihydrolase | - |
| 50 | - | Culture | Rhizobium sp. |
Table 1(b): Morphological and Biochemical Characterization of Rhizobium sp.
Effect of Rhizobium and Azotobacter sp . on black gram growth parameters
Both strains showed positive results in the experiments carried out on “Effect of Azotobacter , Rhizobium and mixed culture of both (Azotobacter and Rhizobium sp .) on the growth and biochemical aspects of black gram” were discussed in this section. To enhance a significant plant growth response, it is necessary to recognize the prominent strains of PGPRs for the sowing condition. It was in this context that an efforts were made to study the PGPRs of black gram with special reference to Rhizobium and Azotobacter and their mixed culture. The effects of enriched microbial inoculants in soil and on plant growth, biomass and biochemical characteristics were studied in polybag culture under natural condition. Soil which augmented with mix microbial inoculants was found to significantly increase shoot length, root length, number of leaf, number of nodules and fresh and dry weight of shoot and root, total fresh and dry weight of the plant. The microbial inoculants provide high-quality of plant nutrients has supported plant growth.
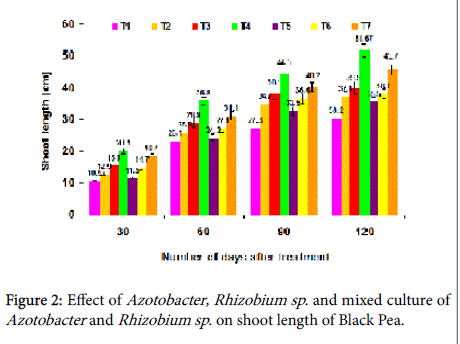
Effect on shoot length: In this experiment, the result showed that shoot length was greatly increased with the mixed culture of Rhizobium and Azotobacter sp . when compared with single culture of Azotobacter sp ., and Rhizobium sp ., at 30th, 60th 90th and 120th days after planting. The maximum shoot length was achieved in the T4 treatment with 20.1, 35.8, 44.3 and 51.6 cm, respectively (Figure 2). The mixed culture inoculations of prominent microorganisms have been reported to perform better than the single culture treatments.
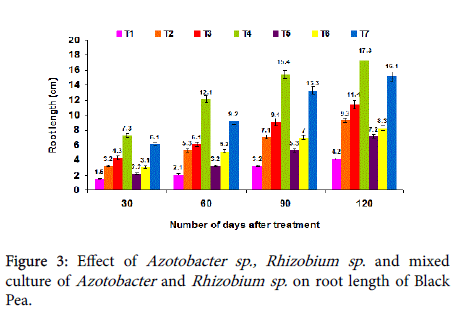
Effect on root length: The plant root length was increased considerably with the mixed culture of Rhizobium and Azotobacter sp . when compared with single culture of Azotobacter sp ., and Rhizobium sp ., at 30th, 60th 90th and 120th days after planting. The maximum average root length was achieved in the T4 treatment with 7.3, 12.1, 15.4 and 17.3 cm, respectively (Figure 3). The combined inoculations of beneficial organisms have been reported to perform better than the single inoculation treatments.
Effect on leaves number: The plant leaves number were increased significantly with the mixed culture of Rhizobium and Azotobacter sp . when compared with single culture of Azotobacter sp ., and Rhizobium sp ., at 30th, 60th 90th and 120th days after planting. The maximum average leaves number was recorded in the treatment, T4 with 8.4, 12.3, 16.3 and 20.4, respectively (Figure 4). The combined inoculations of beneficial organisms have been reported to perform better than the single treatment.
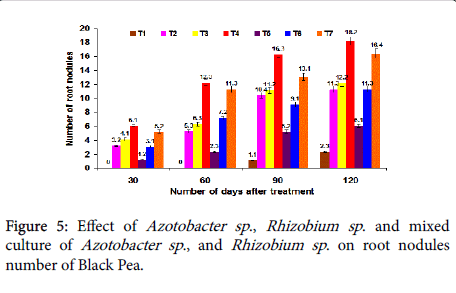
Effect on nodules number: The plant root nodules number were increased significantly with the mixed culture of Rhizobium and Azotobacter sp . when compared with single culture of Azotobacter sp ., and Rhizobium sp ., at 30th, 60th 90th and 120th days after planting. The maximum average root nodules number was recorded in the T4 treatment with 6.1, 12.3, 16.3 and 18.2 respectively (Figure 5). The combined inoculations of beneficial organisms have been reported to perform better than the single inoculation treatments.
