Kuijiean Suppress Inflammation in Ulcerative Colitis Rat Models by Phosphorylation Level of HuR
Received: 14-Dec-2017 / Accepted Date: 26-Jan-2018 / Published Date: 29-Jan-2018 DOI: 10.4172/2476-2024.1000133
Abstract
In this study used a TNBS/ethanol method and combinated complex factors established the rat model of ulcerative colitis and treatment with the Xipayi Kuijiean (KJA) to conduct whole process. We detected the expression level of hnRNA and mRNA in the five inflammation factor such as IL-1α, IL-1β, IL-10, IL-17, TNF-α and regulatory protein NF-κB and HuR in UC with complex factor group (UC group), normal group, UC treated with KJA group (KJA group) and negative control group. In UC group, we assesed the colonic damage and colon length by naked eye decision scores, KJA significantly reduced the severity of colitis inflammation. The immune dysfunction tends to be basically restored in colon tissue of the KJA group. Furthermore, in UC group hnRNA and mRNA level are increased, but mRNA level significantly higher than hnRNA. The post-transcriptional level of inflammatory related factors was mainly followed by the reduction of mRNA level after treated with KJA. These results demonstrate that post-regulation level of HuR has an important role in the development of UC.
Keywords: TNBS/ethanol method and combinated complex factors induce rat model; Ulcerative colitis; qRT-PCR; Xipayi Kuijiean; HuR
Introduction
Inflammatory bowel disease (IBD) is a chronic inflammatory disease of the gastrointestinal tract, which clinically contains Crohn’s disease (CD), ulcerative colitis (UC), and other conditions [1,2]. In recent years, the UC disease obviously increasing both at home and abroad, and closely associated with the onset of colorectal cancer, the main risk of colorectal cancer is found to be high in UC patients include longer duration, greater anatomical extent of colonic involvement [3].
The etiology of UC includes immunity, heredity, environment, intestinal flora and mental factors, but the pathogenesis is not clear [4]. Among them, immunological mechanism is concerned by more and more researchers, especially the intestinal mucosal immune system plays a key role in the occurrence and development of UC [5,6].
The imbalance between pro-inflammatory and anti-inflammatory cytokines that occurs in UC results in disease progression and tissue damage and limits the resolution of inflammation. Cytokines play an important role in the immune pathogenesis of ulcerative colitis, where they drive and regulate multiple aspects of intestinal inflammation [7,8].
There are pro-inflammatory cytokines which include IL-1, IL-2, IL-6, IL-8, IL-12, TNF-a, IFN-gamma etc, and anti-inflammatory cytokines with transforming growth factor beta (TGF-β), IL-1 receptor antagonist (IL-lRa), IL-4, IL-10, IL-13, etc. These cytokines in intestinal immune regulation of UC patients, play an important role in the inflammatory response. The functions instead of balance between the two kinds of cytokines were damaged and balance disorders or various cytokines not normally present in a cell, which can lead to the occurrence of UC [9].
Xipayi Kuijiean (KJA) is a prescription for treating UC [10]. Here, we provide data supporting such studies, indicating that KJA administered in the TNBS/ethanol and combined with complex factors established rat model of UC. In previous research KJA can suppress ulcerative colitis in rat model because of the strong anti-inflammatory properties of KJA [11].
This study mainly aims at observing the curative effectiveness of KJA on pro-inflammatory cytokines (IL-1α, IL-1β and TNF-α) as well as on anti-inflammatory cytokine (IL-10) in UC rats to confirm and explore the mechanism in treating UC. The expression level of hnRNA and mRNA of IL-1α, IL-1β, IL-10, TNF-α, mRNA of IL-17 increased in colon tissue of UC group.
The expression of cytokines was regulated by NF-κB transcriptional and HuR post-transcriptional levels. NF-κB performs a pivotal function in the expression of many genes involved in immune and inflammatory responses, including ones that contribute to UC.
In normal intestinal epithelial cells, inactive NF-κB present in the cytoplasm by binding to their inhibitory subunit, IκB. In response to stimuli, IκB proteins are phosphorylated, ubiquitinated, and degraded. the ultimate result is the translocation of NF-κB into the nucleus, where it can bind specific DNA binding sites and regulate the transcription of target genes, such as IL-1βand TNF-α [12-15].
In our previous study, the phosphorylation level of NF-κB is increased in UC model group, that indicate changes of transcriptional levels of various genes in UC are likely to be related to the regulation of NF-κB [16].
In UC, activation of NF-κB and the release of inflammatory mediators, leads to the damage of the colonic mucosa. NF-κB often needs of specific sequences with some other regulatory factor to form a complex, the role of NF-κB promote or inhibit depends on the combination of other regulatory factors, however which regulatory factors affect to the NF-κB activity need to further study. RNA binding proteins associated with RNA binding protein HuR, Tristetraproline (TTP) and other proteins in cell [17].
HuR is one of the important post-transcription factors to regulate gene expression and has very important biological function in the body, it is not only the important regulatory factors of life activity, or important regulatory factors in the process of activated immune cells and its dysfunction related to inflammation, tumor and other diseases [18].
Therefore, the study on the expression and regulation of HuR and its inflammatory factors is of great significance in the development of UC and the research on drug intervention. In this study using Western blot detected the phosphorylation levels of NF-κB in nucleus and HuR in cytoplasm. The phosphorylation level of NF-κB and HuR are raised in negative control group but they were decreased in the KJA group [16].
