Review Article Open Access
Medical Management of “Uncomplicated” Colonic Diverticular Disease: A Review on Poorly Absorbed Antibiotics
Virginia Festa*, Marco Bianchi, Angelo Dezi, Roberto Luchetti and Maurizio KochGastroenterology and Liver Unit, Azienda Ospedaliera San Filippo Neri, 00135 Rome, Italy
- *Corresponding Author:
- Virginia Festa
Gastroenterology and Liver Unit
Azienda Ospedaliera San Filippo Neri
Via Martinotti 20, 00135 Rome, Italy
Tel: +39-63-3062444
Fax: +39-63-3062641
E-mail: v.festa@sanfilipponeri.roma.it
Received date: March 11, 2014; Accepted date: April 28, 2014; Published date: May 05, 2014
Citation: Festa V, Bianchi M, Dezi A, Luchetti R, Koch M (2014) Medical Management of “Uncomplicated” Colonic Diverticular Disease: A Review on Poorly Absorbed Antibiotics. J Gastroint Dig Syst 4:195. doi:10.4172/2161-069X.1000195
Copyright: © 2014 Festa V, et al. This is an open-access article distributed under the terms of the CreativeCommons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Diverticular disease of the colon is a common gastrointestinal disease. Although most patients remain asymptomatic for their whole life, about 20%-25% present symptoms related to “diverticular disease”. Current guidelines recommend only the use of high spectrum antibiotics in the initial treatment of acute diverticulitis. Several randomized trials suggest a role for a poorly absorbed antibiotic, such as rifaximin, in soothing symptoms and preventing complications such as diverticulitis. This review will highlight the role of long term administration of rifaximin in the treatment of symptomatic uncomplicated diverticular disease. The evidence suggests that rifaximin is effective for obtaining symptomatic relief and shows a positive trend in preventing complications.
Keywords
Rifaximin; Laparoscopic lavage; Diverticular disease
Introduction
“In the past few years, our understanding of diverticulitis has been turned on its head. Causative factors? Say goodbye to the "no seeds and pits" diet. Need for surgery? The simple rule of "2 attacks then operate" is gone. Free perforation? Instead of colostomy, do a laparoscopic lavage. And while the incidence of acute diverticulitis in the young is increasing, the need for aggressive surgical management in this age group is now open to question” by Madoff [1].
The Changing Face of Epidemiology
Diverticular disease of the colon is one of the most common and costly gastrointestinal disease, and its face is sharply changing, its prevalence increases with age from 5% in the fifth decade of life to 50% in the ninth decade [2,3]. Overall annual age-adjusted admissions for acute diverticulitis are strikingly increasing. In the United States population, a 26% increase between 1998 and 2005 has been recorded [4]. Rates of admission increased more rapidly within patients aged 18-44 years (+82%) and 45-74 years (+36%). Elective operations for diverticulitis rose from 16100 to 22500 per year during the same time period (+29%), also with a more rapid increase (73%) in rates of surgery for individuals aged 18 to 44 years [4].
Sandler et al. [5] have been estimated that, in United States, 3400 deaths could be attributed to diverticular disease, with an economic burden in term of direct health care costs of $2.4 billion [6], and the medical impact of this disorder is likely to increase substantially as the population get older. In Europe also, the incidence per 100000 person-years of colonic diverticular bleeding increased over time (from 3.3 in 1996 to 8.0 events in 2005). A small increasing trend was observed for the incidence per 100 000 person-years of intestinal perforations (from 1.5 to 2.3 events) [7,8]. Although most patients remain asymptomatic for their whole life, about 20%-25% present symptoms related to “diverticular disease” at some point [9-11]. Diverticular disease is usually classified as symptomatic uncomplicated disease (diverticulosis), recurrent symptomatic disease or complicated disease [12,13]. Symptomatic uncomplicated disease is characterized by abdominal pain (principally colicky left iliac fossa pain), and altered bowel habits [12-14]. After a first symptomatic episode, 20% of the treated patients develop recurrent symptoms [13]. Among patients with diverticular disease, 25% develop complications [4,15].