Fresh and dry shoot and root biomass: Accordingly, the root and shoot growth, the fresh and dry content in root and shoot as well as total dry contents of black gram were also increased due to the combined action of both strain. The maximum root and shoot fresh and dry weight was achieved in the T4 treatment. The T4 Treatment enhanced the root fresh and dry content of 3.54 and 0.99 g per plant and shoot fresh and dry content of 12.99 and 3.21 g per plant over the control (Tables 2 and 3). Mix-culture could increase the total root and shoot fresh and dry biomass of 16.5 and 4.2 g per plant, respectively (Tables 2 and 3).
| Treatments | Shoot fresh biomass | Root fresh biomass | Total fresh biomass | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Days after treatment | ||||||||||||
| 30 | 60 | 90 | 120 | 30 | 60 | 90 | 120 | 30 | 60 | 90 | 120 | |
| T1 | 0.85 | 1.46 | 2.11 | 4.81 | 0.32 | 0.77 | 1.01 | 1.6 | 1.17 | 2.23 | 3.12 | 6.1 |
| T2 | 1.33 | 2.73 | 4.27 | 7.55 | 0.45 | 1.18 | 1.31 | 2.1 | 1.78 | 4.53 | 5.58 | 9.65 |
| T3 | 1.68 | 3.98 | 5.41 | 9.01 | 0.51 | 1.23 | 1.51 | 2.40 | 2.19 | 6.91 | 6.92 | 11.41 |
| T4 | 2.87 | 6.95 | 9.53 | 12.9 | 0.67 | 1.48 | 1.73 | 3.54 | 2.58 | 8.45 | 10.26 | 16.53 |
| T5 | 1.21 | 2.38 | 3.87 | 5.90 | 0.40 | 1.0 | 1.28 | 2.0 | 1.61 | 3.30 | 5.69 | 7.9 |
| T6 | 1.42 | 2.77 | 4.91 | 7.01 | 0.5 | 1.08 | 1.38 | 2.2 | 1.92 | 3.85 | 6.29 | 9.21 |
| T7 | 1.58 | 3.75 | 6.72 | 10.1 | 0.62 | 1.28 | 1.41 | 2.38 | 2.2 | 5.03 | 8.13 | 12.43 |
Table 2: Effect of Azotobacter sp., Rhizobium sp. and mixed culture of Azotobacter, and Rhizobium sp. on fresh bio mass weight (g) of Black Pea.
| Treat-ments | Shoot dry biomass | Root dry biomass | Total dry biomass | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Days after treatment | ||||||||||||
| 30 | 60 | 90 | 120 | 30 | 60 | 90 | 120 | 30 | 60 | 90 | 120 | |
| T1 | 0.29 | 0.67 | 0.91 | 1.48 | 0.11 | 0.26 | 0.44 | 0.65 | 0.40 | 0.93 | 1.35 | 2.13 |
| T2 | 0.43 | 0.84 | 1.08 | 1.95 | 0.21 | 0.4 | 0.55 | 0.85 | 0.64 | 1.24 | 1.63 | 2.8 |
| T3 | 0.68 | 1.0 | 1.35 | 2.90 | 0.29 | 0.47 | 0.58 | 0.89 | 0.97 | 1.47 | 1.93 | 3.79 |
| T4 | 0.81 | 1.13 | 2.56 | 3.21 | 0.33 | 0.5 | 0.69 | 0.99 | 1.14 | 1.63 | 3.25 | 4.2 |
| T5 | 0.39 | 0.76 | 0.9 | 1.5 | 0.18 | 0.37 | 0.44 | 0.65 | 0.57 | 1.13 | 1.34 | 2.15 |
| T6 | 0.58 | 0.92 | 1.12 | 2.38 | 0.26 | 0.41 | 0.49 | 0.82 | 0.84 | 1.33 | 1.61 | 3.2 |
| T7 | 0.78 | 1.0 | 2.29 | 3.0 | 0.3 | 0.42 | 0.5 | 0.78 | 1.08 | 1.42 | 2.79 | 3.78 |
Table 3: Effect of Azotobacter sp., Rhizobium sp. and mixed culture of Azotobacter sp., and Rhizobium sp. on dry biomass (g) of Black Pea.
