Case Report Open Access
Localized Periodontal Disease Induced by Bacterial Plaque and Palatal Radicular Groove: Treatment and Considerations
Jose Ricardo Kina*
Department of Surgery and Integrated Clinic, Araçatuba School of Dentistry, São Paulo State University - UNESP, Araçatuba, SP, Brazil
- *Corresponding Author:
- José Ricardo Kina, DDS, MS, PhD
Department of Surgery and Integrated Clinic
Araçatuba School of Dentistry, São Paulo State University - UNESP
Araçatuba, SP, Brazil
Tel: +55-18-3636-3200
Fax: +55-18-3636-3200
E-mail: kinajr@hotmail.com
Received date: January 27, 2014; Accepted date: May 8, 2014; Published date: May 15, 2014
Citation: Jose Ricardo Kina (2014) Localized Periodontal Disease Induced by Bacterial Plaque and Palatal Radicular Groove: Treatment and Considerations. J Interdiscipl Med Dent Sci 2:122. doi:10.4172/2376-032X.1000122
Copyright: © 2014 Kina JR. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at JBR Journal of Interdisciplinary Medicine and Dental Science
Abstract
The palatal radicular groove is a morphological tooth defect, which act as local predisposing risk factor favoring accumulation of the bacterial plaque, permitting microbial invasion via root groove way, directly into periodontal structures. A patient diagnosed with palatal radicular groove and a localized periodontal disease was treated by procedures to control bacterial action and procedures to eliminate local predisposing risk factor. To treat the periodontal bone defect a sequelae of periodontal disease, a guided tissue regeneration technique was applied by using allograft and xenograft materials associated with a resorbable demineralized bovine cortical bone membrane. The objective of surgical regenerative procedure was to recover the periodontal tissues nearly as they were before periodontal disease destruction.
Keywords
Bacteria; Etiology; Guided tissue regeneration; Periodontal disease
Introduction
The etiology of inflammatory periodontal disease is a complex interaction of bacteria, and predisposing risk factors as local factors and systemic factors [1-6]. Bacteria colonizing and growing at the gingival margin may be the main cause of the periodontal tissue inflammation, pocket development, and periodontal tissue destruction [7-9]. However, the role of local predisposing risk factors in the etiology of periodontal disease may be determinant to induce localized destruction of periodontal tissues [1,2,10,11]. An association between bacteria and local predisposing risk factors seems to be necessary to induce localized periodontal disease by favoring microbial colonization and growth or/and altering the local susceptibility of the periodontal tissues to be damaged by the bacteria onslaught [10,11]. One of the local predisposing factors may be maxillary incisors radicular grooves. The radicular grooves may be a morphological defect located in palatal region of the upper incisors teeth, extending beyond the cementoenamel junction, along the root surface [12-14]. Palatal radicular groove has been implicated as the local predisposing risk factor for periodontal disease, due the extreme difficult to maintain the area free of microbial deposits [12,14]. Moreover, such sites have a high frequency of pocket formation due bacteria and microbial products invasion directly via radicular groove way into periodontal tissues [13]. Consequently to treat a localized periodontal disease which is provoked by bacteria and palatal radicular groove is necessary to establish a plan of treatment that needs to be focused in plaque control and also in elimination of local predisposing risk factor. After providing a control in all etiologic factors associated with local periodontal disease, the effort to reach health in periodontal tissues was centered in eliminating the anatomic defects produced during periodontal disease activity. Then a regenerative periodontal procedure as guided tissue regeneration and bone graft was applied, attempting to gain new clinical attachment, improve bone level, and minimize postoperative recession [15].