That indicated the NF-κB and HuR are activated in UC group, treated with KJA could be inhibit the activation or phosphorylation of NF-κB and HuR, and they regulate transcription of cytokines and stability of the mRNA respectively, could affected the expression level of cytokines.
qRT-PCR used to detected the expression level of hnRNA and mRNA of IL-1α, IL-1β, IL-10, TNF-α, mRNA of IL-17 from different group rats colon tissue, analyze the regulation mechanism of the expression level of candidate genes expression in the whole process of UC.
Material And Methods
Materials
Chinese traditional medicine: Xipayi KuiJie’an (KJA) Enema (Preparation Standard of Medical Institutions Formulated by the Food and Drug Administration of Xinjiang Uygur Autonomous Region ZZJ-0001-2013) is composed of gallic (liquid herbal extract, 50 mL/bag includes 2 g of crude drug), and the prescription of this drug detail in our previous study [17].
Chemical reagent: TNBS(sigmaUSA, number#SLB 6263V)Chloral hydrate, anhydrous ethanol, liquid paraffin, ether and physiological saline were provided by genomics technology service headquarters; Trizol from Invitrogen used for RNA extraction; 2 x SYBR Green - Mix (QIAGEN ,Germany), 2 x Taq PCR MasterMix purchased from Beijing TIANGEN; primers using Primer 5.0 software design.
Instruments and equipment: FLI- 2999 HT artificial climate box; a gradient PCR (BIO- RAD in Cycler TM Thermal Cycler, USA); Realtime fluorescent quantitative PCR (ABI 7500 Real-time PCR, United States); High-speed centrifuge at low temperature ( Eppendorf centrifuge 5417r, Germany); coagulation glue imager (BIO-RAD, USA); Ultramicro spectrophotometer (Thermo Scientific Nanodrop 2000,USA); -80°C ultra-low temperature freezer (Thermo Forma series, The United States).
Primer design: For mRNA and hnRNA primer sequences were identified from the genomic database (NCBI, GenBank), using Primer 5 design software or blast Primer.
Antibody and reagent for Western blot: Phospho-NF-kB p65 antibody from Cell signaling technology, HuR antibody and beta actin antibody from Santa Cruz Biotechnology. SDS, TEMED, Tris-base and BCA protein assay reagent kit was provided by Shanghai sangon biological engineering co. LTD.
Method
The grouping experimental animals: SPF male Wistar rats (180 ± 30 g) aged 6 to 8 weeks provided by Animal Experimental Center of Xinjiang Medical University. The rats were randomly divided into normal group (10 rats), TNBS induced ulcerative colitis model group (15 rats), complex factor induced ulcerative colitis and treated with KJA (15 rats) and negative control group (20 rats) each 5 rats per cage. They were adaptively fed for seven days before building models.
Establish experiment rat models: Control group were housed in a controlled environment (temperature 24-25°C, humidity 70%-75%) and were fed on a normal laboratory diet. All experiments were conducted according to the guidelines of the local ethics committee at Xinjiang Medical University.
Establish the complex factors induced UC rat model group and KJA group: According to the literature [19] using artificial climate box to create an environment (relative temperature is 26-28°C, relative humidity of 36-40%, and light), put animals to 10 h a day [21-23], feed 300 g per cage for daily and 200 ml water [24,25], according to body weight, rats feed with 2 ml/100 g dose gastric medicine per daily.
A chronic stimulation is given that include: intermittent plantar stimulation (output voltage is 20-40 V, the interval time of voltage is 2-5 V, 30 min per day) [26] clip tail (clamp the tip of the rat tail within 2- 3 cm, each to the bite, Fighting, treatment once a day for 5 min) [27]; give noise to rats with 100 db with fast rhythm of playback music, continues to play 2 hours per day for 10-13 days [28].
TNBS enema was used to induce UC model, the rats fasted for 1day but were allowed to drink water freely. After anesthetized by 10% chloralhydrate solution (According to the weight of 0.3 ml/100 g, an intraperitoneal injection), a catheter 3 mm in diameter was slowly inserted about 8 cm into the anus, then 50% ethanol solution with 70 mg/kg TNBS was slowly injected.
Drugs were slowly administrated to make sure that the liquid did not flow out, and the rats were kept upside down for 1 min after the injection, in order to facilitate the distribution of TNBS in the gut [29].
With the above method to establish the complex factor induced UC model. At the same time every day morning and night per day enema to UC rats 1.35 ml/100 g dose of KJA total of 10 days. After established UC models, fasting 3 days, during these days a dose of 2 ml/100 g dose lavage of 20% glucose is given twice a day in the morning and evening to fill the stomach. Every cage supply 200 ml of 5% glucose water. For negative control groups, using distilled water instead of KJA.
Collection of tissue samples: The rat was anesthetized and the abdomen opened and the blood was taken from the abdominal aorta. Take colon tissue from the anus 5 cm-8 cm, cut open along the mesenteric margin, and gently wash the intestine with saline solution (feces, secretions, and blood Liquid), rinse clean and unfold the colon, surface of the mucosa, to observe the colonic mucosal injury [30].
For histopathological observation, the lesion area was taken and immediately placed in the 40 g/L formaldehyde solution fixed 24 h, the paraffin bag is buried, stain with HE dye. The colon tissues placed and kept in -80°C.
Total RNA extraction: The total RNA was extracted from the colon tissues by the Trizol (Invitrogen, Gaithersburg, MD, USA) one-step method. After enrichment of RNA by isopropyl alcohol precipitation, total RNA was column purified by using a NucleoSpin® RNA clean-up kit (MACHEREY-NAGEL, Germany). The concentration and purity of total RNA were determined by a spectrophotometer, and the quality assessment was conducted by the integrity of 28S and 18S rRNA.