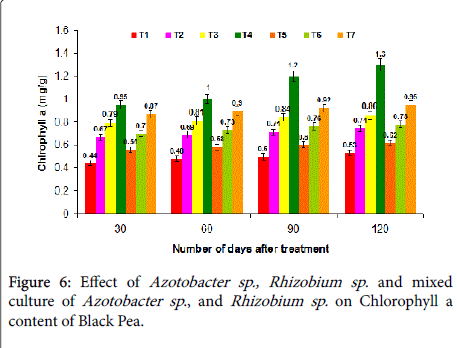
Effect on chlorophyll content: Chlorophyll content was increased in T4 treatment (1.3 mg/g) followed by T7 treatment (0.95 mg/g) at 30, 60, 90 and 120 days after sowing which was significantly better over the control (0.53 mg/g). Enhanced chlorophyll content can be achieved due to the existence of rhizomicrobes in the rhizosphere, promoting the crop roots to secrete growth promoting substances, which in turn might have improved the growth of N2-fixers, P-solubilizing microorganisms in situ, while a synergistic effect might have achieved in the T4 and T7 treatment (Figure 6). Enhancement of rhizospheric microorganism in case of treatment T4 might be due to inoculation of Azotobacter sp . and Rhizobium sp . consortium, where more root hairs become liable for rhizo-microbial infection and also might be due to better condition for P-availability by P-solubilizers.
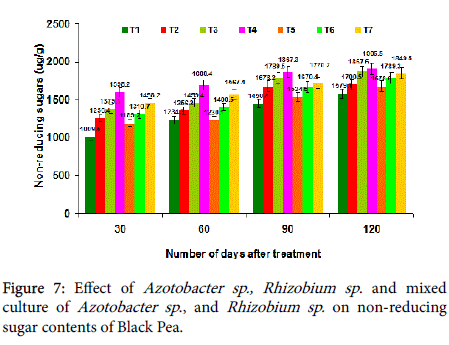
Effect on non-reducing and reducing sugar content: In the present study, mixed culture of Azotobacter and Rhizobium sp . treatment (T4) increased reducing (867.8 mg/g) and non-reducing (1509.5 mg/g) sugars quantity (Figures 7 and 8). Non-reducing sugar contents were increased due to the possible reasons to enhance in carbon fixation, activation of enzymes and improved photosynthetic rate.
Discussion
Application of biofertilizers is a satisfactory approach for higher yield and better quality of crops, which is healthy for human consumption. Our results showed that mixed culture of Azotobacter and Rhizobium gave positive result to the different studied parameters. The plant shoot length, root length, leaves numbers and nodules numbers were increased significantly with the mixed culture of Rhizobium and Azotobacter sp . when compared with single culture of Azotobacter sp., and Rhizobium sp . Kamil et al. [28] have also reported that the mixed culture inoculations of beneficial strains were always performed better than the single inoculation treatments. Mixed culture of Azotobacter and Rhizobium significantly enhanced the fresh and dry shoot and root biomass along with the other parameters (chlorophyll content and reducing and non-reducing sugar content).
Combined inoculations further enhanced the total root and shoot fresh and dry biomass of 16.5 and 4.2 g per plant (Tables 2 and 3). It is well documented that the non-symbiotic microorganisms have two beneficial aspect for plants, first is the nitrogen fixing capability and second is to produced growth promoting substances (vitamins, hormones and amino acids) [29,30] which attributed the combined effects of the consortium reside with friendly associations by receiving more colonization in the rhizosphere of the crops. The friendly association of Rhizobium and Azotobacter may be suggested for better results instead of single culture.