Clinical Report
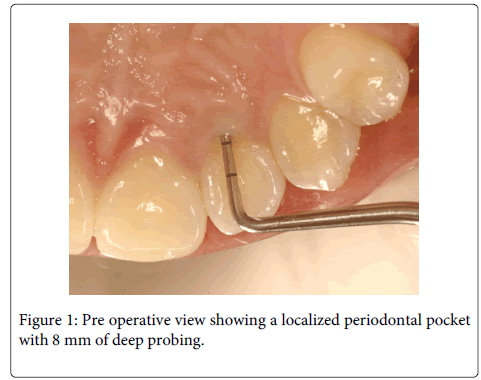
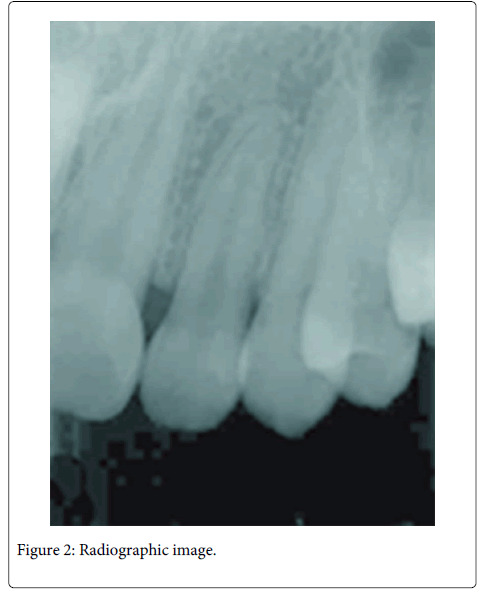
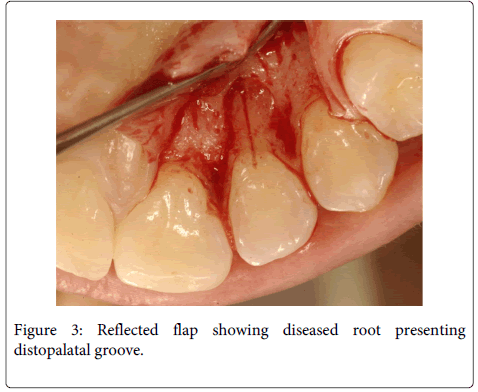
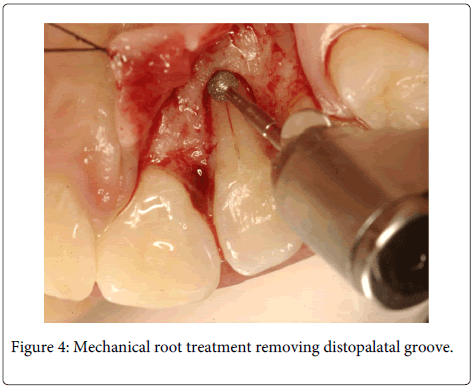
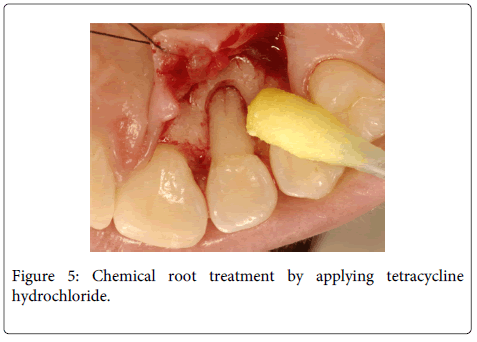
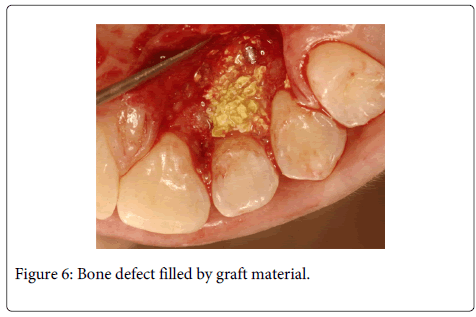
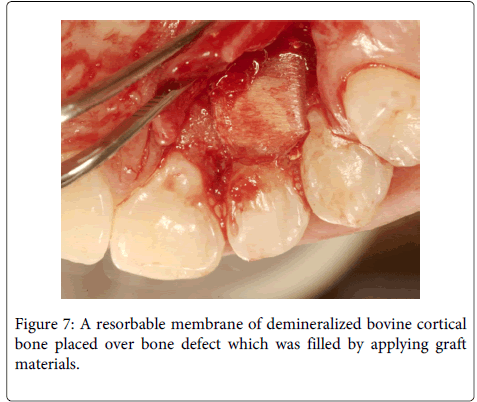
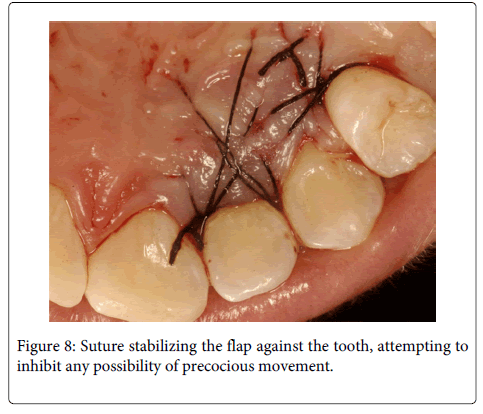
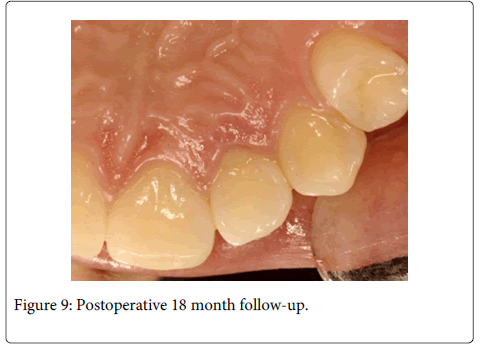
A 20 years old female individual was referred to the FOA-UNESP-ARAÇATUBA DENTAL SCHOOL (BRASIL) with history of pain and suppuration in upper anterior region. Clinical examination revealed a localized deep periodontal pocket and a palatal radicular groove in the left upper lateral incisor (Figure 1). The radiographic image showed a normal appearance due the periodontal alteration was localized in the lingual side (Figure 2). The therapy of localized periodontal disease was based in plaque control and root groove elimination. To achieve these goals, a full-thickness mucoperiosteal flap was reflected to access diseased root surface and then, all diseased periodontal soft tissue were curetted carefully (Figure 3). Subsequently, were applied mechanical and chemical root biomodification to promote favorable characteristics on a pathologically root surface which was inside of the contaminated periodontal pocket. The mechanical root biomodification was applied by using scaling and root planning, including the use of rotatory instruments (Kavo, Joinville, Brazil) to remove mainly