Acute diverticulitis is the most common complication of diverticular disease: it will develop in 10%-25% of people with diverticula [3]. Diverticulitis recurrence occurs in 7%-42% of people with diverticular disease, and after the first episode the calculated yearly risk of relapse is 3% [16]. Fifty percent of recurrence occurs within 1 year of the initial episode, and 90% within 5 years [17,18]. A cyclical increase in diverticulitis during the summer months has been noted: Rocco Ricciardi and coll. monitored rates of non elective diverticulitis admissions from 1997 through 2005, as recorded in the United States Nationwide Inpatient Sample (NIS) database, they have shown fewer non-elective diverticulitis admissions in February, with 25% increased rate in August [19]. Surgery, when performed in urgency and in septic complications, can achieve high mortality rate, up to 26% [20-22].
The Main Risk Factor for Complications: Aspirin or Non-Steroidal Antinflammatory Drugs
The more and more wider use of Aspirin or Nonsteroidal anti-inflammatory drugs (NSAIDs) is a possible cause for the incremental rate of diverticular disease complications.
NSAIDs, including aspirin, are a well-known cause of upper gastrointestinal tract complications, and are also implicated in lower gastrointestinal injury. In randomized trials of patients with rheumatoid or osteoarthritis, 30%–50% of all serious gastrointestinal events associated with NSAIDs were localized to the lower gastrointestinal tract, with diverticulitis and diverticular bleeding as the most common aetiologies [23,24].
Although a number of case-control studies have shown a significantly higher prevalence of NSAID use among cases with complications of diverticular disease (diverticulitis and bleeding) compared with controls, risk estimates vary widely, with odds ratios ranging from 1.8 to 16.0 [24-29]. Harder risk estimates come from the Health Professionals Study cohort [30]. This is a report on a cohort of 47.210 US men, who were 40-75 years old at baseline in 1986, and presented 939 cases of diverticulitis during a 22-year period of follow-up evaluation. After adjustment for risk factors, men who used aspirin regularly (> 2 times/wk) had a multivariable hazard ratio (HR) of 1.25 (95%CI: 1.05-1.47) for diverticulitis, compared with nonusers of aspirin and NSAIDs. Use of aspirin on a daily basis was associated with the highest risk of diverticulitis (multivariable HR, 1.46; 95%CI: 1.13-1.88). Increased risk of diverticulitis is reported in regular users of nonaspirin NSAIDs (multivariable HR, 1.72; 95%CI: 1.40-2.11), compared with “non users” [31]. Although Aspirin and NSAIDs use is a relevant risk factor for diverticulitis, current use of aspirin does not result in an increased risk of diverticular perforation [32] and probably plays a role in older patients only, since complicated diverticulitis incidence is rising in younger patients.
What are the Effects of Medical Treatments for Uncomplicated Diverticular Disease?
Fibres and laxatives
In uncomplicated diverticular disease, Bran or ispaghula husk appear not to be superior to placebo in relieving symptoms at 16 weeks (very low-quality evidence) [12]. Methylcellulose results no more effective at 3 mo at reducing mean symptom scores in people with uncomplicated diverticular disease compared with placebo (low-quality evidence) [12].
Antispasmodics
Clinical Evidence found no direct results from randomized controlled trial (RCTs) about antispasmodics in the treatment of people with uncomplicated colonic diverticular disease [12].
Mesalazine
Compared with no treatment, Mesalazine may be more effective at 4 years than no treatment at reducing recurrence of symptoms of diverticulitis in people previously treated for an episode of acute diverticulitis (very low-quality evidence) [12]. Clinical Evidence found no systematic review, but some RCTs.
A RCT, by Trespi and Coll [33] compared 8 weeks of treatment with oral mesalazine (400 mg twice daily) versus no treatment. People in both groups had received intramuscular sulbactam–ampicillin (1.5 g twice daily) and oral Rifaximin (400 mg twice daily) for 7 d before randomisation. They found that mesalazine reduced symptomatic recurrence of diverticulitis at 4 years compared with no treatment [12/81 (15%) with mesalazine vs 39/85 (46%) with no treatment; Relative Risk (RR) 0.32, 95%CI: 0.18-0.57; Number Needed to Treat (NNT)=4, 95%CI: 3-6]. However, the RCT provided insufficient information on several factors. Methods for determining symptom scores, including the assessment and diagnosis of pain, were not reported. In patients with symptomatic uncomplicated diverticular disease, two recent trials have shown that cyclic treatment with mesalazine (800 mg of mesalamine b.i., for 10 d) seems to be clinical, although not statistically effective in reducing the incidence of diverticulitis [33-35].
