Lasiodiplodia theobromae in the Root Rot Disease Complex of Rice
Received: 15-Jul-2018 / Accepted Date: 18-Aug-2018 / Published Date: 20-Aug-2018 DOI: 10.4172/2375-4338.1000197
Keywords: Fusarium ; Leaf chlorosis; NERICA ; Root rot; Upland rice
Introduction
Rice is the most widely, consumed staple food for a large part of the world's human population especially Asia where more than 90% of the world’s rice is grown and consumed [1]. In Nigeria, rice is the sixth major crop in cultivated land area after sorghum, millet, cowpea, cassava and yam [2,3]. It is the only crop grown nationwide and in all agro ecological zones from Sahel to the coastal swamps. Nigeria is the highest rice producer in West Africa, producing an average of 3.2 million tons of paddy rice. Rice yield are however very low compared to the regions average [4]. This low productivity is due to various constraints among which are pests and diseases. The country is also the largest consuming nation in the region, with the growing demand amounting to 4.1 million tons of rice [4]. Nigeria is the World’s second largest rice importer, spending over US$ 300 million on rice imports annually [5]. In order to reduce expenses on rice import, and provide income for local growers, successive governments of Nigeria established initiatives for increased rice production [6]. This increase in production is likely to increase pest pressure and growers need to be aware and prepared for pest appropriate management.
Several diseases have been implicated in reduced productivity of rice. Rice can be attacked by one or more pathogens at any stage, from germination to physiological maturity. Rice can seriously be affected by a large range of pests and diseases which can destroy up to 40% of the world’s rice crops [7]. The diseases range from foliar diseases such as brown leaf spot, rice blast, bacterial blight to root diseases caused by Pythium , Fusarium and nematodes [8]. Lasidiplodia theobromae (Syn. Botryodiplodia theobromae ) and F. oxysporum have also been reported to be isolated from rice [9] although losses attributed to them have not been well described. Fusarium species have been implicated in rots and vascular wilts of various plants including rice [8], while L. theobromae is associated with postharvest degeneration of various produce [10] and root rot of several crops [11-13]. L. theobromae has a wide host range. It affects both tree crops and arable crops L. theobromae is found in the tropics and sub tropics causing numerous kinds of diseases especially rot of fruits and tuber crops during storage [14]. With the very little literature on the incidence and pathogenicity of L. theobromae on rice, this study was carried out to evaluate the incidence and pathogenicity of L. theobromae on rice cultivars in selected locations in Nigeria.
Material and Methods
Field sampling
Soil and root samples were collected from ten farms each in two rice growing zones of Oyo (Iddo and Iseyin) and Ogun (Ijebu-North and Ikenne) states in southwestern Nigeria. The selected local government areas in Ogun state are in the rainforest agro-ecology while those in Oyo state are in the derived savanna. The sampling was conducted on upland rice only. Soil samples per farm were made up of 20-30 randomly taken cores. Plant roots were samples systematically along a W shaped path per farm with root samples taken at every 10 min interval. Both soil and root samples were collected in polythene bags, labeled and placed in an insulated box.
Isolation of fungi from soil and root samples of rice
In the laboratory, soil samples were thoroughly mixed and three separate samples were taken out for analysis. A sample of 10 g was added to 50 ml of sterile distilled water and agitated vigorously for 30 sec. The supernatant was immediately poured into labeled test tubes from which 1 ml was taken out with a sterile pipette and spread on preprepared acidified potato dextrose agar (PDA) plates. The plates were incubated for 7 days at 28 ± 2°C with daily observation of colonies. Single colonies were sub-cultured in pure cultures for identification. Fungi were identified using colony characteristics, microscopic morphological characteristics in comparison with descriptors [15,16].
Root samples were washed and evaluated for rot symptoms, and then sections of roots showing symptoms were cut with a sterile scalpel. The root pieces were surface sterilized, plated in acidified PDA and incubated for 7 days at 28 ± 2°C with daily observation. Pure cultures were made from single colonies observed then identified as previously described. Incidence of root rot was calculated using no of samples with root rot/total number of samples collected. Frequency of occurrence of each identified pathogen was computed following the method of Francis-Otokito [9].