Primer design: IL-1αIL-1βIL-10, IL-17, TNF-α primers designed 2-3 pairs of primers are designed for mRNA and hnRNA candidates for candidate genes (Tables 1 and 2). Applied the method of quantitative RT-PCR to detect the expression level of hnRNA and mRNA in the five inflammation factor such as IL-1α, IL-1β, IL-10, IL-17, TNF-α in normal group, UC model group, KJA group and negative control group. The condition of qPCR reaction: degeneration at 95°C for 10 min, 95°C for 15 s, each primer annealed temperature is 1 min.
| Gene | Primer sequences | Lenthg(bp) | Anneling T(°C) |
|---|---|---|---|
| IL-1α | Forward: ACATGTATGCCTACTCATCGGG Reward: TCCGGAATCTCCTTCAGCCAAC |
127 | 57 |
| IL-1β | Forward: AGGAGAGACAAGCAACGACAA Reward: GTTTGGGATCCACACTCTCCA |
122 | 57 |
| IL-10 | Forward: GCTCAGCACTGCTATGTTGC Reward: TGTTGTCCAGCTGGTCCTTC |
183 | 67 |
| IL-17 | Forward: TCCATGTGCCTGATGCTGTT Reward: AGGGTGAAGTGGAACGGTTG |
208 | 67 |
| TNF-α | Forward: CCACCACGCTCTTCTGTCTA Reward: GGGCTTGTCACTCGAGTTTTG |
143 | 59 |
| β-actin | Forward: AGCCATGTACGTAGCCATCC Reward: ACCCTCATAGATGGGCACAG |
115 | 57 |
Table 1: The primer list of qRT-PCRf (for mRNA).
| Gene | Primer sequences | Lenthg(bp) | Anneling T(°C) |
|---|---|---|---|
| IL-1α | Forward: ACGGCTAAGTTTCAATCA Reward: CACCGAAGACCTTTACAT |
270 | 58 |
| IL-1β | Forward: ACTGTTCCAGACCCATAC Reward: CCTCTGAAAGTAGATAACCC |
288 | 58 |
| IL-10 | Forward: TCCCTTTCCCTACTGACC Reward: ACACCTTTGTCTTGGAGC |
232 | 60 |
| TNF-α | Forward: GTGCCTCAGCCTCTTCTC Reward: CACGCTTGTTCGTTCATT |
298 | 62 |
| IL-17 | Forward: AGACTACCTCAACCGTTCCACTT Reward: TACAGGCTTCTTGGATAGAGCAGG |
219 | 63 |
Table 2: The primer list of qRT-PCR (for hnRNA).
Statistical analysis
Data analysis was performed using SPSS software v13.0. Values were reported as mean ± SD. ANOVA followed by Student’s t-test was used for multiple comparisons of the data. Statistical significance was set at an alpha value of p<0.05.
Results
Compared with the normal group, in UC group general state has undergone obvious changes, these characterize are indicated in Table 3A and 3B. Compare to negative control group, in KJA group, the rat hair becoming lustrous, mental state is better, have more activity, eat regular, the food quantity is normal, formed defecate.
| GROUP | N | Level of degree | X2 | P | ||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||||
| KJA group | 9 | 0 | 3 | 5 | 1 | 0 | 15 | 0.02 |
| Negative control group | 6 | 0 | 0 | 0 | 0 | 6 | ||
Table 3A: The inflammation scores of colon tissue from KJA and negative control group.
N:number of rats; 0: no damage of intestinal wall; 1: intestinal wall mild hyperemia, edema, smooth surface, no erosion or ulcer; 2: intestinal wall congestive edema, the mucosa is coarse and granular, have erosive or enteric adhesion; 3: lining of the intestine is swollen and swollen, surface necrosis and ulcer formation, wall thickened or the surface has necrosis and inflammation; 4: high blood edema in intestinal wall, mucosal necrosis and ulcer formation, diameter of ulcer is greater than 1 cm, giant colon death by toxic.(P<0.05).
| Histological parameters and description | |||
|---|---|---|---|
| Score | observe the colon tissue by naked eye | Epithelial cell | Inflammatory cell |
| 0 | no damage | normal | not infiltration |
| 1 | mildly congested, edema | loss of goblet cells | infiltrate to bottom of the mucosal base, fibrosis |
| 2 | intestine swollen, mucosa is coarse and granular | loss large part of goblet cells | a large number of chronic inflammatory |
| 3 | intestine swollen with necrosis and ulcer | loss of part of goblet, columnar cell | the mucosa and has a large number of neutrophils infiltrating |
| 4 | hyperemic, mucosal necrosis and ulceration | loss of part of goblet, columnar cell | mucosal necrosis, colon wall necrosis, ulceration is greater than 1 cm |
Table 3B: The inflammation score and its description.
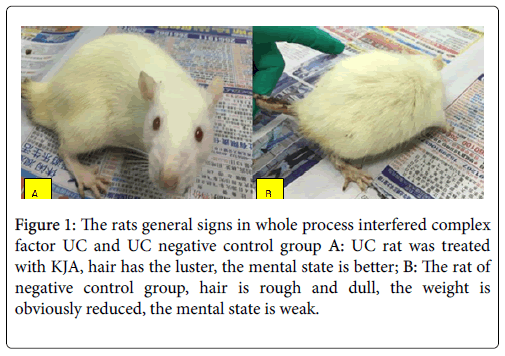
The mortality was obviously lower than negative control group (Figures 1A and 1B). Those symptoms in negative control group are appearing seriously.
Figure 1: The rats general signs in whole process interfered complex factor UC and UC negative control group A: UC rat was treated with KJA, hair has the luster, the mental state is better; B: The rat of negative control group, hair is rough and dull, the weight is obviously reduced, the mental state is weak.
