Review Article Open Access
Japanese Encephalitis and its Epidemiology
| Malhotra S*, Sharma S and Hans C | |
| Department of Microbiology, Dr. Ram Manohar Lohia Hospital and PGIMER, New Delhi | |
| Corresponding Author : | Malhotra S Department of Microbiology, PGIMER, New Delhi, India Tel: 7428177617 E-mail: drshalinimalhotra@yahoo.com |
| Received: August 22, 2015 Accepted: October 05, 2015 Published: October 25, 2015 | |
| Citation: Malhotra S, Sharma S, Hans C (2015) Japanese Encephalitis and its Epidemiology. J Infect Dis Ther 3:243. doi:10.4172/2332-0877.1000243 | |
| Copyright: © 2015 Malhotra et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at Journal of Infectious Diseases & Therapy
Abstract
Japanese encephalitis (JE) is among the most common cause of viral encephalitis in human beings and is found worldwide. JE, a zoonotic disease, is caused by JE virus (JEV), a mosquito-borne flavivirus and belongs to the family Flaviviridae. About 1% of human JEV infections result in JE, but 20–30% of these cases are fatal and 30-50% of survivors have significant neurologic or psychiatric sequelae. WHO estimated that approximately 67 900 JE cases occur annually in the 24 JE-endemic countries with an overall incidence of 1.8 per 100 000 population and 75% of these cases occur in children aged 0-14 years, which gives an estimated overall annual incidence of 5.4 per 100 000 in this age group. Five genotypes of JEV (1 to 5), based on structural protein sequences, with geographically distinct distributions, have been described. Diagnosis of JE can be made by virus isolation in cell/ tissue culture, antigen detection, and antibody detection. There is no specific treatment of JE, and only supportive care is provided to patient thus prevention of JE is considered as an important intervention in JE. Hence a strong surveillance system along with implementation of high quality vaccination program for children is the key ingredients to reduce the number of JE cases in endemic areas.
| Keywords |
| Japanese encephalitis; Epidemiology; Endemic; Vaccination |
| Introduction |
| Japanese encephalitis (JE) is among the most common cause of viral encephalitis in human beings and is found worldwide, especially in South-east Asia and less commonly in the Western Pacific regions and Australia [1]. JEV continues to invade other geographical areas and becoming a serious public health problem. JE is caused by JE virus (JEV), a mosquito-borne flavivirus and belongs to the family Flaviviridae, a singlestranded positive-sense RNA virus [2]. JE is primarily a zoonotic disease and vertebrate hosts such as pigs and birds play an important role in the maintenance and amplification of the virus while invertebrate host, mosquitoes are responsible for the transmission of virus [3]. About 1% of human JEV infections result in JE, but 20-30% of these cases are fatal and 30-50% of survivors have significant neurologic or psychiatric sequelae [4]. JE is primarily a disease of children however all age groups are affected. It is estimated that approximately 67,900 JE cases have occurred annually in 24 countries, with only 10,426 cases reported in 2011 [4,5]. This review briefly discusses the epidemiology of JE along with diagnosis and prevention of this deadly disease. |
| Historical perspective |
| The first outbreak of encephalitis was recognized in Tokyo, Japan during 1871 in which the causative agent of JE was mosquitoes Culex tritaeniorhynchus [6,7]. Major epidemics have been reported about every ten years and in 1924, over 6,000 cases were documented in a severe epidemic in Japan. Due to changes in agricultural and pigrearing practices, increased use of pesticides, and widespread immunization, the incidence of Japanese encephalitis decreased in Japan [6]. In 1935, Nakayama strain was isolated from the brain of a patient suffering from encephalitis. Thereafter, the virus had been classified with other flaviviruses as a group B arbovirus in the family Togaviridae, Originally the term “type B” encephalitis was used to distinguish it from sleeping sickness, commonly known as type A encephalitis,5 which occurs in winter with a different clinical presentation. Later in 1985, JEV was designated under a separate family Flaviviridae, as a member of genus Flavivirus [8]. |
| Vector and transmission |
| The JEV is transmitted to vertebrates by mosquitoes which was first suspected in 1930s; it was isolated from Culex tritaeniorynchus in 1938 [9]. For Southern Asia, Eastern Asia, and Southeastern Asia, the main vector of JE is C. tritaeniorhynchus while for Northern Australia, the main vector is C. annulirostris. However, JE virus has been isolated from different mosquito species belonging to the genus Culex, Anopheles and Mansonia but Culex vishnui complex (Culex tritaeniorhynchus, Culex vishnui and Culex pseudovishnui)are found to be the chief vectors of JEV which breed in bodies of stagnant water such as paddy fields [10,11]. Various other secondary vectors have been revealed from different studies including Mansonia indiana, C. whitmorei, C. gelidus, C. epidesmus, Anopheles subpictus, A. peditaeniatus, and M. uniform [12]. The natural cycle of JE virus involves water birds and Culex mosquitoes while pigs are considered to be the most important amplifying host, providing a link to humans through their proximity to housing [13]. Humans are generally thought to be dead-end hosts, i.e. they seldom develop enough viremia to infect feeding mosquitoes. |