Chlorophyll content was increased in the mixed culture inoculation treatment (T4) over the uninoculated control. Similar results in respect to increase in chlorophyll content in several plants have also been reported by several workers [31]. Enhanced chlorophyll content can be certified that the occurrence of microorganisms in the rhizosphere promoting the plant roots to produce growth promoting substances, which improved the growth of N2-fixers, P-solubilizing microorganisms in situ and a synergistic effect might have attained. Improvement of rhizomicrobial growth in case of treated plant might be due to inoculation of Azotobacter sp . and Rhizobium sp . consortium, where more root hairs become susceptible for rhizomicrobial infection and also might be due to better provision for P-availability by P-slubilizers [32]. Furthermore, N-fixers like Azotobacter and Rhizobium sp. is recognized to improve the plant growth [33]. Enhanced quantity of reducing and non-reducing sugars is due to improved carbon fixation, activation of enzymes and improved photosynthetic rate [34,35]. Growth parameters improved due to the mixed culture treatments.
Several methods have been recommended to elucidate the fact of plant growth enhancement by Azotobacter is due to increase in the nitrogen fixation, production of different hormones (auxins, gibberellins, cytokinin, and ethylene), phosphorus solubilization, sulfur oxidation, accessibility of nitrate, production of antibiotics, lytic enzyme, hydrocyanic acid, increase in root permeability, firm antagonism for the existing and root spot, inhibition of harmful rhizobacteria and improvement in the uptake of fundamental plant nutrients etc. [36-39].
It clearly indicate that Rhizobium nodulation (number and size) might have due to the presence was Azotobacter which could fix atmospheric N2 and supported plant growth from initial growth of seedlings. In the early stage, plant roots might have supported the Azotobacter population. Such finding indicate that Rhizobium does not complete far soil organic compound and other minerals with the rhizobium because Rhizobium get its nutrients farm the croups plant and that is why did not grow effect the Azotobacter and perhaps due to such condition, Azotobacter increase 5-6% time. The significant finding must be study along with other nutrients like Cr, Mg, Zn ability in soil Mg gives other finding regarding some combination further it’s also important that how Azotobacter could permitted the plant growth higher it is through and plant growth hormones in the experiment the Azotobacter are isolate and tested for N2 fixing.
The Azotobacter sp . is an aerobic organism that can fix atmospheric nitrogen as demonstrated by the grown in the flask at the liquid/air interface. The presence of oxygen can inactivate the nitrogenase enzyme and inhibit the nitrogen fixation. The Azotobacter sp . compensates for this problem by maintaining a very high respiration rate which effectively uses the oxygen as soon as it enters into the cell. Rhizobium sp . can only fix atmospheric nitrogen under microaerophilic conditions, such as inside the root nodule. It would be necessary to duplicate conditions found in nature in a strict artificial environment to prove that the Rhizobium sp. was able to fix atmospheric nitrogen.
Conclusion
The novel concept of this study is that combined application of Azotobacter and Rhizobium is more effective than single one. It is always found that Rhizobium is recommended for leguminous crops along due to node forming status by symbiotic relations. Rhizobium require more time in nodule formation and then N2 fixation starts, while Azotobacter starts fixing N2 asymbiotically in the soil and help better plant growth at the initial stage of seedling growth. Such effects make plants healthy and diseases free. Therefore, application of such combinations should be recommended to the formers for better yield response. The consortium of Azotobacter sp., and Rhizobium sp . could improve the growth and yield response of black gram by improving physiology of the crop by supplying nitrogen through symbiotically and asymbiotically. Therefore, the shoot length, root length, number of leaves, number of root nodules, fresh and dry biomass of root and shoot were improved as compared to individual culture of Azotobacter sp., and Rhizobium sp . The chlorophyll and carbohydrates contents were also increased in the treatment plants. The application of consortium of Azotobacter sp ., and Rhizobium sp. as bioinoculants in agriculture, horticulture and other land plants should be recommended for better yield of crops and improvement of soil health through increasing the biotic component in order to reduce the risk of chemical toxicity.
References
- Selvakumar G, Reetha S, Thamizhiniyan P (2012) Response of Biofertilizers on Growth, Yield Attributes and Associated Protein Profiling Changes of Blackgram (Vigna mungo L. Hepper). World Appl Sci J 16: 1368-1374.