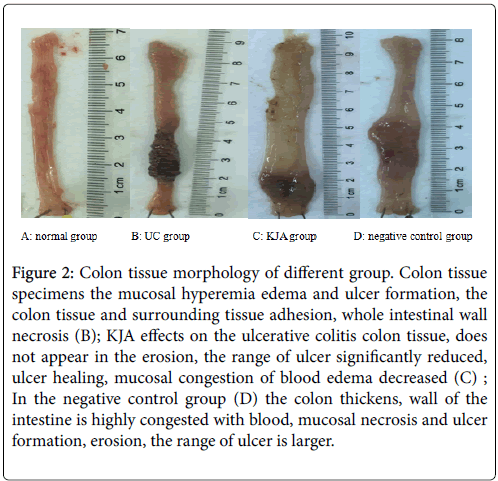
The KJA on the ulcerative colitis is very effectiveness (Figures 2A-2D). Observed each group’s colon tissue can found the therapeutic effect of KJA. To investigate mucosal inflammation, we performed H&E staining and demonstrated the KJA group and negative control group pathological results.
Figure 2: Colon tissue morphology of different group. Colon tissue specimens the mucosal hyperemia edema and ulcer formation, the colon tissue and surrounding tissue adhesion, whole intestinal wall necrosis (B); KJA effects on the ulcerative colitis colon tissue, does not appear in the erosion, the range of ulcer significantly reduced, ulcer healing, mucosal congestion of blood edema decreased (C) ; In the negative control group (D) the colon thickens, wall of the intestine is highly congested with blood, mucosal necrosis and ulcer formation, erosion, the range of ulcer is larger.
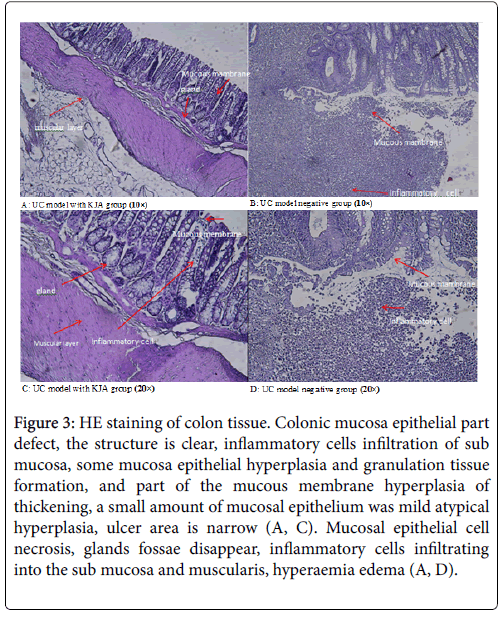
Colon tissue in KJA group (Figures 3A and 3C), all the colonic mucosa epithelial part defect, some glandular arrangement rules, the structure is clear, inflammatory cells infiltration of sub mucosa, and part of the mucous membrane hyperplasia of thickening, a small amount of mucosal epithelium was mild hyperplasia, ulcer area is narrow.
Figure 3: HE staining of colon tissue. Colonic mucosa epithelial part defect, the structure is clear, inflammatory cells infiltration of sub mucosa, some mucosa epithelial hyperplasia and granulation tissue formation, and part of the mucous membrane hyperplasia of thickening, a small amount of mucosal epithelium was mild atypical hyperplasia, ulcer area is narrow (A, C). Mucosal epithelial cell necrosis, glands fossae disappear, inflammatory cells infiltrating into the sub mucosa and muscularis, hyperaemia edema (A, D).
In the negative control group (Figures 3B and 3D), mucosal epithelial cell necrosis, inflammatory cells infiltrating into the sub mucosa and muscularis, there are a large number of neutrophil infiltration, and accompanied by obvious hyperaemia edema.
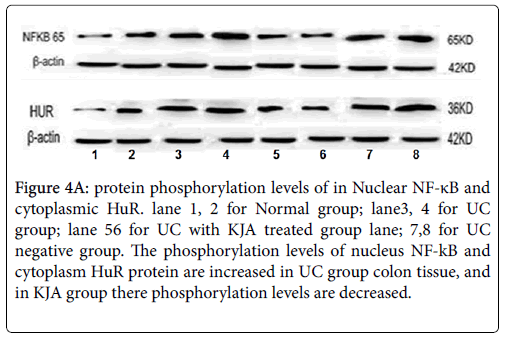
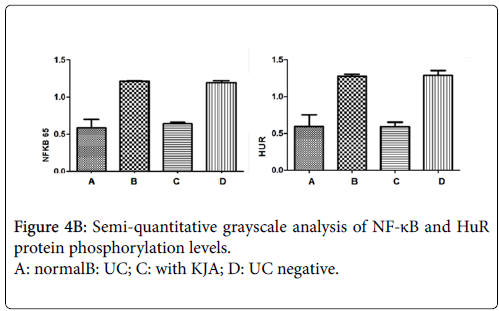
Using western blot analysis, detect phosphorylation levels of each groups of the nucleus NF-kB and cytoplasm HuR proteins. Compared with the control group, phosphorylation levels of NF-kB and HuR proteins are obviously increased in UC group and negative control group. But both of NF-kB and HuR proteins are decreased in KJA group. The evaluation results of their expression levels in each experiment groups showed in Figure 4a and 4b.