radicular groove [16,17] (Figure 4). The chemical root biomodification was centered in application of acid therapy by using tetracycline hydrochloride 500mg diluted in 5ml of distilled solution on root surface mechanically treated [18] (Figure 5). Then regenerative procedures techniques were applied to treat the sequel produced by periodontal disease activity [15,19-21]. After mechanical and chemical root biomodification, the anatomic defect produced by periodontal disease was filled by using a bovine cortical bone; particle size 250-1000mm (Gen-Ox, Baumer Co., Mogi Mirim, Brazil) blended 1:1 with a microgranular hydroxyapatite, mean particle size 5 µm (Gean-Pro, Baumer Co., Mogi Mirim, Brazil) homogeinized with blood [19,20] (Figure 6). A resorbable demineralized bovine cortical bone membrane (Gen-Derm, Baumer Co., Mogi Mirim, Brazil) was applied over bone defect and graft material [21] (Figure 7). The material was tightly secured to the tooth by a sling flap suture (Figure 8). Postoperative care included systemic minocycline 100 mg orally every 12 hours for 5 days and local (Chlorhexidine digluconate solution 0.12) antimicrobial therapy. The objective of surgical procedure was to gain new clinical attachment, improve bone level, and minimize postoperative recession. The present clinical results after 1 year and 6 months of periodic control allow concluding that the procedures and material applied to treat this case may be valuable (Figure 9).