Evaluation of NERICA rice lines for their reaction to Lasiodiplodia theobromae
Nine NERICA (1,2,4,5,7,8,10,11,14) with WAB 56/104 and OS6 were obtained from the Africa Rice Centre. The latter two cultivars served as checks for susceptibility and resistance respectively based on their reaction to rice blast. Soil was sterilized and filled into 7 kg capacity plastic pots. Four seeds of each rice cultivar were planted per pot and thinned to two plants per pot two weeks after planting where necessary. A week after transplanting, Lasiodiplodia theobromae suspension from pure cultures isolated from the field sampling was inoculated into soil surrounding the rice seedlings. The suspension was prepared by adding 10 ml of sterile distilled water to 7 day old pure culture plates. The agar surface was scraped with a sterile spatula and the conidia suspension filtered through three layers of sterile cotton gauze. The concentration of spores was adjusted to 2.2 × 106 after counting spores with the aid of haemocytometer. The experiment was arranged in Completely Randomized Design (CRD) and was replicated six times. Data were collected on plant height, number of leaves, chlorophyll content and number of tillers. A disease index of root lesion formed by the fungi was determined by the length and severity of the lesion [17] using the following scale; 0=no visible lesion; 1=lesion 1 mm long; 2=lesion 1 to 5 mm long, brown; 3=lesion longer than 5 mm, dark brown; 4=root totally infected and brown; 5=root destroyed and plant dying or dead. Foliar Diseases symptoms was scored using a 0-5 where 0=No damage on the leaves; 1=1-10% damage; 2=11-20% damage; 3=21-35% damage; 4=36-60% damage; 5=>60 % damage. Height of plant was recorded with the aid of meter rule; number of leaves was counted on each plant. Chlorophyll content was measured with a SPAD meter. Root lesion length was measured with the aid of a flexible measuring tape and used to score for root lesion.
Data were processed using Microsoft Excel. Descriptive statistics was used for data collected from the field sampling. Quantitative data from the pot experiment was analyzed using SAS statistical package for ANOVA using the procedure for generalized linear model. The significant means were separated using LSD at 5% level of significance.
Results and Discussion
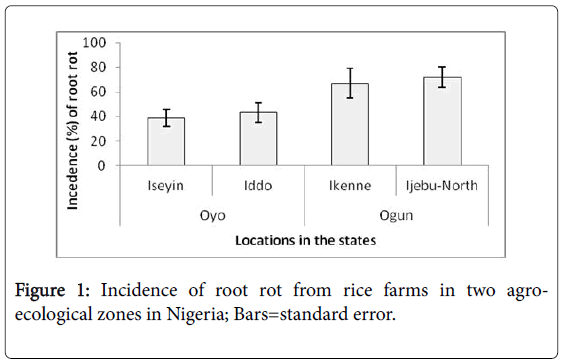
There was significantly higher incidence of root rot in the locations in Ogun state which is in the rainforest agroecological zone compared to root rot incidence in Oyo state in the derived savanna (Figure 1). Fungal pathogens are favoured in warm humid conditions which is the predominant environmental condition in both locations of Ogun state [18]. This maybe the reason for the higher incidence of root rot encountered in the area. In comparison, the other locations are in the less humid plains of the derived savanna.
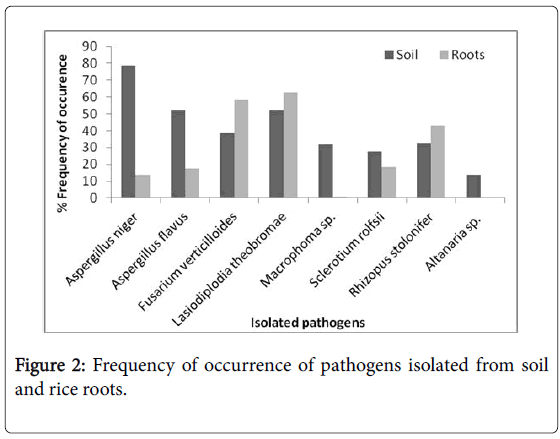
Pathogens identified in soil samples were Aspergillus niger , A flavus , Fusarium verticilloides , L. theobromae , Macrophoma sp. Sclerotium rolfsii , Rhizopus stolonifer and Alternaria sp (Figure 2). The same organisms were found associated with the roots except for Macrophoma sp. and Alternaria sp. A. niger was the most often encountered in the soil and the least encountered was Alternaria sp. whereas in the roots L. theobromae followed by F. verticilloides were the most frequently encountered at 62% and 58% respectively. These pathogens have been previously listed as associated with rice plants growing on the field [9,19]. The high level of occurrence of F. verticilloides and L. theobromae could be responsible for the incidence of root rot in all the locations. Both pathogen species were the second most frequently occurring pathogen is root rot disease complex of cassava [20]. Especially as these two pathogens were found in the majority of the locations sampled. In addition they were found associated with roots of the rice plants. The higher occurrence in roots rather than soil also indicates their association with roots as possible causal agents of root the rot observed.