| Epidemiology of JE |
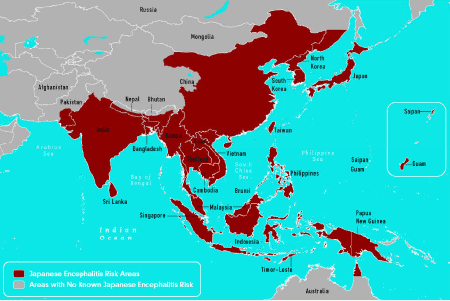
| The global incidence of JE is unknown because the intensity and quality of JE surveillance and the availability of diagnostic laboratory testing vary throughout the world. Although JE is reportable to the World Health Organization (WHO) by its Member States, reporting is highly variable and incomplete [14]. In the past 50 years, the geographic areas affected by JEV have expanded. Postulated explanations for JE expansion are bird migration, certain irrigation projects, animal smuggling, global warming and rice plantations creating a favorable environment for vector proliferation. JE cases are seen presently in North Australia, Bangladesh, Burma, Cambodia, China, Guam, India, Indonesia, Japan, Laos, Malaysia, Nepal, North and South Korea, Pakistan, New Guinea Papua, Philippines, Russia, Saipan, Singapore, SriLanka, Taiwan, Thailand, Timor-Leste and Vietnam as shown in Figure 1 [15]. Epidemic activity in Northern India, Central India, and Nepal has increased since the early 1970s. In 1990s, the virus continued to spread in Pakistan, Nepal and also in continental Australia [16,17] In unvaccinated populations in endemic areas, JE is largely a paediatric disease and most people have acquired active immunity by adulthood. However, in areas with long-standing, high-quality childhood vaccination programmes, JE is usually a rare disease of non-immune adults, especially the elderly. Hence for better understanding JE affected areas were classified into various categories as follows [14]: |
| Group A: Historically high incidence areas with high quality vaccination programmes. It includes Japan, Korea and Taiwan. Here overall incidence is reduced to 0.003 per 100 000 and the child (≤ 14 years) to adult (>14 years) case frequency ratio is 7:1 [18,19]. |
| Group B: Extremely low incidence areas with rare human cases and minimal or no vaccination programmes. It includes Australia, Pakistan, Russia and Singapore. JE is rare and an overall incidence is 0.003 per 100 000 with the child to adult case frequency ratio of 7:1 [18]. |
| Group C: Historically medium to high incidence areas with expanding vaccination programmes as seen in China. Overall incidence is around 3.3 per 100 000 and the child to adult case frequency ratio is 3:1 [20,21]. |
| Group D: High incidence areas with minimal or no vaccination programmes. It includes Cambodia, Indonesia, Laos, Malaysia, Myanmar, Philippines, Timor Leste. Incidence in these areas is 10.6 per 100 000 and the child to adult case frequency ratio is 7:1 [22,23]. |
| Group E: Medium incidence areas with no vaccination programmes. It includes Malaysia (Peninsular) and New Guinea Papua. Here incidence is assumed to be 5.3 per 100000 population [22,23]. |
| Group F: Historically high incidence areas with expanding vaccination programmes. It includes India (high incidence stratum) and Nepal. Overall Incidence in these areas is 2.8 per 100 000 and the child to adult case frequency ratio is 5:4 [24,25]. |
| Group G: Low incidence areas with minimal or no vaccination programmes. It includes Bangladesh, Bhutan, Brunei and Nepal (lower incidence stratum). Incidence in these areas is 1 per 100 000 and the child to adult case frequency ratio is 4:1 [24-26]. |
| Group H: Historically medium to high incidence areas with expanding vaccination programmes. It includes India (medium incidence stratum), Malaysia (Sarawak), Korea, SriLanka, Thailand and Vietnam. Overall Incidence in these areas is 1.5 per 100 000 and the child to adult case frequency ratio is 7:1 [27]. |
| WHO estimated that approximately 67 900 JE cases occur annually in the 24 JE-endemic countries with an overall incidence of 1.8 per 100 000 and 50% of these cases occur in China (excluding Taiwan). Approximately 55 000 (81%) occur in areas with well established or developing JE vaccination programmes, while approximately 12 900 (19%) occur in areas with minimal or no JE vaccination programmes. 51 000 (75%) of these cases occur in children aged 0–14 years, which gives an estimated overall annual incidence of 5.4 per 100 000 in this age group [14]. A fatality rate of 30% to 50% has been attributed to JE in Asian countries and 30% to 60% of the survivors suffer from longterm neurological manifestations like convulsions, tremors, paralysis, ataxia etc. Five genotypes of JEV (1 to 5), based on structural protein sequences, with geographically distinct distributions, have been described [28]. Genotype 3 (G3) is most common and has been widely distributed throughout Asia. The characteristics of JE, incidence, vaccination status and JEV genotypes in various JE affected areas in Table 1 [14,29]. |