Rifaximin
Concerning medical therapy, current guidelines actually recommend only the use of high spectrum antibiotics in the initial treatment of acute diverticulitis [13,36,37].
Rifaximin is the only oral antibiotic listed as potentially useful also on Clinical Evidence (http://clinicalevidence.bmj.com/ceweb) or Dynamed (http://DynaMedEditor@ebscohost.com) [37]. Clinical trials have provided evidence of the substantial benefit of Rifaximin-alfa (R-α), a poor absorbable antibiotic, in diverticular disease, showing the efficacy of the drug in reducing symptoms in most patients with uncomplicated disease [38-41].
In 2011 our group carried out a meta-analysis of RCTs with R- α plus fiber supplementation, to provide an evidence-based assessment of its potential efficacy in modifying the clinical course of the disease, and in primary prevention of diverticulitis [42].
The objective of this meta-analysis was to compare the efficacy of R-α plus fiber supplementation vs placebo on 1-year symptom disappearance and complication rate in patients with symptomatic uncomplicated diverticular disease.
This study included RCTs of patients with symptomatic uncomplicated diverticular disease with the following design: R-α therapy, or placebo, followed by clinical re-evaluation (at least every 3 mo) to assess symptom relief and complications.
We found 4 RCTs [38-41]. A total of 1660 patients were enrolled: 970 were randomized to treatment with the poorly absorbed antibiotic, and 690 were randomized to no treatment. In all studies the antibiotic used was R-α 400 mg bid for 7 d every month; all patients in both the treated group and in control group, received a standard supplement of dietary fibers.
In all studies the diagnosis of symptomatic uncomplicated diverticular disease was made by double contrast barium enema and/or colonoscopy. Clinical evaluation was performed on admission and at 2-4 mo interval, for the following 12 mo in 4 studies. All studies used different symptom score system based on several clinical variables. However, the review focused only the dichotomous analysis (presence/absence of any symptom).
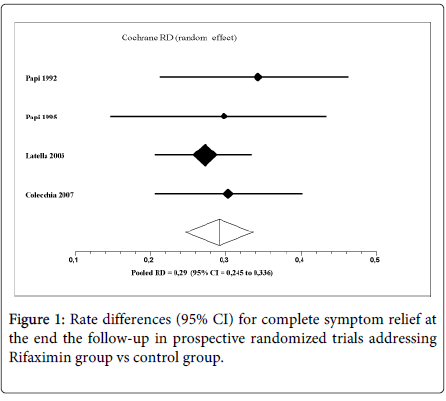
Two hundred forty-one out of 690 patients in control group (pooled rate 34.9%) were symptom-free at end the follow-up, compared to 621 out of 970 patients in the treatment group (pooled rate 64.0%). The pooled rate difference (RD) for complete symptom relief in favor of R- α group was 29.0% (95% CI 24.5%-33.6%; P < 0.0001; NNT= 3). No heterogeneity was found (Q=1.12, df=3, P=0.77; I2=0%) (Figure 1).
Twenty-two out of 690 patients in control group (pooled rate 3.2%) suffered at least one complication, during 1-year follow up, compared to 15 out of 970 patients in the treatment group (pooled rate 1.5%).