Discussion
The etiology includes the sum of evidences related to the causes of a disease. The etiological concept of the inflammatory periodontal disease is an exceedingly complex interaction of bacteria and predisposing risk factors [1-6]. The predisposing risk factor may be an inherent characteristic associated with an increased rate of a subsequently occurring disease, but does not necessarily cause the disease. In periodontal disease, the predisposing risk factors may be defined as local environmental factors, behavioral factors in nature and systemic factors, which may be responsible in providing an ideal environment for bacterial colonization and/or fragility in a determinate tooth or teeth and adjacent periodontal tissues and/or interference in the inflammatory process [10,11]. Local environmental factor may interfere in the fragile equilibrium of the gingival sulcus defense by favoring microbial colonization and growth or/and altering the local susceptibility of the periodontal tissues to be damaged by the bacterial onslaught [10,11]. Then opportunist bacteria may initialize periodontal tissue destruction by intense interaction with cells of the inflammatory process. When predisposing risk systemic factors affect the individual, a deficient interaction of the bacteria with cells of the inflammatory process may occur, inducing an incomplete defensive curse, leading to the periodontal destruction [5,6,11]. Periodontal disease could be considered as sequel of the inflammatory reaction, which must be always active, protecting individual against infection and possible septicemia, by bacteria present in the gingival sulcus, a critical area where junctional epithelium is an exclusive and fragile structure, separating connective tissue from an infected humid and warm oral environment [22]. Periodontitis begins with microbial challenge, which induce a host-mediate response and destruction of periodontal tissue, caused by bursts of clastic cell activity, triggered by hyperactivated or primed polymorphonuclear leukocytes and factors generated during the inflammatory acute phase, such as eicosanoides and various proteins as enzymes that cause damage and rupture of the periodontium, promoting periodontal pocket establishment [23,24]. Periodontal pocket development is the most important clinical and pathologic alteration associated with inflammatory periodontal disease and also may be considered as a local predisposing risk factor for periodontal disease progression, by generating an anaerobic environment to be contaminated as a result of repeated infection by the various species or combination of the species as exogenous anaerobic and facultative bacteria [25-27]. These putative periodontal pathogens and their products may induce substantial pathological alterations, essentially in root surface exposed to the contaminated periodontal pocket [10,28-31]. On the other side, due bacterial approximation to the ulcerated pocket epithelium, infected periodontal pocket also could be an infectious focus linked to the various systemic disorders, probably led by anachoresis, a process associated with dissemination of the microorganisms or/and toxics products into blood stream, assisting or causing infection in the various vital organs [32]. To prevent or to treat any disease, all etiologic factors must be controled and/or host defense improved to promote homeostasis in diseased areas through a long stated period [11]. When a localized periodontal pocket is diagnosed, the treatment must be via eliminating or controlling all etiological and also predisposing risk factors, which may aid bacteria to develop a specific, localized and destructive periodontal disease [1-6]. The palatal radicular groove may be a local predisposing risk factor, inducing the area to develop a localized periodontal disease, for providing via radicular groove way, bacterial and microbial products invasion directly into periodontal structures [12-14]. In all periodontal disease treatment is essential to eliminate or/and to control all etiologic factors evolved with disease progression as bacteria and palatal radicular groove [13]. However, in determinate cases also is necessary to treat the disease sequel by using regenerative procedures to try to recover periodontal tissues destructed during the disease activity [15,19-21]. In this case was applied guided tissue regeneration associated with grafts techniques to treat the sequel promoted by periodontal disease [19-21]. An essential step in regenerative therapy is to alter the periodontitis-affected root surface to make it a hospitable substrate to support and encourage migration, proliferation, proper phenotypic expression of periodontal connective tissue progenitor cells and attachment [16,17,28-31,33-35]. Periodontitis produces considerable changes of the tooth root surface, as loss of collagen and consequently hipermineralization [33,34]. Bacterial plaque and calculus penetrate the cementum and/or dentin of the root [10,33,34].The root surface thus becomes toxic and unsuitable for the new connective tissue attachment necessary for periodontal regeneration [33]. Mechanical and chemical therapy may alter the periodontitis affected root surface to make it a hospitable substrate to sustain and stimulate migration, attachment, proliferation, and proper phenotypic expression of periodontal connective tissue progenitor cells [31,33-35]. Mechanical root biomodification was targeted in scaling and root planning including the use of rotatory instruments to