| Problem in India |
| JE is considered as a major pediatric problem in many parts of India. It was first recognised via serological survey in 1955, in Tamil Nadu [30]. A major outbreak resulting in a 42.6% fatality rate was reported in the Bankura District of West Bengal in 1973. Subsequently, the disease spread to other states causing series of outbreaks in southern, eastern, and western states of the country. Surveys carried out by the National Institute of Virology, Pune indicated that approximately half of the population in Southern India has neutralizing antibodies to the virus. In 1978, cases were reported from 21 states and union territories [31] In Uttar Pradesh, the first major JE epidemic occurred in Gorakhpur in 1978, with 1,002 cases and 297 deaths reported. Since 1978 to 2005, this encephalitis has taken more than 10,000 lives in the state [32]. In 2005, Uttar Pradesh faced a devastating outbreak of JE, mostly confined to Gorakhpur, with 6,061 cases and 1,500 deaths. The clinical features of the cases were severe, and hospital-based acute encephalitis syndrome (AES) surveillance in north and northeast India showed that ~25% of cases were positive for JE, which were prevalent mainly in children.63–65. Similarly, JE cases in Uttar Pradesh were confined predominantly to Gorakhpur during 2007, with 3,024 cases and 645 deaths [32]. Till 2007 there have been 103,389 reported cases in India, and 33,729 deaths [33]. JE cases are seen from 24 states in India however 75% of the cases were contributed from Uttar Pradesh. In year 2011, around 891 people, including 508 in Uttar Pradesh alone and 200 in Bihar, died due to encephalitis [34]. Between 2010 and 2014, the number of annual cases rose from 154 to 744, with deaths rising from 41 to 160, according to data from the Assam health department. In India, annual incidence ranged between 1,765 and 3,428 cases and deaths ranged between 466 and 707 according to the National Vector Borne Disease Control Programme of the Ministry of Health and Family Welfare [32]. Laboratory tests confirmed that the JE cases occurred throughout the year, with more cases in the rainy season.68,69 A routine vaccination program was implemented in JE epidemic area starting from 2006 using the LAV-SA 14-14-2. Presently three types of JE vaccines are licensed in India: one LAV-SA 14-14-2 and two inactivated vaccines namely vero cell culture derived SA 14-14-2 JE vaccine ( JEEV by BE India) and vero cell culture derived 821564XY, JE vaccine ( JENVAC by Bharat Biotech) [35]. |
| Clinical presentation of JE |
| The incubation period of JEV ranges from 6-16 days after the bite of mosquito. Infection is mostly asymptomatic, and 1 in 300 cases present with symptoms like fever, muscle pain, headache, vomiting and abdominal pain. Serious features include confusion, paralysis, abnormal posturing, seizures and coma. Fatality rate is 20-30% and is caused due to acute cerebral edema or severe respiratory distress from pulmonary edema. 30-50% of the recovered cases develop serious behavioral and neurological sequelae in the form of altered sensorium, extrapyramidal syndrome, epileptic seizures, and severe mental retardation in children [36,37]. |
| Diagnosis of JE |
| Patients with JE present with symptoms of acute encephalitic syndrome which are also shared by other Flaviviruses. Even viruses responsible for causing sore throat, mumps, measles, and chickenpox which are common in childhood can sometimes present with encephalitis symptoms. |
| Thus, laboratory confirmation is essential for the accurate diagnosis of JE, which is difficult due to the very low viremia and rapid removal of transient viremia by neutralizing antibodies [38]. Diagnosis of JE can be made by virus isolation in cell/tissue culture, antigen detection, and antibody detection. Various cell cultures that have been used for virus isolation include primary chick, duck embryo cells, and Vero cell lines, LLCMK2, C6/36, PK, and AP61 cells [39]. Antigen detection in CSF can be done using reverse passive hemagglutination, immunofluorescence and staphylococcal coagglutination tests using polyclonal or monoclonal antibodies. Immunohistochemistry and histopathological examination are also helpful to identify viral antigens in the CNS. Recently, antigen capture ELISA method to detect highly conserved E gene in different JEV genotypes was developed which is a very sensitive diagnostic test against Japanese encephalitis [40]. IgM capture enzyme-linked immunosorbent assay (ELISA) has been the most widely used diagnostic method for JEV antibody detection, but it cannot specifically distinguish antibodies of JEV from other flaviviruses. Antibodies can also be detected by the dipstick method [41]. However molecular methods provide better sensitivity and specificity. Real-time polymerase chain reaction (PCR) assay is considerably faster and the results can be read within an hour compared to conventional PCR. In reverse transcriptase PCR, the viral genome can be amplified directly from tissue or blood. Newer techniques like nested reverse transcription-polymerase chain reaction (RTPCR) and reverse transcription loop-mediated isothermal amplification (RT-LAMP) assay are highly specific, and less time consuming for detection of JEV with no cross-reactivity with other flaviviruses [31,42]. However molecular methods have certain limitations in the form of specific requirements of laboratory operations, skilled technicians and special equipments, but it can be used as a diagnostic tool in national laboratories for the surveillance of this disease. |