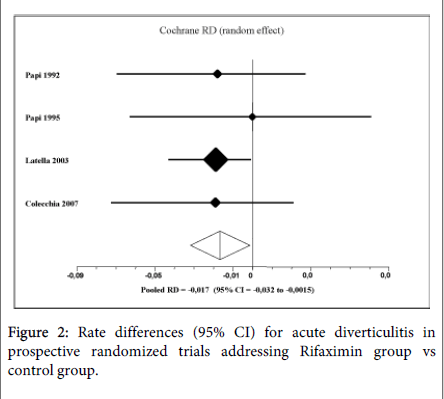
The pooled RD for complication rate in favor of R-α was -1.7% (95%CI: -3.2% to -0.15%, P=0.03; NNT=59). No heterogeneity was found (Q=0.57, df=3, P=0.9; I2=0%). Considering only acute diverticulitis, 20 out of 690 patients in control group (2.8%) suffered of this complication compared to 10 out of 970 patients in the treatment group (1.0%). The pooled RD for diverticulitis rate in the treatment group was -1.9% (95%CI: -3.4% to -0.57%, P = 0.0057; NNT=50) (Figure 2).
Three out of 4 trials reported side effect data. No significant difference was found between control group and treatment group. Twenty-two out of 690 patients in control group (pooled rate 3.2%) suffered at least one.
Discussion on Available Evidence
The effects of treatments on symptoms in uncomplicated diverticular disease are not well documented. Bran or ispaghula husk, methylcellulose, antispasmodics and mesalazine could be of some help [12]. Consistent evidence indicates that dietary fibre, especially the insoluble fibre found mostly in fruits and vegetables rather than cereals, decreases risk of diverticular disease [43,44]. The protective action of dietary fibre would make the stools bulkier, thereby increasing the colon size and decreasing intraluminal pressures, and reducing colonic transit time [45,46].
The administration of the non-absorbable antibiotic R- α is able to reduce most of the clinical manifestations of diverticular disease, when compared with fiber supplementation alone. This effect is reached mainly through the reduction of the intestinal bacterial overgrowth [47].
It has been suggested that the synergistic effect of R-α on a high-fiber diet may be due to a reduced proliferation of gut microflora, with a consequent decrease in bacterial hydrogen (H2) and methane (CH4) production, and/or to an expansion in fecal mass, due to a decrease in bacterial degradation of fiber, thus reducing pain [48]. Furthermore, it has been suggested that these effects could induce acceleration in intestinal transit time, thus reducing constipation, which is frequently present in patients with diverticular disease [46]. R-α administration was shown to be effective in normalizing breath H2 profile in patients with intestinal bacterial overgrowth [46-48].
R-α absorption from the bowel is considered to be less than 1%, even in presence of colitis [49,50]. Our meta-analysis [42] evaluated the long-term efficacy administration of R- α plus fiber supplementation versus fiber supplementation alone, on symptoms and complications in patient with symptomatic uncomplicated diverticular disease.
The results of our study confirm previous observations, that cyclic administration of R-α, a poorly absorbable antibiotic, achieves symptomatic relief in large proportions of patients with uncomplicated diverticular disease, in comparison to control. After 12 months of follow up, 64.0% (pooled rate: 95%CI: 31.4-38.6) of patients treated with R-α plus standard supplement of dietary fibers were symptom-free, in comparison to 34.9% (95%CI: 60.9-67.0) of patients treated with fibers supplement. The 1-year gain in total symptom relief resulted statistically significant and clinically relevant (+29%, NNT 3).
Although a meta-analysis does not replace a large-scale, well-designed, randomized controlled trial, individual studies may be limited by small sample sizes, especially for end points with relatively low incidences. By pooling all available data, meta-analysis allows for a more precise estimate, than that which can be obtained from the results of any individual study. This study suggests that R-α treatment significantly could be of value in reducing complication development: at 1 year, 1.5% of patients treated with R-α plus standard supplement of dietary fibers developed complications, versus 3.2% of patients treatment with supplement of dietary fibers. The 1-year gain in primary prevention of complications was statistically, but not clinically relevant (-1.7%; NNT 59).
Further studies would be appropriate to check if R-α could have a role in modifying the clinical course of the disease. In fact, one-third of patients will proceed to a second attack of diverticulitis [4,51,52]. It is generally believed that the prognosis is worse with a second attack, since some studies have reported that the rate of complicated diverticulitis in such patients approaches 60 percent and the mortality rate are doubled [4,53,54]. Recurrent diverticulitis is expected to range from 7% to 42% of patients [14], and 50% of recurrence occur within 1 year of the initial episode [15].