Figure 4a: protein phosphorylation levels of in Nuclear NF-κB and cytoplasmic HuR. lane 1, 2 for Normal group; lane3, 4 for UC group; lane 56 for UC with KJA treated group lane; 7,8 for UC negative group. The phosphorylation levels of nucleus NF-kB and cytoplasm HuR protein are increased in UC group colon tissue, and in KJA group there phosphorylation levels are decreased.
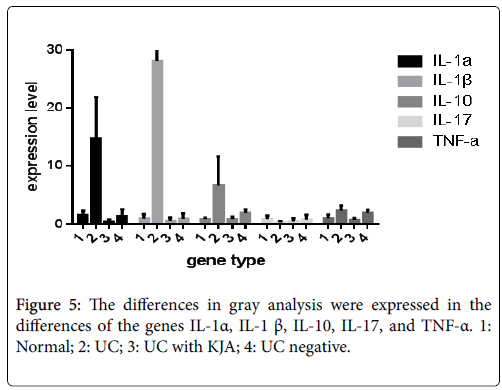
hnRNA expression and mRNA expression of several cytokines such as IL-1α, IL- 1β, IL -10, IL-17 and TNF -α in all experimental model groups (Tables 4 and 5). Also the expression levels of each genes hnRNA in all experimental groups in Figure 5. Pro-inflammatory cytokines are implicated in ulcerative colitis, with anti-inflammatory cytokine therapy are potential targets for remission in ulcerative colitis [19].
| Genes | IL-1α | IL-1β | IL-10 IL-17 | TNF-α |
|---|---|---|---|---|
| Normal group | 1.55 ± 0.71 | 0.99 ± 0.71 | 0.90 ± 0.22, 0.92 ± 0.53 | 1.01 ± 0.62 |
| U C group | 14.79 ± 7.08* | 28.03 ± 11.85* | 6.63 ± 4.96*, 0.36 ± 0.17 | 2.27 ± 0.96* |
| UC treated with KJA | 0.46 ± 0.38 | 0.61 ± 0.52 | 0 .90 ± 0.38*, 0.53 ± 0.52 | 0.75 ± 0.31* |
| UC negative group | 1.34 ± 1.23 | 0.93 ± 0.90 | 1.91 ± 0.62, 0.81 ± 0.77 | 1.92 ± 0.51 |
Table 4: The hnRNA differential expression of IL-1α, IL-1β, IL-10, IL-17, TNF-a in colon tissues from different group. NOTE*compare with the normal groups P<0.05, compare with the negative groups P<0.05.
| Genes | IL-1α | IL-1β | IL-10 | IL-17 | TNF-α |
|---|---|---|---|---|---|
| Normal | 0.83 ± 0.19 | 1.05 ± 0.09 | 1.16 ± 0.25 | 1.09 ± 0.44 | 0.69 ± 0.35 |
| UC group | 114.45 ± 50.59* | 306.06 ± 72.25* | 11.97 ± 6.10* | 5.62 ± 4.27* | 4.93 ± 2.99* |
| UC treated with KJA | 0.48 ± 0.40* | 0.57 ± 0.41* | 1.15 ± 0.83 | 0.30 ± 0.29* | 0.549 ± 0.36* |
| UC negative | 1.45 ± 0.94 | 2.04 ± 0.89 | 1.24 ± 0.56 | 1.19 ± 0.62 | 1.37 ± 0.56 |
Table 5: The mRNA differential expression of IL-1α, IL-1β, IL-10, IL-17, TNF-a in colon tissues from different group NOTE *compare with the normal groups P<0.05, compare with the negative groups P<0.05.
Compare the expression levels of hnRNA and mRNA of IL-1α, IL- 1β, IL -10, IL-17 and TNF-α which is resulted in both of hnRNA and mRNA expression levels are increased, among them the mRNA expression level are significantly higher than hnRNA expression level. The role of post-transcriptional level is very important for the further investigation of treatment of inflammation diseases.
Discussion
Ulcerative colitis is a chronic inflammatory bowel disease that affects millions of people worldwide, and is significantly associated colorectal cancer risk [31-33]. At the present, the explanation of pathogenesis of UC is not yet complete, and the therapeutic effect is not ideal.
Recently the immunomodulatory agent originating from herbal medicine represents a promising approach for UC therapy, as shown by the variety of clinical trials and experimental studies currently underway [34]. Traditional Chinese medicinal enemas can effectively inhibit regional mucosal inflammatory factors and improve disorders associated with immunity [35].
In our previous study investigated and indicated treatment of UC rats with KJA significantly reduced the NF-kB and IL-1β in the intestinal mucosa as compared with UC rats treated with water. In previous research, KJA can significantly effect to the morphology and pathological changes of colon tissue in UC rats [20].
In UC group, rats significantly decreased activity, loss of weight, like crouching, the hair color to dim and lackluster, mental depression, appear the loose, mucous, purulent and bloody stool. In previous study, UC model rats colon inflammatory cells infiltration, the decrease of microvilli in columnar cells, increase of mucous of granula in goblet-cells and mitochondrial vacoules in colon tissue on histomorphology [36].
Colon tissue specimens in UC model, the mucosal hyperemia edema and ulcer formation and the shape of linear or focal, whole intestinal wall necrosis, mucosa thickening, and intestinal stenosis. In the negative control group, rats treated with water and the symptoms clearly same as UC group rats. The colon thickens, wall of the intestine is highly congested with blood, mucosal necrosis and ulcer formation, erosion, appeared big lump.
These pathological symptoms are in KJA group, the range of ulcer significantly reduced, did not see colon thickened, color returns to normal. That shows the KJA could affect to improve the colon tissue and cure the inflammation of ulcer.