eliminate calculus and bacterial plaque and the surface of the cementum and dentin which were infiltrated by these pathogenic deposits, and also to remove radicular groove [10,13,16,17,31,33]. Chemical biomodification was centered on acid therapy by using tetracycline hydrochloride, to promote a lingering antimicrobial action, inhibition of collagenase, augmentation of cells attachment, to remove the smear layer left by mechanical instrumentation and to expose the intrinsic collagen of the root dentin [18,31,35]. To treat anatomic bone defect, a sequel produced by periodontal disease destructive activity phase, were applied the guided tissue regeneration technique associated with the use of a bovine cortical bone, blended with a microgranular hydroxyapatite, homogeinized with blood [15,19-21]. Over bone defect filled with graft materials, was placed a resorbable membrane of demineralized bovine cortical bone, trying to exclude gingival epithelium from the grafts and root surface [15,21]. The resorbable membrane of demineralized bovine cortical bone obtained after organic solvents, peroxides and acid treatment, was constituted mainly by fibrillar type I collagen, reabsorbed at 30 days [21]. The space created by graft materials may allow cells from the periodontal ligament to establish an interaction to populate the root surface in order to achieve new connective tissue attachment to the root surface, preventing epithelial migration and an establishment of the long junctional epithelium until the base of the original periodontal bone defect [15,22]. Another aspect regarding to the biologic mechanism that facilitates healing of lost periodontium by using guided tissue regeneration is attributed to stabilization of the root-clot-graft material interface by resorbable membrane favoring regenerative attempt, due the clot must form and adhere to the root surface for enough time to allow for proper wound maturation, including connective tissue maturation and development [36-39]. In surgical periodontal therapy when periodontal wounds are closed and sutured, one of the wound margins is an avascular and rigid periodontitis-affected and altered root surface and another wound margin is a soft tissue vascular flap margin [18,28-30,33-39]. This detail induces a fibrin clot formation with a fragile initial attachment to the altered root surface, to prevent epithelial down growth and to form a scaffold for development of a cell and collagen fiber attachment mechanism [38,39]. Then a fibrin clot adherent to the altered root surface is a fragile but vital part of early periodontal wound healing. The fibrin clot must form and adhere to the altered root surface for adequate time to allow for proper wound maturation, including connective tissue formation and development, before a new connective tissue attachment can occur [38,39]. If this first series of events is disrupted, or if the initial attachment of fibrin or/and immature connective tissue is ruptured, then a pattern of healing including a long junctional epithelium to the base of the original periodontal pocket is expected to occur. In periodontal surgery procedure the early wound healing stability is easily disturbed inducing a disruption in the fibrin clot, which is frail attached to the altered root surface [39]. This occurrence allows in this unique healing site a communication between the underlying connective tissue and the contaminated, humid and warm oral environment as healing progresses. To prevent infection, epithelial proliferations extend apically on the tooth aspect, establishing a long junctional epithelium adhered to the root surface by hemidesmosomes [11,22,38,39]. The long junctional epithelium is a fragile structural and functional adaptation which substitute attached gingival ligament, enabled to produce a defensive biological mechanism, responsible to control the constant microbial challenge by isolating the exposed connective tissue in the inner surface of the wound from contaminated oral environment [11,22]. Then the suture also may be considered as an important factor during regenerative attempts, stabilizing and protecting the root-clot-graft material interface in earlier period of wound healing. In this case the flap margin was tried to be sutured in a manner that could be well stabilized against the tooth, limiting the possibilities of the movement. Furthermore in this specific case, the periodontal bone defect localized at lingual side, present a design with favorable dimension, having width similar of the root shape and height measurement significantly small, permitting few contact among exposed, altered, avascular and rigid root surface, the graft, the membrane and the soft tissue vascular flap margin meaning that the most part of the soft tissue flap was placed and supported by adjacent healthy and vascular bone tissues which may induce a production of the a stable fibrin clot, an essential step in tissue regeneration. Anyway, the soft tissue flap margin was tried to be sutured in a manner that could be well stabilized against the tooth, limiting the possibilities of the movement. The patient is maintained over periodic plaque control supervision, to keep the area clinically health with absence of recidivism of the localized periodontal disease. The present clinical results after 1 year and 6 months of periodic control allow concluding that the procedures and material applied to treat this case may be biologically and clinically valuable to be used in regenerative periodontal procedures.
