Intestinal Epithelial Organoids: A Platform for Discovering Mucosal Healing Drug Candidates for the Treatment of Inflammatory Bowel Diseases
Received: 20-Jul-2020 / Accepted Date: 03-Aug-2020 / Published Date: 10-Aug-2020 DOI: 10.4172/2476-2024.1000166
Abstract
Inflammatory bowel diseases (IBDs) are chronic, remitting inflammatory disorders of the digestive system. Intestinal epithelial dysfunction constitutes an integral part of the pathogenesis of IBD. Epithelial regeneration is a complex process, and the healing of injured tissue requires the resolution of inflammation followed by the proper and rapid proliferation of epithelial cell groups involving stem cell activation and mobilization. In recent years, an in vitro culture method for intestinal epithelial stem cells has been established. Known as “organoid culture”, this novel culture system regenerates differentiated crypt lineages and reflects the architecture of intestinal mucosa. The mechanisms for controlling the growth and differentiation of intestinal epithelium have been investigated at the molecular level. Furthermore, this approach has been used for studying various intestinal diseases including cancer, based on the fact that functional abnormality of intestinal epithelium contributes to the development of infectious diseases. Therefore, using organoid culture to investigate the mucosal healing of IBD by identifying the molecule that controls the regenerative response induced by intestinal epithelial stem cells may lead to potent therapies for IBD patients.
Keywords: Organoid; Mucosal healing; Stem cell; Inflammatory bowel diseases
Abbreviations
IBD: Inflammatory bowel disease; IL-6: Interleukin-6; IL-22: Interleukin-22; IL-33: Interleukin-33; LncRNA: Long non-coding RNA; DIC: Disseminated Intravascular Coagulation
Introduction
Inflammatory bowel diseases (IBDs), comprised of Crohn's disease and ulcerative colitis, are intractable diseases in which inflammation of uncertain origin occurs in the digestive tract [1]. Symptoms include diarrhea, bloody stools, and abdominal pain, and a subset of patients with IBD are at increased risk to develop colorectal cancer [2]. The occurrence of IBD worldwide has been continuously increasing both in terms of prevalence and incidence for the last few decades [3]. Although the etiology is still unknown, growing evidence indicates that intestinal epithelium integrity is involved in the pathogenesis of IBD [4]. For a long time, the goal of treatment was to alleviate symptoms. Recently, however, the advent of targeted therapeutic agents such as biological agents (anti-TNF antibody, anti-α4 or α4β7 integrin antibody, anti-IL-12/IL-23 antibody), have greatly improved the clinical outcomes of IBD patients [5]. With these now classes of therapeutic agents, achieving long-term remission has become the treatment goal in IBD. However, despite considerable advances in the management of IBD, there are still a significant proportion of patients who remain unable to reach or maintain long-term remission. A therapeutic strategy called "mucosal healing", aimed at the structure and functional recovery of damaged tissues, was developed. Initial clinical studies suggest that "mucosal healing" is not only a marker of long-term remission, but also a predictor of long-term life quality in IBD patients [6-8]. Consequently, research aimed at intestinal mucosal regeneration has garnered a great deal of attention. In the meantime, a major advance has been made utilizing intestinal stem cells as part of three-dimensional (3D) culture system, also known as organoid culture. This system could give rise to all differentiated cell types of the intestinal epithelium and facilitate the replication of physiological structures of tissue in vitro [9,10]. Recent research on the mechanism underlying intestinal tract regeneration using intestinal epithelial stem cell-derived organoids and the development of therapeutic agents based on it have sharply increased.
The development of intestinal epithelial organoids
The intestinal epithelium is a dynamic organ that is continuously renewed every 3 to 5 days through a process of cell proliferation, differentiation and shedding. Proliferative cells (stem cells) that reside at the bottom of the intestinal crypt are the source of all intestinal epithelial cells. Until 13 years ago, in vitro culture of normal intestinal epithelium had been technically impossible, but in 2007, Barker et al. identified LGR5, one of the target molecules of Wnt signaling, as a specific marker for stem cells [11]. Two years later, an in vitro culture method for mouse intestinal epithelial stem cells was established by Sato’s et al. [12]. In addition, in 2012, it became possible to culture normal human intestinal tissue on a culture dish [13].