The pathological alterations in colon of KJA group were manifested in Figures 3A and 3C. Compared to the negative control group, KJA group significantly showed the structure is clear, ulcer is narrowed, part of the mucous membrane hyperplasia. As we analyzed above, the KJA group exhibited a greater inhibitor effect of inflammation score and symptoms.
In the present study, KJA significantly ameliorated inflammatory cell infiltration in KJA group that compared with complex factors induced UC group. The Xipayi Kuijiean (KJA), which was made from gallic, was used for enema to rats model groups, can treat many kinds of ulcers and the usual medicines for inflammation [37]. After gallic tannis bind to proteins, except for the convergence, there are antiinflammatory, analgesia, antivirus, anti-immunity, decreasing blood lipid concentration and blood sugar and other functions [38].
The phosphorylation levels of nucleus NF-kB and cytoplasm HuR protein are increased in UC group colon tissue, and in KJA group they are decreased. This result indicate rats were treated with KJA can be suppress the nucleus NF-kB and cytoplasm HuR protein activation or phosphorylation, than might regulate the transcription activity of cytokine and the stability of mRNA, consequently be affected the gene expression of cytokines. This analysis showed that KJA markedly suppressed phosphorylation of NF-kB p65 and also inhibited NF-kB p65 translocation to the nucleus in the inflamed colon tissue [39].
Compared the normal group and UC group, hnRNA expression level increased in cytokines of IL-1α, IL-β, IL-10 and TNF-α, and these differences were statistically significant (P<0.05). Compared with KJA group and the negative control group, hnRNA expression level of IL-10 and TNF-α are decreased in KJA group, the difference was statistically significant (P<0.05), and hnRNA expression level of IL-1α, IL-β, IL-17 is no statistical significance (P>0.05).
The mRNA expression was significantly increased of IL-1α, IL-1β, IL-10, IL-17 and TNF-α in UC group (P<0.05). In KJA group, the mRNA expression level of IL-1α, IL-1β, IL-17, TNF-α was significantly decreased (P<0.05). But the mRNA expression level of IL-10 was not significantly changed (P>0.05).
IL-1 is an important medium that is closely related to immune regulation, inflammation and tissue injury. The important role of IL-1 in immunity and inflammation induced with many effects in the process of protein expression, for example: chemokines, cytokines, nitric oxide synthase (iNOS), matrix metalloproteinases (MMPS) and an enzyme called cyclooxygenase 2 (cox-2), etc [40].
IL-10 reduce the Major histocompatibility complex (MHC), increase the soluble TNF-α, IL-1Ra and other several antiinflammatory protein expression, inhibition of IL-1β, IL-4, IL-5, IL-6, IL-2, IL-8, TNF-α, IFN-ɣ, and the secretion of iNOS and COX-2 and adjust the differentiation and proliferation of immune cells, thereby limiting and termination of the inflammatory response [42-56].
IL-17 is an important cytokines induced by T cells and promotes inflammation. The mRNA expression level significantly increased of IL-17 in the UC group, but hnRNA expression level of was not statistically significant (P>0.05). That might be the stability of mRNA expression of IL-17 was improved by raising the level of HuR phosphorylation in the UC group. It can also be synergistic with TNF- α to amplify its inflammatory effect [46-47].
Our observations showed KJA inhibits the expression of proinflammatory cytokines, that indicate the regulation of posttranscriptional level and HuR are important to understanding the mechanism of UC. Thereby we conclude that KJA exerts its therapeutic effects by inhibit the HuR phosohorylation level in the UC.
Compare the hnRNA and mRNA expression levels between the UC group and KJA group shown by Table 6. The mRNA expression levels were significantly higher than hnRNA levels in IL-1α, IL-1β, IL-17 (P<0.05). This study reveals that the mRNA expression levels of IL-1α, IL-1β and IL-10 in UC group were all increased. In particular, the increase of mRNA expression of IL-1α, IL-1β in UC group higher than that of normal group, and these two genes expression significantly higher than IL-10.
| Genes | UC group | P | UC treated with KJA | p | ||
|---|---|---|---|---|---|---|
| mRNA | hnRNA | mRNA | hnRNA | |||
| IL-1α | 114.45 ± 50.49 | 14.79 ± 7.08 | 0.03 | 0.48 ± 0.40 | 0.46 ± 0.38 | 0.93 |
| IL-1β | 306.05 ± 72.25 | 28.03 ± 11.85 | 0.00 | 0.61 ± 0.52 | 0.93 ± 0.90 | 0.46 |
| IL-10 | 11.97 ± 6.10 | 6.63 ± 4.96 | 0.10 | 1.15 ± 0.83 | 0.90 ± 0.39 | 0.46 |
| TNF-α | 3.90 ± 1.80 | 2.27 ± 0.96 | 0.14 | 0.55 ± 0.36 | 0.75 ± 0.31 | 0.27 |
| IL-17 | 5.61 ± 4.27 | 0.36 ± 0.16 | 0.04 | 0.30 ± 0.29 | 0.53 ± 0.52 | 0.40 |
Table 6: Compare the hnRNA and mRNA expression levels of IL-1α, IL-1β, IL-10, TNF-a in colon tissues from different group P<0.05.
This result indicates the increased phosphorylation level of HuR causes colon tissue inflammation and lead to increase the mRNA amount in UC group. Treated with KJA, the phosphorylation level of HuR and mRNA decreased, resulted in no any difference on expression level between the mRNA and hnRNA, shown as table 6. In this study, the expression level of mRNA higher than hnRNA of IL-1α, IL-1β which might indicate HuR play an important regulatory role on mechanism of UC.