Assuming the observed Odds Ratio (OR) for 1-year complication rate from this meta-analysis (0.37, 95%CI: 0.17-0.79), the 1-year NNT to prevent a second episode of diverticulitis could be expected to range from 44 for a 1-year risk of 3.5% (50% of 7%, the minimum range we found in the literature) to 8 for a 1-year risk of 21% (50% of 42%, the maximum range).
Conclusion
Cyclic treatment with R-α plus fiber supplementation is more effective in obtaining symptom relief and could prevent more complications, in symptomatic uncomplicated diverticular disease. We conclude that the evidence that cyclic R-α may further reduce symptoms at 12 mo, in comparison to fiber supplementation, should move form level 2 (mid-level), as indicated in reference 37, to level 1 (meta-analysis of multiple well designed, controlled studies), according to the Standards Committee of American Society of Colon and Rectal Surgeons [55].
However, at the moment the evidence of an effect of R-α over fiber supplementation on the clinical course of diverticular disease is poor. RCTs on secondary prevention of diverticulitis are warmly expected.
References
- Madoff RD (2011) Diverticulitis: something new under the sun? Arch Surg 146: 324.
- Schoetz DJ Jr (1999) Diverticular disease of the colon: a century-old problem. Dis Colon Rectum 42: 703-709.
- Parks TG (1975) Natural history of diverticular disease of the colon. Clin Gastroenterol 4: 53-69.
- Etzioni DA, Mack TM, Beart RW Jr, Kaiser AM (2009) Diverticulitis in the United States: 1998-2005: changing patterns of disease and treatment. Ann Surg 249: 210-217.
- Sandler RS, Everhart JE, Donowitz M, Adams E, Cronin K, et al. (2002) The burden of selected digestive diseases in the United States. Gastroenterology 122: 1500-1511.
- Shaheen NJ, Hansen RA, Morgan DR, Gangarosa LM, Ringel Y, et al. (2006) The burden of gastrointestinal and liver diseases, 2006. Am J Gastroenterol 101: 2128-2138.
- Lanas A, García-Rodríguez LA, Polo-Tomás M, Ponce M, Quintero E, et al. (2011) The changing face of hospitalisation due to gastrointestinal bleeding and perforation. Aliment Pharmacol Ther 33: 585-591.
- Rafferty J, Shellito P, Hyman NH, Buie WD; Standards Committee of American Society of Colon and Rectal Surgeons (2006) Practice parameters for sigmoid diverticulitis. Dis Colon Rectum 49: 939-944.
- Bogardus ST Jr (2006) What do we know about diverticular disease? A brief overview. J Clin Gastroenterol 40 Suppl 3: S108-111.
- Simpson J, Neal KR, Scholefield JH, Spiller RC (2003) Patterns of pain in diverticular disease and the influence of acute diverticulitis. Eur J Gastroenterol Hepatol 15: 1005-1010.
- Tursi A, Papagrigoriadis S (2009) Review article: the current and evolving treatment of colonic diverticular disease. Aliment Pharmacol Ther 30: 532-546.
- Petruzziello L, Iacopini F, Bulajic M, Shah S, Costamagna G (2006) Review article: uncomplicated diverticular disease of the colon. Aliment Pharmacol Ther 23: 1379-1391.
- Humes D, Simpson J, Spiller R. Colonic diverticular disease.
- Annibale B, Lahner E, Maconi G, Usai P, Marchi S, et al. (2012) Clinical features of symptomatic uncomplicated diverticular disease: a multicenter Italian survey. Int J Colorectal Dis 27: 1151-1159.
- Haglund U, Hellberg R, Johnsén C, Hultén L (1979) Complicated diverticular disease of the sigmoid colon. An analysis of short and long term outcome in 392 patients. Ann Chir Gynaecol 68: 41-46.
- Parks TG, Connell AM (1970) The outcome in 455 patients admitted for treatment of diverticular disease of the colon. Br J Surg 57: 775-778.
- BOLES RS Jr, JORDAN SM (1958) The clinical significance of diverticulosis. Gastroenterology 35: 579-582.