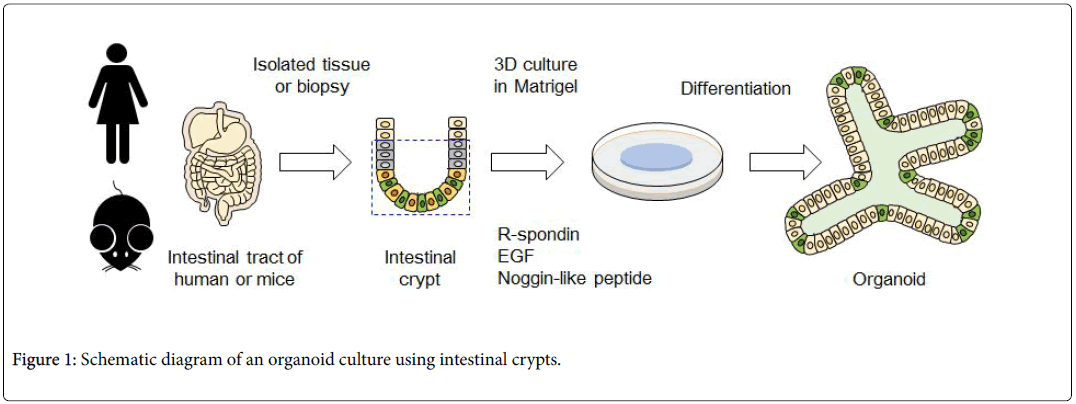
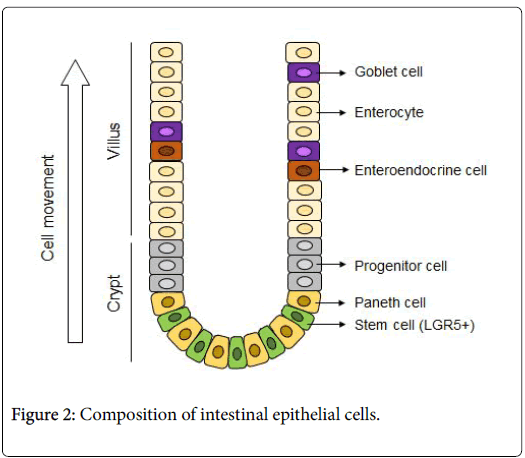
The intestinal epithelial stem cells produced by this culturing method and the mass of epithelial cells differentiated from stem cells are called "intestinal epithelial organoids". To cultivate organoid, primarily intestinal epithelial stem cells are isolated from the intestinal tract of a living body. These are embedded into a specific basement membrane matrix (extracellular matrix) known as a 3D tissue culture, such as Matrigel, in order to maintain stem cell replication and differentiation [14,15]. Embedded crypts were then cultured in a culture medium containing various molecules (R-spondin, EGF, Noggin-like peptide, etc.) (Figure 1). Thus, when a single isolated epithelial stem cell self-renews, it migrates to the villus region, where it differentiates into Paneth cells required for stem cell niche formation in the crypt region, and for mature functional intestinal epithelial cell formation (absorbing epithelial cells, goblet cells, enteroendocrine cells) (Figure 2). Therefore, the intestinal epithelial organoid has a crypt-villus structure similar to that of the intestinal epithelium of a living body. At the same time, is smaller than a living organ and is more suitable for experimental research.
The use of intestinal epithelial organoids for studying mucosal healing
Increasing evidence indicates that dysregulation of the intestinal epithelium is one of the characteristics of IBD. During inflammation, proinflammatory cytokines cytokines such as IFN- and TNF-α, which promote apoptosis in epithelial cells, could increase intestinal permeability and substantially destroy the epithelium [16,17]. The structural basis of reestablishing mucosal homeostasis is an integrated gut epithelium; here, epithelial regeneration plays a critical role in intestine barrier maintenance and function following tissue damage [18]. By mimicking the tissue of origin, the intestine epithelial organoid is a very useful organ model that can be used as a substitute for the living body, elucidating the mechanisms underlying wound healing and leading to the development of therapeutic drugs for IBD patients.