In general, L. theobromae was found to be pathogenic on the rice cultivars evaluated. The pathogen significantly (P<0.05) reduced plant height, number of leaves and number of tillers of the inoculated rice cultivars, compared to uninoculated cultivars (Table 1). The infection caused by the pathogen also induced chlorosis in the leaves observed from the significantly lower chlorophyll content measured in infected plants. L. theobromae has been known as an important pathogen of root and tuber crops and stored produce in the tropics [14]. The awareness of its pathogenicity on various crops has been increasing as seen in various first reports [12,13,21]. The list of host of the fungus also includes banana, mangoes, cocoa [14], cassava, yams [22], peanut [23], coffee and coconut [24].
| Treatment | Number of leaves | Leaf chlorophyll content | Plant height (cm) | Number of tillers |
|---|---|---|---|---|
| L. theobromae | 9.6b | 29.4 | 17.7b | 3.9b |
| Control | 19.8b | 41.3 | 31.6a | 9.3a |
| LSD | 3.7 | 8.3 | 5.3 | 1.5 |
Values are means of six replicates with two stands each. Means followed by the same letters within a column are not significantly different at 5% level of significance using LSD
Table 1: Plant height, number of leaves and number of tillers of rice cultivars inoculated with Lasiodiplodia theobromae.
Plant height, number of leaves and number of tillers of plant inoculated with L. theobromae were generally lower than the uninoculated rice cultivars (Table 2). Without inoculation with L. theobromae , NERICA 15 , NERICA 7 and WAB 56-104 were the tallest plants while NERICA 14 was the shortest. Among the inoculated plants, however, NERICA 10 was the tallest followed by NERICA 4 . Significant reduction in plant height in L. theobromae inoculated plants occurred in NERICA 1 , 11 , 15 , 7 , and 8, in addition, both checks also showed stunting when inoculated. All plant except for NERICA 10 had significantly fewer leaves and number of tillers. Plant height and other growth parameters are generally impacted negatively when root function is affected. Impairment of root function caused by root rot results in the inability of the plant to carry various physiological functions leading to reduced measurement of growth parameters. Root rot of cucumber when not managed caused a significant reduction of all growth parameters [25].
| Cultivar | Plant height (cm) | No. of leaves | No. of tillers | |||
|---|---|---|---|---|---|---|
| Control | L. theobromae | Control | L. theobromae | Control | L. theobromae | |
| NERICA 1 | 29.04 | 18.16* | 18.1 | 8.89* | 7.02 | 3.54* |
| NERICA 10 | 28.49 | 25.94 | 17.92 | 14.88 | 6.76 | 4.67 |
| NERICA 11 | 30.23 | 6.38* | 20.7 | 2.06* | 8.83 | 1.15* |
| NERICA 14 | 23.85 | 21.86 | 16.92 | 11.56* | 7.06 | 3.78* |
| NERICA 15 | 40.97 | 20.60* | 26.02 | 9.91* | 9.63 | 2.69* |
| NERICA 2 | 30.84 | 17.21* | 20.69 | 10.38* | 8.37 | 4.59* |
| NERICA 4 | 28.71 | 23.21 | 18.8 | 11.60* | 7.96 | 4.54* |
| NERICA 7 | 40.69 | 17.91* | 25.77 | 9.79* | 10 | 3.29* |
| NERICA 8 | 27.36 | 12.27* | 14.89 | 7.73* | 6.31 | 3.65* |
| OS6 | 28.53 | 10.19* | 15.19 | 3.60* | 9.06 | 0.83* |
| WAB 56 -104 | 38.86 | 20.72* | 23.09 | 11.28* | 10.37 | 4.11* |
| LSD | 3.75 | 3.8 | 2.48 | 2.37 | 0.9 | 0.85 |
Values are means of six replicates per treatment with two stands per replicate *=significant difference within the row and per parameter at 5% level of significance using LSD
Table 2: Effect of Lasiodiplodia theobromae on plant height, number of leaves and number of tillers of selected NERICA cultivars.