- Selvakumar G, Joshi P, Suyal P, Mishra PK, Joshi GK, et al. (2010) Pseudomonas lurida M2RH3 (MTCC 9245), a psychrotolerant bacterium from the Uttarakhand Himalayas, solubilizes phosphate and promotes wheat seedling growth. World J Microbiol Biotechnol 27: 1129-1135.
- Lemanski K, Scheu S (2014) Incorporation of 13C labelled glucose into soil microorganisms of grassland: Effects of fertilizer addition and plant functional group composition. Soil Biol Biochem 69: 38-45.
- Kloepper JW, Schroth MN (1978) Plant growth-promoting rhizobacteria on radishes. Proc 4th Intern Conf Plant Pathogen Bacteria 2: 879-882.
- Vessey JK (2003) Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255: 571-586.
- Nalawde A, Bhalerao S (2015) Response of Black gram Vigna mungo (L. Hepper) to Biofertilizer. Inter J Life Sciences 3: 81-84.
- Lutenberg B, Kamilova F (2009) Plant growth promoting rhizobacteria. Anna Review of Microbiol 63: 541-556.
- Bhattacharya PN, Jha DK (2012) Plant growth promoting rhizobacteria (PGPR): Emergence in agriculture. World J Microbiol Biotechnol 28: 1327-1350.
- Ahmad E, Khan MS, Zaidi A (2013) ACC deaminase producing Pseudomonas putida strain PSE3 and Rhizobium leguminosarum strain RP2 in synergism improves growth, nodulation and yield of pea grown in alluvial soils. Symbiosis 61: 93-104.
- Peix A, Ramirez-Bahena MH, Velazquez E, Bedmar EJ (2015) Bacterial association with legumes. Cri Rev Plant Sci 34: 17-42.
- Zaidi A, Khan MS, Ahemad M, Oves M (2009) Plant growth promotion by phosphate solubilizing bacteria. Acta Microbiologica et Immunologica Hungarica 56: 263-284.
- Nosrati R, Parviz O, Horieh S, Iraj R, Malboobi MA (2014) Phosphate solubilization characteristics of efficient nitrogen fixing soil Azotobacter strains. Iranian J Microbiol 6: 285-295.
- Mohite B (2013) Isolation and characterization of indole acetic acid (IAA) producing bacteria from rhizospheric soil and its effect on plant growth. J Soil Sci Plant Nutrit 13: 638-649.
- Viruel E, Erazzú LE, Calsina LM, Ferrero MA, Lucca ME, et al. (2014) Inoculation of maize with phosphate solubilizing bacteria: effect on plant growth and yield. J Soil Sci Plant Nutrit 14: 819-831.
- Noreen S, Ali B, Hasnain S (2012) Growth promotion of Vigna mungo (L.) by Pseudomonas spp. exhibiting auxin production and ACC deaminase activity. Â Ann Microbiol 62: 411-417.
- Singh R, Arora NK, Gautam P, Lal S (2013) Enhancement of plant growth of Trigonella foenum-graecum by coinculation of fluorescent Pseudomonas and Rhizobium for the sustainability of agriculture. Asian J Plant Sci Res 3: 74-79.
- Khurana AL, Dudeja SS (1997) Biological Nitrogen Fixation Technology for Pulses Production in India. Indian Institute of Pulses Research, Kanpur, India.
- Goel AK, Laura RD, Pathak DV, Anuradha G, Goel A (1999) Use of biofertilizers: potential, constraints and future strategies review. International J Tropic Agri 17: 1-18.
- Hedge DM, Dwivedi BS, Babu SNS (1999) Biofertilizers for cereal production in India-A review. Industrial J Agri Sci 69: 73-83.
- Ahmad F Iqbal A, Khan MS (2005) Indole acetic acid production by the indigenous isolates of Azotobacter and fluorescent Pseudomonas in the presence and absence of tryptophan. Turk J Biol 29: 29-34.
- Lenin L, Jayanthi M (2012) Indole acetic acid, gibberellic acid and siderophore production by PGPR isolates from rhizospheric soils of Catharanthus roseus. Intern J Pharmaceu Biol Arch 3: 933-938.