By utilizing in vitro intestinal organoids, the experiment of Jeffery et al. [19]. demonstrated that interleukin (IL)-6 could induce the proliferation of stem cells by activating the phosphorylation of STAT3 in Paneth cells. Mahapatro M [20] et al. reported that overexpression of IL-33 induces the increased secretory lineage of the gut epithelium. In addition, a previous study suggested that IL-22 promotes reestablishment of intestinal epithelium by the stem cell mediated IL-22R/STAT3 signaling pathway [21]. Moreover, levels of IL-22 were found to be elevated at the outset and then gradually decreased with the onset of inflammation. Although IL-22 mediates organoid growth by activating cell proliferation, such as occurs with transit-amplifying cells, it inhibits the differentiation of intestinal epithelial cells and potentially reduces the survival of LGR5-positive stem cells [22,23]. Thus, the intestinal organoid culture system could also provide a platform for clarifying the roles played by other cytokines in the mucosal healing of inflamed intestinal tissue. The study of Geng et al. provided evidence that long non-coding RNA (lncRNA) H19 plays a crucial role in IL-22-dependent epithelial regeneration, showing that the lack of H19 impedes re-establishment of the intestinal epithelium [24]. In addition, lncRNA uc.173 has been reported to function as an essential regulator of mucosal renewal, stimulating gut mucosal growth by downregulating miR195 in intestinal epithelial cells [25].
Taken together, intestinal organoid representing a promising platform for novel drug discovery in IBD. Our laboratory is currently conducting basic research aimed at methods for regenerating of intestinal epithelial cells by elucidating of their regenerative control mechanism using small intestinal epithelial organoids. Our preliminary data have shown that recombinant thrombomodulin (Recomodulin™, ART-123), a therapeutic biologic agent approved for the treatment of disseminated intravascular coagulation (DIC) in sepsis, positively regulates stem cell function. Recomodulin ™ may promote stem cell proliferation and differentiation using the established mouse intestinal epithelial organoid culture method.
Based on these studies, the utilization of intestinal organoid cultures may offer a sound basis for exploring novel treatment strategies for mucosal healing in clinical practice. In addition, intestinal epithelial organoid transplantation has been used to treat mucosal injuries in mice under pathological conditions resulting from failed regeneration processes such as occurs in severe IBD and radioactive enteritis [26]. If these effects on regenerative treatment can be confirmed, their clinical application is envisioned in the near future.
Conclusion
As described above, we have realized a microenvironment that reproduces the stem cell niche of an intestinal epithelium crypt in vitro , maintaining the original replication and differentiation functions of LGR5-positive stem cells, and enabling a long-term culture system. These cultured tissues can subsequently be used as an effective tool not only for functional analyses of the small and large intestines under normal state, such as drug evaluation and gene expression analysis, but also for elucidating the pathological conditions of IBD. Furthermore, there is a possibility that intestinal-like cultured tissue transplantation could be applied to organ function regeneration. Therefore, elucidating the pathophysiology and treatment paradigms of gastrointestinal tract diseases by using intestinal epithelial organoids holds tremendous promise in the quest to realize mucosal healing of IBD.
References
- Maloy KJ, Powrie F (2011) Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 474: 298-306.
- Baek SJ, Kim SH (2017) Colitis-associated colorectal cancer in patients with inflammatory bowel disease. Minerva Chir 72: 520-529.
- Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, et al. (2018) Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 390: 2769-2778.
- Koch S, Nusrat A (2012) The life and death of epithelia during inflammation: lessons learned from the gut. Annu Rev Pathol 7: 35-60.
- Philipp S, Markus FN, Siew CN, Emad ME, Ala IS (2019) Mechanism-based treatment strategies for IBD: cytokines, cell adhesion molecules, JAK inhibitors, gut flora, and more. Inflamm Intest Dis 4: 79-96.
- Ardizzone S, Cassinotti A, Duca P, Mazzali C, Penati C, et al. (2011) Mucosal healing predicts late outcomes after the first course of corticosteroids for newly diagnosed ulcerative colitis. Clin Gastroenterol Hepatol 9: 483-489.
- Schnitzler F, Fidder H, Ferrante M, Noman M, Arijs I, et al. (2009) Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn’s disease. Inflamm Bowel Dis 15: 1295-1301.