Inoculated plants showed various levels of both foliar and root symptoms of infection (Table 3). Foliar symptom of L. theobromae was lowest in NERICA 10 compared to all other cultivars including the checks. The foliar symptoms began as chlorosis on the leaf tips which later turned necrotic with an active chlorotic border. NERICA 10 also showed the lowest root lesion score compared to the other cultivars and it was not significantly different from the uninoculated treatment. Observations on the reaction of NERICA 10 in terms of the growth parameters and disease response indicated that it possesses an appreciable level of resistance to L. theobromae . Evidences from this study shows that L. theobromae is pathogenic on several rice cultivars and is implicated in the root rot complex of rice. The pathogen is reported to be a tropical and subtropical cosmopolitan soil borne fungus [26]. It causes black rot on roots of strawberries, which led to wilting and eventually to blackened, necrotic discoloration in the crown of daughter plants [13], in Yucca plants it causes leaf tip dies back [21] a symptom similar to the one observed on the rice plants in this study. L. theobromae is also involved in a complex of root diseases affecting rice along with other pathogens [19,27].
| Cultivar | Foliar symptoms | Root lesion | ||
|---|---|---|---|---|
| Control | L. theobromae | Control | L. theobromae | |
| NERICA 1 | 0.5 | 2.8* | 0.2 | 3.2* |
| NERICA 10 | 0.8 | 1.8* | 0.2 | 0.7 |
| NERICA 11 | 0.7 | 2.5* | 0.5 | 2.3* |
| NERICA 14 | 0.5 | 3.0* | 0.2 | 3.8* |
| NERICA 15 | 1.1 | 2.7* | 0.7 | 3.0* |
| NERICA 2 | 0.6 | 3.3* | 0.5 | 3.5* |
| NERICA 4 | 0.7 | 3.2* | 0.2 | 4.2* |
| NERICA 7 | 1 | 2.9* | 0.3 | 3.5* |
| NERICA 8 | 0.6 | 2.5* | 0.7 | 3.0* |
| OS6 | 0.5 | 2.9* | 0.2 | 1.7* |
| WAB56-104 | 0.8 | 3.2* | 0.3 | 3.3* |
| LSD | 0.13 | 0.52 | 0.12 | 0.61 |
Values are means of six replicates per treatment. *=significant difference within the row and per parameter at 5% level of significance using LSD. Foliar Diseases symptoms scored from 0-5 using ; 0=No damage on the leaves; 1=1-10 % damage; 2=11-20 % damage; 3=21-35 % damage; 4=36-60 % damage; 5= >60 % damage; Root lesion damage scale was 0=no visible lesion; 1=lesion 1 mm long on few roots; 2=lesion 1 to 5 mm long on few roots, brown; 3=lesion longer than 5 mm dark brown on many roots; 4=root totally infected and brown; 5=root destroyed and plant dying or dead.
Table 3: Disease severity caused by Lasiodiploidia theobromae on selected NERICA cultivars.
This study presents the presence of L. theobromae in the root rot disease complex of rice in the majority of rice growing locations sampled in south western Nigeria. It is also a confirmation of the pathogenicity of L. theobromae on rice cultivars with an attendant reduction in growth parameters of rice and possibly yield. This should be put into consideration in developing any management strategy for rice diseases.
References
- Dauda SM, Dvzivama AU (2004) Comparative performance of a locally developed rice thresher with an imported Votex Rice Fan. In: Proceedings of the 5 th International Conference of the Nigerian Institution of Agricultural Engineers. Ilorin, pp 29-32.
- Oladele S, Awodun M (2014) Response of Lowland Rice to-Biofertilizer Inoculation and their Effects on Growth and Yield in Southwestern Nigeria. J Agri Environ Sci 3: 371-390.
- Ezedinma C (2008) Impact of trade on domestic rice production and the challenge of self-sufficiency in Nigeria. In: Kormawa P, Toure AA (eds) Rice policy and food security in sub-Saharan Africa. Proceedings of a workshop held on 7-9 November 2005, Cotonou, Benin. Africa Rice Centre, Cotonou, Benin, pp 141–157.
- Bamidele FS, Abayomi OO, Esther OA (2010) Economic Analysis of Rice Consumption Patterns in Nigeria. J Agr Sci Tech 12: 135-144.
- Daramola B (2008) Government policies and competitiveness on Nigerian rice economy. In: Kormawa P, Toure AA (eds) Rice policy and food security in sub-Saharan Africa. Proceedings of a workshop held on 7-9 November 2005, Cotonou, Benin. Africa Rice center, Cotonou, Benin, pp 125-140.
- Oerke E-C (2006) Crop losses to pests. The Journal of Agricultural Science 144: 31.
- Ou SH (1985) Rice diseases, 2nd ed. Commonwealth Mycological Institute, Kew, Surrey, UK.