- Floch MH, White JA (2006) Management of diverticular disease is changing. World J Gastroenterol 12: 3225-3228.
- Ricciardi R, Roberts PL, Read TE, Marcello PW, Hall JF, et al. (2011) Cyclical increase in diverticulitis during the summer months. Arch Surg 146: 319-323.
- Kronborg O (1993) Treatment of perforated sigmoid diverticulitis: a prospective randomized trial. Br J Surg 80: 505-507.
- Zeitoun G, Laurent A, Rouffet F, Hay J, Fingerhut A, et al. (2000) Multicentre, randomized clinical trial of primary versus secondary sigmoid resection in generalized peritonitis complicating sigmoid diverticulitis. Br J Surg 87: 1366-1374.
- Van Arendonk KJ, Tymitz KM, Gearhart SL, Stem M, Lidor AO (2013) Outcomes and costs of elective surgery for diverticular disease: a comparison with other diseases requiring colectomy. JAMA Surg 148: 316-321.
- Laine L, Connors LG, Reicin A, Hawkey CJ, Burgos-Vargas R, et al. (2003) Serious lower gastrointestinal clinical events with nonselective NSAID or coxib use. Gastroenterology 124: 288-292.
- Laine L, Curtis SP, Langman M, Jensen DM, Cryer B, et al. (2008) Lower gastrointestinal events in a double-blind trial of the cyclo-oxygenase-2 selective inhibitor etoricoxib and the traditional nonsteroidal anti-inflammatory drug diclofenac. Gastroenterology 135: 1517-1525.
- Campbell K, Steele RJ (1991) Non-steroidal anti-inflammatory drugs and complicated diverticular disease: a case-control study. Br J Surg 78: 190-191.
- Goh H, Bourne R (2002) Non-steroidal anti-inflammatory drugs and perforated diverticular disease: a case-control study. Ann R Coll Surg Engl 84: 93-96.
- Morris CR, Harvey IM, Stebbings WS, Speakman CT, Kennedy HJ, et al. (2003) Anti-inflammatory drugs, analgesics and the risk of perforated colonic diverticular disease. Br J Surg 90: 1267-1272.
- Mpofu S, Mpofu CM, Hutchinson D, Maier AE, Dodd SR, et al. (2004) Steroids, non-steroidal anti-inflammatory drugs, and sigmoid diverticular abscess perforation in rheumatic conditions. Ann Rheum Dis 63: 588-590.
- Wilson RG, Smith AN, Macintyre IM (1990) Complications of diverticular disease and non-steroidal anti-inflammatory drugs: a prospective study. Br J Surg 77: 1103-1104.
- Yamada A, Sugimoto T, Kondo S, Ohta M, Watabe H, et al. (2008) Assessment of the risk factors for colonic diverticular hemorrhage. Dis Colon Rectum 51: 116-120.
- Strate LL, Liu YL, Huang ES, Giovannucci EL, Chan AT (2011) Use of aspirin or nonsteroidal anti-inflammatory drugs increases risk for diverticulitis and diverticular bleeding. Gastroenterology 140: 1427-1433.
- Humes DJ, Fleming KM, Spiller RC, West J (2011) Concurrent drug use and the risk of perforated colonic diverticular disease: a population-based case-control study. Gut 60: 219-224.
- Trepsi E, Colla C, Panizza P, Polino MG, Venturini A, et al. (1999) [Therapeutic and prophylactic role of mesalazine (5-ASA) in symptomatic diverticular disease of the large intestine. 4 year follow-up results]. Minerva Gastroenterol Dietol 45: 245-252.
- Kruis W, Meier E, Schumacher M, Mickisch O, Greinwald R, et al. (2013) Randomised clinical trial: mesalazine (Salofalk granules) for uncomplicated diverticular disease of the colon-a placebo-controlled study. Aliment Pharmacol Ther 37: 680-690.
- Gatta L, Di Mario F, Curlo M, Vaira D, Pilotto A, et al. (2012) Long-term treatment with mesalazine in patients with symptomatic uncomplicated diverticular disease. Intern Emerg Med 7: 133-137.