The mRNA and hnRNA of TNF-α expression levels significantly increased in UC group (P<0.05). TNF-α activity and the mRNA stability in UC group might improve by phosphorylation level of nucleus NF-κB and cytoplasmic HuR represented. TNF-α is recognized as the pro-inflammatory cytokines mediated by UC. TNF-α can also aggravate intestinal mucosal injury after the action of inflammatory cells [48]. Komatsu et al. [49] showed compare to non-active period in active period the expression level of TNF-α in the serum of UC patients, was significantly higher 380 times.
TNF-α promoting IL-1, IL-10, IL-17 and the other by macrophages, fibroblasts and epithelial cells and endothelial cytokines producing cytokines, indirectly cause tissue damage and lead to the effect of overlap. Therefore TNF-α plays a very important role in promoting inflammation and development of UC.
In conclusion, gene expression of IL-1α, IL-1β and IL-17 and mRNA stability might be regulated by HuR; The TNF-α expression activity mainly based on NF-κB regulation. Since in the further study, the role of HuR post-transcription regulating mRNA is very important than transcription regulation of NF-kB. HuR can lead to another new way to relieve inflammation in UC, and is suitable method for curing UC.
References
- Szigethy E, McLafferty L, Goyal A (2010) Inflammatory bowel disease. Child Adolesc Psychiatr Clin N Am 19: 301–318.
- de Souza HS, Fiocchi C (2016) Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol 13: 13-27.
- Yashiro M (2014) Ulcerative colitis-associated colorectal cancer. World J Gastroenterol 20: 16389-16397.
- Ming Hui W, Hua LY, Xiao-Huan C (2011) Research progress in ulcerative colitis. Chinese traditional Chin Med Mod Remote Edu 9: 170.
- Toyoda H, Yang H, Rotter JI (1993) Ulcerative colitis: A genetically heterogeneous disorder defined by genetic (HLAclassII) and subclinical (antineutrophil cytoplasmic antibodies) markers. J Clin Invest 92: 1080-1084.
- Yamamoto-Furusho JK, Uscanga LF, Vargas-Alarcón G, Ruiz-Morales JA, Higuera L, et al. (2003) Clinical and Genetic Heterogeneity in Mexican Patients With Ulcerative Colitis. Hum Immunol 64: 119-123.
- Tang SK, Jin WH, Shi DR (2006) Interleukin and ulcerative colitis. World Chin J Digestol 14: 405-411.
- Sanchez-Munoz F, Dominguez-Lopez A, Yamamoto-Furusho JK (2008) Role of cytokines in inflammatory bowel disease. World J Gastroenterol 14: 4280-4288.
- Jing XC, Hua ZY, Long LY (2013) The research progress of immune factors in the pathogenesis of ulcerative colitis. Chin J Cell Mol Immunol 29: 889-892.
- Committee CP (1998) Drug Standards of the Ministry of Public Health of the People’s Republic of China. In: Book Drug Standards of the Ministry of Public Health of the People’s Republic of China. City: Uygur Pharmaceutical Section.
- Iminjan M, Yunus K, Hizbilla M, Hupur H, Li Y (2011) Experimental Study of Effects of Uygur Medicine Xipayi Kuijie’an on Colon Mucosa Apoptosis and the Mechanism of Treating Ulcerative Colitis. Sci Technol Rev 29: 29–35.
- Guo Xia YK, Kerim A (2009) Effect of Uyghur compound Xipayi KuiJie’an on the ultrastructure of small intestinal epithelial cell in Rat Model of Ulcerative Colitis. J Xinjiang Med Univ 32: 893–895.
- Upur H, Yunus K, Mijiti Y, Huang JJ, He J, et al. (2011) The histologic effects of the Uyghur medicine xipayi kuijiean on ulcerative colitis in rats. J Aust Tradit-Med So 17: 219–223.
- Hoffmann A, Levchenko A, Scot ML (2002) The NF-KB signaling module :temporal control and selective gene activation. Science 298: 1241-1245
- Karin M, Ben-Neriah Y (2000) Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol 18: 621–663.
- Lopez-Franco O, Suzuki Y, Sanjuan G (2002) Nuclear factor kappa B inhibition as potential novel anti-inflammatory agentes for the treatment of immune glomerulonephritis. Am J Pathol 16: 1497-l505.
- Yamamoto Y, Gaynor RB (2001) Therapeutic potential of inhibition of the NF-KB pathway in the treatment of inflammation and cancer. J Clin Invest 107: 135-142
- Titmothy S, Blaekwell A, Chrisama JW (1997) The Role of Nuclear Factor-kB in Cytokine Gene Regulation. Am J Reapir Cell Mol Biol 17: 3-9.
- Barreau C, Pailiard L, Osborne HB (2005) AU-rich elements andassociated factors: are there unifying principles? Nucleic Acids Res 33: 7138-7150
- Yuan G, Peng MD (2012) The mechanism of RNA binding protein to regulate mRNA stability. Prog Microbiol Immunol 40: 79-84.
- Yiming A, Jie-Chun H, Upuer H (2010) Several different methods were used to establish the study of the syndrome model of impotence syndrome. J Xinjiang Med Univ 33: 1279-1280.
- Upuer H, Ayinuer M, Nuermaimaiti A (2006) The establishment of the animal model of abnormal Sawda and its natural recovery. J Xinjiang Med Univ 29: 910-914.
- Wang Lu Z, Li H, Wang J (2004) Effects and control of ventilation, temperature and humidity on animal welfare. Chin J Compar Med 8: 234-236.
- Liu Hong Y, Zhao H, Guo W (2000) The effects of gender and ambient temperature on acute hypoxia tolerance in mice. J Bethune Univ 26: 123-125.