References
- Pennel BM, Keagle JG (1977) Predisposing factors in the etiology of chronic inflammatory periodontal disease. J Periodontol 48: 517-532.
- Genco RJ (1996) Current view of risk factors for periodontal diseases. J Periodontol 67: 1041-1049.
- Dowsett SA, Archila L, Foroud T, Koller D, Eckert GJ, et al. (2002) The effect of shared genetic and environmental factors on periodontal disease parameters in untreated adult siblings in Guatemala. J Periodontol 73: 1160-1168.
- Polson AM, Meitner SW, Zander HA (1976) Trauma and progression of marginal periodontitis in squirrel monkeys. IV Reversibility of bone loss due to trauma alone and trauma superimposed upon periodontitis. J Periodontal Res 11: 290-298.
- Sollecito TP, Sullivan KE, Pinto A, Stewart J, Korostoff J (2005) Systemic conditions associated with periodontitis in childhood and adolescence. A review of diagnostic possibilities. Med Oral Patol Oral Cir Bucal 10: 142-150.
- Preshaw PM, Bissett SM (2013) Periodontitis: oral complication of diabetes. EndocrinolMetabClin North Am 42: 849-867.
- LOE H, THEILADE E, JENSEN SB (1965) EXPERIMENTAL GINGIVITIS IN MAN. J Periodontol 36: 177-187.
- Theilade E, Wright WH, Jensen SB, Löe H (1966) Experimental gingivitis in man. II. A longitudinal clinical and bacteriological investigation. J Periodontal Res 1: 1-13.
- Lindhe J, Hamp S, Löe H (1973) Experimental periodontitis in the beagle dog. J Periodontal Res 8: 1-10.
- Kina JR, Kina J, Kina EF, Kina M, Soubhia AM (2008) Presence of bacteria in dentinal tubules. J Appl Oral Sci 16: 205-208.
- Kina JR, Suzuki TYU, Kina J, Kina M, Kina EFU (2013) Reparative phase events on periodontal disease progression: interpretation and considerations. Int J Microbiol Res 5: 439-44.
- Hou GL, Tsai CC (1993) Relationship between palato-radicular grooves and localized periodontitis. J ClinPeriodontol 20: 678-682.
- Estrela C, Pereira HL, Pécora JD (1995) Radicular grooves in maxillary lateral incisor: case report. Braz Dent J 6: 143-146.
- Everett FG, Kramer GM (1972) Thedisto-lingual groove in the maxillary lateral incisor; a periodontal hazard. J Periodontol 43: 352-361.
- Aukhil I, Pettersson E, Suggs C (1986) Guided tissue regeneration. An experimental procedure in beagle dogs. J Periodontol 57: 727-734.
- Cercek JF, Kiger RD, Garrett S, Egelberg J (1983) Relative effects of plaque control and instrumentation on the clinical parameters of human periodontal disease. J ClinPeriodontol 10: 46-56.
- Proye M, Caton J, Polson A (1982) Initial healing of periodontal pockets after a single episode of root planing monitored by controlled probing forces. J Periodontol 53: 296-301.
- Frantz B, Polson A (1988) Tissue interactions with dentin specimens after demineralization using tetracycline. J Periodontol 59: 714-21.
- Ferreira GR, Cestari TM, Granjeiro JM, Taga R (2004) Lack of repair of rat skull critical size defect treated with bovine morphometric protein bound to microgranularbioabsorbable hydroxyapatite. Braz Dent J 15: 175-180.