- World Gastroenterology Organisation. Practice Guidelines: Diverticular Disease, 2007.
- Diverticulitis. In DynaMed, http: //dynaweb.ebscohost.com. Updated 2010, Dec 13; accessed on 2013 Aug 3.
- Papi C, Ciaco A, Koch M, Capurso L (1992) Efficacy of rifaximin on symptoms of uncomplicated diverticular disease of the colon. A pilot multicentre open trial. Diverticular Disease Study Group. Ital J Gastroenterol 24: 452-456.
- Papi C, Ciaco A, Koch M, Capurso L (1995) Efficacy of rifaximin in the treatment of symptomatic diverticular disease of the colon. A multicentre double-blind placebo-controlled trial. Aliment Pharmacol Ther 9: 33-39.
- Latella G, Pimpo MT, Sottili S, Zippi M, Viscido A, et al. (2003) Rifaximin improves symptoms of acquired uncomplicated diverticular disease of the colon. Int J Colorectal Dis 18: 55-62.
- Colecchia A, Vestito A, Pasqui F, Mazzella G, Roda E, et al. (2007) Efficacy of long term cyclic administration of the poorly absorbed antibiotic Rifaximin in symptomatic, uncomplicated colonic diverticular disease. World J Gastroenterol 13: 264-269.
- Bianchi M, Festa V, Moretti A, Ciaco A, Mangone M, et al. (2011) Meta-analysis: long-term therapy with rifaximin in the management of uncomplicated diverticular disease. Aliment Pharmacol Ther 33: 902-910.
- Aldoori W, Ryan-Harshman M (2002) Preventing diverticular disease. Review of recent evidence on high-fibre diets. Can Fam Physician 48: 1632-1637.
- Painter NS (1985) The cause of diverticular disease of the colon, its symptoms and its complications. Review and hypothesis. J R Coll Surg Edinb 30: 118-122.
- Lupton JR, Turner ND (1999) Potential protective mechanisms of wheat bran fiber. Am J Med 106: 24S-27S.
- Ventrucci M, Ferrieri A, Bergami R, Roda E (1994) Evaluation of the effect of rifaximin in colon diverticular disease by means of lactulose hydrogen breath test. Curr Med Res Opin 13: 202-206.
- Corazza GR, Ventrucci M, Strocchi A, Sorge M, Pranzo L, et al. (1988) Treatment of small intestine bacterial overgrowth with rifaximin, a non-absorbable rifamycin. J Int Med Res 16: 312-316.
- Di Stefano M, Malservisi S, Veneto G, Ferrieri A, Corazza GR (2000) Rifaximin versus chlortetracycline in the short-term treatment of small intestinal bacterial overgrowth. Aliment Pharmacol Ther 14: 551-556.
- Descombe JJ, Dubourg D, Picard M, Palazzini E (1994) Pharmacokinetic study of rifaximin after oral administration in healthy volunteers. Int J Clin Pharmacol Res 14: 51-56.
- Rizzello F, Gionchetti P, Venturi A, Ferretti M, Peruzzo S, et al. (1998) Rifaximin systemic absorption in patients with ulcerative colitis. Eur J Clin Pharmacol 54: 91-93.
- Larson DM, Masters SS, Spiro HM (1976) Medical and surgical therapy in diverticular disease: a comparative study. Gastroenterology 71: 734-737.
- Rege RV, Nahrwold DL (1989) Diverticular disease. Curr Probl Surg 26: 133-189.
- Rodkey GV, Welch CE (1984) Changing patterns in the surgical treatment of diverticular disease. Ann Surg 200: 466-478.
- Sarin S, Boulos PB (1994) Long-term outcome of patients presenting with acute complications of diverticular disease. Ann R Coll Surg Engl 76: 117-120.
- Stollman NH, Raskin JB (1999) Diagnosis and management of diverticular disease of the colon in adults. Ad Hoc Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol 94: 3110-3121.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 15439
- [From(publication date):
June-2014 - Aug 08, 2025] - Breakdown by view type
- HTML page views : 10823
- PDF downloads : 4616