- Oskar AP, Bashan Y, de-Bashan LE (2014) Proven and potential involvement of vitamins in interactions of plants with plant growth-promoting bacteria-an overview. Biol Fertil Soils 50: 415-432.
- Narula N, Gupta KG (1986). Ammonia excretion by Azotobacter chroococcum in liquid culture and soil in the presence of manganese and clay minerals. Plant Soil 93: 205-209.
- Saini P (2012) Preliminary screening for plant disease suppression by plant growth promoting rhizobacteria. Sci Res Rep 2: 246-250.
- Arnon DI (1949) Copper enzymes in isolated chloroplasts, polyphenol oxidase in Beta vulgaris. Plant Physiology 24: 1-15.
- Highkin HR, Frankel F (1962) Studies on growth and metabolism of barley mutant lacking chlorophyll b. Plant Physiol 37: 314-320.
- Holt JG, Krieg NR, Sneath PHA, Staley JT, Williams ST (1994) Bergey’s Manual of Determinative Systematic Bacteriology.  Lippincott Williams and Wilkins, A Wolters Kluwer Company, Philadelphia.
- Kamil P, Yami KD, Singh A (2008) Plant growth promotional effect of Azotobacter chroococcum, Piriformospora indica and vermicompost on rice plant. Nepal J Sci Technol 9: 85-90.
- Shende ST, Apte RG, Singh T (1977) Influence of Azotobacter on germination of rice and cotton seeds. Current Science 46: 675.
- Mogle UP, Chamle DR (2011) Evaluation of Biofertilizers and Parthenium Vermicompost on Tomato Crop. J Eco-biotechnol 3: 11-13.
- Poi SC, Ghosh G, Kabi MC (1989). Response of chickpea (Cicer arietinum L.) to combined inoculation with Rhizobium, phosphobacteria and mycorrhizal organisms. Zentralblatt fur Mikrobidogie 144: 249-253.
- Planzinski J, Rolfe BG (1985) Interaction of Azospirillum and Rhizobium strains leading to inhibition of nodulation. Appl Environ Microbiol 49: 990-993.
- Krishna KR, Bagyaraj DJ (1981) Note on the effect of VA mycorrhizal and soluble phosphate fertilizers on Sorghum. Ind J Agric Sci 51: 688-690.
- Patil NM (2008) Biofertilizers effect on growth, protein and carbohydrate content in Stevia rebaudiana var bertoni. Recent Res Sci Technol 2: 42-44.
- Vijayakumari B, Yadav R, Hiranmai Raja A (2009) Influence of Fresh, Composted and Vermicomposted Parthenium hysterphorus and Poultry Droppings on Quality Parameters of Radish. J Appl Sci Environ 13: 79-82.
- Van Loon LC, Bakker PAHM, Pieterse CMJ (1998) Systemic resistance induced by rhizosphere bacteria. Annu Rev Phytopathol 36: 453-483.
- Ramamoorthy V, Samiyappan R (2001) Induction of defense related genes in Pseudomonas fluorescence treated chili plants in response to infection by Colletrichum cupsici. J Mycol Plant Pathol 31: 146-155.
- Rao PP, Birthal PS, Reddy BVS, Rai KN, Ramesh S (2006) Diagnostics of Sorghum and Pearl Millet Grains-based Nutrition in India. J SAT Agri Res 2: 313-321.
- Selvakumar G, Lenin M, Thamizhiniyan P, Ravimycin T (2009) Response of Biofertilizers on the Growth and yield of Black gram (Vigna mungo L.). Recent Res Sci Technol 1: 169-175.
Citation: Tiwari S, Chauhan RK, Singh R, Shukla R, Gaur R (2017) Integrated Effect of Rhizobium and Azotobacter Cultures on the Leguminous Crop Black Gram (Vigna mungo). Adv Crop Sci Tech 5:289. DOI: 10.4172/2329-8863.1000289
Copyright: © 2017 Tiwari S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 12107
- [From(publication date): 0-2017 - Sep 01, 2025]
- Breakdown by view type
- HTML page views: 10806
- PDF downloads: 1301