- Francis-Otokito GL, Umechuruba CI (2003) Fungal diseases of rice in nursery farms in Bayelsa state of Nigeria. Global J Agri Sci 2: 90-92.
- Ejechi BO, Souzey JA (1999) Inhibition of biodeterioration of yam tuber Dioscorea rotundata Poir in storage with phenolic extract of Acalypha hispida Burm f leaves. J Stored Products Res 35: 127-134.
- Prajapati HN, Patel JK, Patil RK (2014) Lasiodiplodia theobromae: the causal agent of root rot and collar rot of biofuel plant (Jatropha curcas) and its variability. Plant Disease Res 29: 174-177.
- Xie HH, Wei JG, Liu F, Pan XH, Yang XB (2014) First Report of Mulberry Root Rot Caused by Lasiodiplodia theobromae in China. Plant Disease 98: 1581-1581.
- Nam MH, Park MS, Kim HS, Kim T-il, Lee EM, et al. (2016) First Report of Dieback Caused by Lasiodiplodia theobromae in Strawberry Plants in Korea. Mycobiol 44: 319-324.
- Twumasi PA, Ohene-Mensa G, Moses E (2014) The rot fungus Botryodiplodia theobromae strains cross infect cocoa, mango, banana and yam with significant tissue damage and economic losses. African J Agri Res 9: 613-619.
- Dugan FM (2017) The Identification of Fungi: An Illustrated Introduction with Keys, Glossary, and Guide to Literature. The American Phytopathological Society. St Paul, USA.
- McGee DC, Kellock AW (1974) Fusarium avenaceum, a seed-borne pathogen of subterranean clover roots. Australian J Agri Res 25: 549.
- Osabuohien ES, Okorie UE, Osabohien RA (2018) Rice production and Processing in Ogun State, Nigeria: Qualitative Insights from Farmers’ Association. In: Obayelu AE (editors) Food Systems Sustainability and Environmental Policies in Modern Economies: IGI Global, Hershey PA, pp 188–215.
- Oyetunji OE, Peluola CO, Nwilene FE, Akinwale G, Togola A, et al. (2012) Effect of fungi–termite interaction on the chlorophyll content of three rice varieties grown on ultisol soil of Ikenne, south-west Nigeria. Archives Of Phytopathology And Plant Protection 45: 1292-1303.
- Msikita W, Bissang B, James BD, Baimey H, Wilkinson HT, et al. (2005) Prevalence and Severity of Nattrassia mangiferae Root and Stem Rot Pathogen of Cassava in Bénin. Plant Disease 89: 12-16.
- Aigbokhan O, Claudius- Cole A, Ikotun B (2017) Leaf Tip Die- Back of Yucca elephantipes by Lasiodiplodia theobromae Pat. and Production of Phytotoxin in Filtrate and Infected Leaves. Journal of Experimental Agriculture International 16: 1-8.
- Ogundana SK (1983) Life Cycle of Botryodiplodia theobromae, a Soft Rot Pathogen of Yam. Journal of Phytopathology 106: 204-213.
- Phipps PM, Porter DM (1998) Collar Rot of Peanut Caused by Lasiodiplodia theobromae. Plant Disease 82: 1205-1209.
- Rosado AWC, Machado AR, Freire F das CO, Pereira OL (2016) Phylogeny, Identification, and Pathogenicity of Lasiodiplodia Associated with Postharvest Stem-End Rot of Coconut in Brazil. Plant Disease 100: 561-568.
- Rose S, Parker M, Punja ZK (2003) Efficacy of Biological and Chemical Treatments for Control of Fusarium Root and Stem Rot on Greenhouse Cucumber. Plant Disease 87: 1462-1470.
- Burgess TI, Barber PA, Mohali S, Pegg G, de Weer W, et al. (2006) Three new Lasiodiplodia spp. from the tropics, recognized based on DNA sequence comparisons and morphology. Mycologia 98: 423-435.
- Akpheokhai LI (2013) Pathogenicity of Heterodera sacchari (Luc And Merny) and its interaction with Botryodiplodia theobromae (PAT) on some nerica rice cultivars. PhD, University of Ibadan.
Citation: Claudius-Cole AO (2018) Lasiodiplodia theobromae in the Root Rot Disease Complex of Rice. J Rice Res 6: 197. DOI: 10.4172/2375-4338.1000197
Copyright: © 2018 Claudius-Cole AO. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4240
- [From(publication date): 0-2018 - Apr 30, 2025]
- Breakdown by view type
- HTML page views: 3331
- PDF downloads: 909