| Prevention and control |
| There is no specific treatment of JE, and only supportive care is provided to patient hence prevention of JE is considered as an important intervention in JE. There are two strategies for prevention of transmission of virus: vector control and immunization in endemic areas. |
| Vector control |
| Vector control is done by thermal fogging with ultra low volume insecticides such as pyrethrum or malathion during epidemics and regular use of bed nets in endemic areas [39]. Effective measures to prevent larval development like novel water management and irrigation practices such as periodic lowering of the water level, intermittent irrigation, and constant flow systems have reduced the incidence of disease in certain areas. However it is difficult to control mosquito density in the rural areas, which are the worst affected due to poor socioeconomic conditions and hence vector control alone cannot be relied upon to prevent JE [43]. |
| Immunization |
| Implementation of large scale vaccination in endemic areas is necessary for the control of JE. Several groups of vaccines are currently in use: Inactivated mouse-brain derived, inactivated cell-culture derived and live attenuated cell-culture derived vaccines.15 Mousebrain derived inactivated vaccines (MBDV) are based on the Nakayama and Beijing-1 strains, are effective and safe with seroconversion rate of 80% to 90% [9]. This is the only vaccine against JE approved by the World Health Organization. This vaccine is independently produced in Japan, China, India, Thailand, and Taiwan. It is available in lyophilized form, in which gelatin and sodium glutamate are used as stabilizers, and thiomersal is used as a preservative [44]. Primary vaccination is done between 1-3 years and it requires three primary doses on days zero, seven, and 30 with a booster after one year and thereafter every three years until age 10. MBDV has high production cost, lack of long-term immunity, and adverse allergic reactions and hence new improved vaccines were developed. |
| Inactivated hamster kidney cell-culture-derived vaccine, based on Beijing-3 strain was developed in China and has been in use since 1967. It has relatively fewer side effects, easy to manufacture, with an efficacy ranging from 76% to 90% [45]. Vero-cell culture derived formalin inactivated vaccine using an attenuated SA14-14-2 strain, induced high titers of neutralizing antibodies after two injections [46] Recently, Vero-cell culture derived formaldehyde inactivated JE vaccine using P20778 (Indian isolate) has been developed, and has generated high titers of anti-JEV antibodies in mice; sera from immunized mice neutralized different JEV strains with varying efficacies [47]. |
| Cell-culture derived live attenuated vero cell culture derived SA14-14-2 JE vaccine are developed which showed 95% protection after two doses in children [31]. At present, the licensed JE vaccines used in different countries include inactivated MBD (Nakayama and Beijing-1), inactivated Vero cell-based (Beijing-1, P-3, SA 14-14-2, Kolar strain-JEV 821564XY), live attenuated vaccine (SA14-14-2) from Chengdu Institute of Biological Products, and the chimeric live attenuated SA14-14-2 vaccine [29]. The live attenuated SA14-14-2 vaccine is the first Chinese vaccine to have its safety and quality endorsed by the World Health Organization (WHO) for use with children [29]. |
| Conclusion |
| Presently, Japanese encephalitis is a public health problem, not only for Asia but it has spread to the entire world with high fatality rate of 20-30%. Prevention of the disease is the main target for controlling the spread of this devastating disease because specific treatment is not available. Hence a strong surveillance system along with implementation of high quality vaccination program for children is the key ingredients to reduce the number of JE cases in endemic areas. |
References
|
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
| Figure 1 |
Relevant Topics
- Advanced Therapies
- Chicken Pox
- Ciprofloxacin
- Colon Infection
- Conjunctivitis
- Herpes Virus
- HIV and AIDS Research
- Human Papilloma Virus
- Infection
- Infection in Blood
- Infections Prevention
- Infectious Diseases in Children
- Influenza
- Liver Diseases
- Respiratory Tract Infections
- T Cell Lymphomatic Virus
- Treatment for Infectious Diseases
- Viral Encephalitis
- Yeast Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 20145
- [From(publication date):
October-2015 - Aug 30, 2025] - Breakdown by view type
- HTML page views : 14861
- PDF downloads : 5284