- Li Chao Y, Zhao Wen G, Yuan Xin H (2013) Effects of temperature, humidity, feeding density and noise on animal welfare. J Henan Inst Sci Technol 34: 24-25.
- Geir A, May-Bente B, Morten HV (2013) Can temperature explain the latitudinal gradient of ulcerative colitis Cohort of Norway Aamodt. BMC Public Health 13: 2-8.
- Zainule A (2010) Baiyi medical book (uygur language). Xinjiang people's publication.
- Yin S, Shuang L, Gan H, Wu H (2007) Interventional effect of electro acupuncture combined with medicine on monoamine neurotransmitters in hypothalamus of rats with ulcerative colitis. Neural Regen Res 2: 536-539.
- Kellett J (2004) Extinction deficit and fear reinstatement after elect rical stimulation of the amygdale: implications for kindling associated fear and anxiety. Neuroscience 127: 277-287.
- Han Q (2001) Establishment of the animal model of liver and spleen deficiency syndrome. J Guiyang Trad Chin Med Col 23: 59-61.
- Zou J, Jiang H, Guo H (2007) The effects of noise on vasopressin, corticotrophin and oxytocin levels in the psychological and plasma pituitary gland. Chin Hehavioral Med Sci 16: 135-139.
- Li N, Wang X, Ji Y (2008) Establishment and evaluation of immune stress ulcerative colitis model. J Clin Med 36: 496-498.
- Wang X (2011) Development of rat model of ulcerative colitis. Shanxi J Trad Chin Med 27: 54-56.
- Obermeier F, Dunger N, Strauch UG (2003) Contrasting activity of cytosine-guanosin dinucleotide oligonucleotides in mice with experimental colitis. Clin Exp Immunol 134: 217-224.
- Sartor RB (2006) Mechanisms of disease:pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol 3: 390-407.
- Fiocchi C (2008) Future of IBD pathogenesis:how much work is left to do?. Inflamm Bowel Dis 2: S145-S147.
- Kaser A, Zeissig AB, Blumberg RS (2010) Inflammatory bowel disease. Annu Rev Immunol 28: 573-621.
- Wei N, Zhang X, Liu L (2003) Immunological study of pathogenesis of ulcerative colitis in active stage. Nat J Mod Med 8: 74-79.
- Fe K, Yadav PK, Ju LZ (2012) Herbal medicine in the treatment of ulcerative colitis. Saudi journal of gastroenterology. Saudi J Gastroenterol 18: 3–10.
- Huang J, Zhang J, Lixiaoxi E (2016) The establishment of the rat model of abnormal Sapra syndrome. J Xinjiang Med Univ 39: 1353-1358.
- Mairepaiti A (2016) Changes and mechanism of inflammatIion related factors in colon tissue from rat model of ulcerative colitis with abnormal Sapra syndrome. J Xinjiang Med Univ 2: 11-18.
- Hamulati U (2011) Pharmacognostics of uygur medicine. Xinjiang Sci Technol Health Press 1041-1042.
- Wang C, Liu Y (2001) Research progress of tannin physiological activity. Foreign Chin Med 23: 278
- Anand AC, Adya CM (2014) Cytokines and inflammatory bowel disease. Nat Rev Immunol 14: 97-106.
- Dinarello CN (2002) The IL-1 family and inflammatory diseases. Clin Exp Rheumatol 20: S1–13.
- Rosenwasser LZ, Borish L (1997) Genetics of Atopy and Asthma:The Rationale behind Promoter-based Candidate Gene Studies (IL-4 and IL-10). Am J Respir Crit Care Med 156: 152-155.
- Peng H, Wang W, Zhou M, Li R, Pan HF, et al. (2013) Role of interleukin-10 and interleukin-10 receptor in systemic lupuserythematosus. Clin Rheumatol 32: 1255-66.
- Borish L (1998) IL-10: Evolving concepts. J Allergy Clin Immunol 101: 293-297.
- Trifunović J, Miller L, Debeljak Z, Horvat H (2015) Pathologic patterns of interleukin 10 expression A review. Biochemia Med(Zagreb) 25: 36–48.
- Zhang JX, Dang SC, Qu JG, Wang XQ, Chen GZ (2005) Changes of gastric and intestinal blood flow,serum phospholipase A2 and interleukin1B in rats with acute necrotizing pancreatitis. World J Gastroenterol 11: 3578-3581.
- KomiyamaYS, Nakae T, Matsuki A (2006) IL-17 plays an important role In the development of experimental autoimmunity encephalomyelitis. J Immunol 177: 566-573.
- Momeleone G, MacDonald TT (2000) Manipulation of cytokines in the managment of patients with inflammative bowel disease. Ann Med 32: 552-560.
- Li l, Yang W, Xu M (2012) The expression of TNF-a and COX2 in the intestinal mucosa of ulcerative colitis and its relationship with apoptosis. Med Clin Stu 29: 1094-1097.
- Komatsu M, Kobayashi D, Saito H (2001) TNF-a in serum of patients with inflammatory bowel disease as measuredby a highly sensitive immune-PCR. Clin Chem 47: 1297-1301.
Citation: Wushouer X, Kadeer N, Chenbo X, Aximujiang K, Jingping Z, et al. (2018) Kuijiean Suppress Inflammation in Ulcerative Colitis Rat Models by Phosphorylation Level of HuR . Diagn Pathol Open 3: 133. DOI: 10.4172/2476-2024.1000133
Copyright: © 2016 Wushouer X, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 5031
- [From(publication date): 0-2018 - Aug 04, 2025]
- Breakdown by view type
- HTML page views: 4120
- PDF downloads: 911