- Sicca CM, Oliveira RC, Silva TL (2000) Microscopic and biochemical analysis of the cellular response to cortical bovine grafts implanted in rat subcutaneous. Effect of particle sizes. Revista da Faculdade de Odontologia de Bauru 8:1-10.
- de Oliveira RC, Menezes R, Cestari TM, Taga EM, Taga R, et al. (2004) Tissue response to a membrane of demineralized bovine cortical bone implanted in the subcutaneous tissue of rats. Braz Dent J 15: 3-8.
- Bosshardt DD, Lang NP (2005) Thejunctional epithelium: from health to disease. J Dent Res 84: 9-20.
- Craig RG, Yip JK, So MK, Boylan RJ, Socransky SS, et al. (2003) Relationship of destructive periodontal disease to the acute-phase response. J Periodontol 74: 1007-1016.
- Dennison DK, Van Dyke TE (1997) The acute inflammatory response and the role of phagocytic cells in periodontal health and disease. Periodontol 2000 14: 54-78.
- Takata T, Donath K (1988) The mechanism of pocket formation. A light microscopic study on undecalcified human material. J Periodontol 59: 215-221.
- Socransky SS, Haffajee AD (2005) Periodontal microbial ecology. Periodontol 2000 38: 135-187.
- Dzink JL, Socransky SS, Haffajee AD (1988) The predominant cultivable microbiota of active and inactive lesions of destructive periodontal diseases. J ClinPeriodontol 15: 316-323.
- Aleo JJ, De Renzis FA, Farber PA, Varboncoeur AP (1974) The presence and biologic activity of cementum-bound endotoxin. J Periodontol 45: 672-675.
- Aleo JJ, De Renzis FA, Farber PA (1975) In vitro attachment of human gingival fibroblasts to root surfaces. J Periodontol 46: 639-645.
- Aleo JD, De Renzis FA (1976) Proliferation of cells in vitro after long-term exposure to endotoxin. J Dent Res 55: 1139.
- Aleo JJ, Vandersall DC (1980) Cementum. Recent concepts related to periodontal disease therapy. Dent Clin North Am 24: 627-650.
- Matthews DC (2000) Periodontal medicine: a new paradigm. J Can Dent Assoc 66: 488-491.
- Polson AM, Caton J (1982) Factors influencing periodontal repair and regeneration. J Periodontol 53: 617-625.
- Polson AM (1986) The root surface and regeneration; present therapeutic limitations and future biologic potentials. J ClinPeriodontol 13: 995-999.
- Wikesjö UM, Claffey N, Nilvéus R, Egelberg J (1991) Periodontal repair in dogs: effect of root surface treatment with stannous fluoride or citric acid on root resorption. J Periodontol 62: 180-184.
- Polson AM, Proye MP (1982) Effect of root surface alterations on periodontal healing. II. Citric acid treatment of the denuded root. J ClinPeriodontol 9: 441-454.
- Clark RAF (1993) Biology of dermal wound repair dermatological clinics. J Invest Dermatol 11:647-61.
- Polimeni G, Xiropaidis AV, Wikesjö UM (2006) Biology and principles of periodontal wound healing/regeneration. Periodontol 2000 41: 30-47.
- Wikesjö UM, Selvig KA (1999) Periodontal wound healing and regeneration. Periodontol 2000 19: 21-39.
Relevant Topics
- Cementogenesis
- Coronal Fractures
- Dental Debonding
- Dental Fear
- Dental Implant
- Dental Malocclusion
- Dental Pulp Capping
- Dental Radiography
- Dental Science
- Dental Surgery
- Dental Trauma
- Dentistry
- Emergency Dental Care
- Forensic Dentistry
- Laser Dentistry
- Leukoplakia
- Occlusion
- Oral Cancer
- Oral Precancer
- Osseointegration
- Pulpotomy
- Tooth Replantation
Recommended Journals
Article Tools
Article Usage
- Total views: 17108
- [From(publication date):
June-2014 - Aug 16, 2025] - Breakdown by view type
- HTML page views : 12480
- PDF downloads : 4628