- Colombel JF, Rutgeerts P, Reinisch W, Esser D, Wang Y, et al. (2011) Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology 141: 1194-1201.
- Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, et al. (2010) Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6: 25-36.
- Múnera JO, Sundaram N, Rankin SA, Hill D, Watson C, et al. (2017) Differentiation of human pluripotent stem cells into colonic organoids via transient activation of BMP signaling. Cell Stem Cell 21: 51-64.
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, et al. (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003-1007.
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, et al. (2009) Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459: 262-265.
- Fujii M, Matano M, Toshimitsu K, Takano A, Mikami Y, et al. (2018) Human intestinal organoids maintain self-renewal capacity and cellular diversity in niche-inspired culture condition. Cell Stem Cell 23: 787-793.
- Jabaji Z, Brinkley GJ, Khalil HA, Sears CM, Lei NY, et al. (2014) Type I collagen as an extracellular matrix for the in vitro growth of human small intestinal epithelium. PLoS One 9: e107814.
- Sugimoto S, Sato T (2017) Establishment of 3D intestinal organoid cultures from intestinal stem cells. Methods Mol Biol 1612: 97-105.
- Eriguchi Y, Nakamura K, Yokoi Y, Sugimoto R, Takahashi S, et al. (2018) Essential role of IFN- in T cell-associated intestinal inflammation. JCI Insight 3: e121886.
- Grabinger T, Luks L, Kostadinova F, Zimberlin C, Medema JP, et al. (2014) Ex vivo culture of intestinal crypt organoids as a model system for assessing cell death induction in intestinal epithelial cells and enteropathy. Cell Death & Disease 5: e1228.
- Barker N (2014) Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol 15: 19-33.
- Jeffery V, Goldson AJ, Dainty JR, Chieppa M, Sobolewski A (2017) IL-6 signaling regulates small intestinal crypt homeostasis. J Immunol 199: 304-311.
- effery V, Goldson AJ, Dainty JR, Chieppa M, Sobolewski A (2017) IL-6 signaling regulates small intestinal crypt homeostasis. J Immunol 199: 304-311.
- Mahapatro M, Foersch S, Hefele M, He GW, Ventura E, et al. (2016) Programming of intestinal epithelial differentiation by IL-33 derived from pericryptal fibroblasts in response to systemic infection. Cell Rep 15: 1743-1756.
- Lindemans CA, Calafiore M, Mertelsmann AM, O'Connor MH, Dudakov J, et al. (2015) Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature 528: 560-564.
- Zha JM, Li HS, Lin Q, Kuo WT, Jiang ZH, et al. (2019) Interleukin-22 expands transit-amplifying cells while depleting Lgr5+ stem cells via inhibition of Wnt and Notch signaling. Cell Mol Gastroenterol Hepatol 7: 255-274.
- Zhang X, Liu S, Wang Y, Hu H, Li L, et al. (2019) Interleukin 22 regulates the homeostasis of the intestinal epithelium during inflammation. Int J Mol Med 43: 1657-1668.
- Geng H, Bu HF, Liu F, Wu L, Pfeifer K, et al. (2018) In inflamed intestinal tissues and epithelial cells, interleukin 22 signaling increases expression of H19 long noncoding RNA, which promotes mucosal regeneration. Gastroenterology 155: 144-155.
- Xiao L, Wu J, Wang JY, Chung HK, Kalakonda S, et al. (2018) Long noncoding RNA uc.173 promotes renewal of the intestinal mucosa by inducing degradation of MicroRNA 195. Gastroenterology 54: 599-611.
Citation: Yan Q, Gaowa A, Kawamoto E, Park EJ, Shimaoka M (2020) Intestinal Epithelial Organoids: A Platform for Discovering Mucosal Healing Drug Candidates for the Treatment of Inflammatory Bowel Diseases. Diagn Pathol Open 5: 166. DOI: 10.4172/2476-2024.1000166
Copyright: © 2020 Yan Q, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 3280
- [From(publication date): 0-2020 - Dec 20, 2025]
- Breakdown by view type
- HTML page views: 2427
- PDF downloads: 853