Mesenchymal Stem Cell Therapy on Tendon/Ligament Healing
Received: 01-Nov-2016 / Accepted Date: 29-Dec-2016 / Published Date: 07-Jan-2017 DOI: 10.4172/2576-3881.1000112
Abstract
A normal healing response after ligament and tendon rupture results in scar formation and an inferior tissue that fails to emulate its original structure, composition, and function. More regenerative healing (closer to the original) can be obtained through early suppression of inflammatory cells and associated cytokines. Examination of the immune mediated response of mesenchymal stem/stromal cells (MSCs) during healing indicates that MSCs reprogram macrophages from a pro-inflammatory M1 phenotype to an anti-inflammatory M2 phenotype. Based on these studies our objective was to treat ligament and tendon injuries with MSCs in order to modulate their inflammatory response. Our initial studies using allogeneic cells demonstrated an in vivo dose dependency of MSCs on ligament healing. Medial collateral ligaments (MCLs) treated with 1 × 106 (low dose) MSCs exhibited less inflammation and a reduced number of M1 macrophages compared to ligaments treated with 4 × 106 (high dose) MSCs. Strength of ligament was also improved with the low dose treatment. We then examined the in vivo effects of MSCs that had been preconditioned to be more anti-inflammatory. Treatment with these preconditioned MSCs was compared with normally processed (unconditioned) MSCs using the rat Achilles tendon and MCL healing models. Pre-conditioned MSCs significantly reduced inflammation by increasing the M2 macrophages and decreasing the M1 macrophages. Most importantly, treatment with pre-conditioned MSCs improved tissue strength to levels comparable to intact tissue. Overall, pre-conditioned MSC-treatment out-performed unconditioned MSCs to improve ligament and tendon healing by stimulating a more robust, paracrine-mediated immunosuppressive response.
Ligament and Tendon Healing
After rupture, normal ligament and tendon healing undergoes inflammation, proliferation, and remodeling. During inflammation, neutrophils, M1 and M2 macrophages, and to a lesser degree, t-lymphocytes infiltrate the wound. Fibroblasts, endothelial cells and additional macrophages accrue thereafter and form granulation tissue during proliferation. As healing progresses, production of type I pro-collagen decreases while type III collagen increases [1]. The newly formed type III collagen produces a weak transient connection for injured tissue. As remodeling continues, the ratio of type I to type III collagen improves along with the tensile strength of the compromised region, but nevertheless results in a mechanically inferior tissue that fails to mimic its original structure. An improved healing scenario would involve no scar formation, reduced inflammation, organized collagen fibers, restored concentrations of type I collagen, and mechanical function that mimics native tissue. Previous studies indicate that early suppression of inflammatory cells and associated cytokines promotes regenerative healing of tendons/ligaments towards their original structure [2-4]. Based on these studies our objective was to treat musculoskeletal injuries with MSCs which are known modulators of inflammation.
Immunosuppressive effects of Mesenchymal Stem Cells on Musculoskeletal Healing
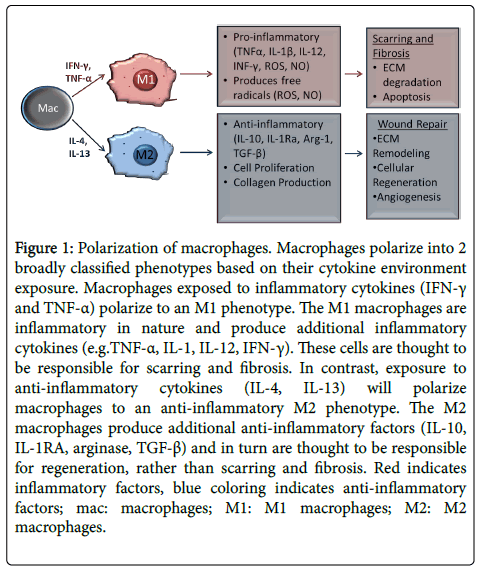
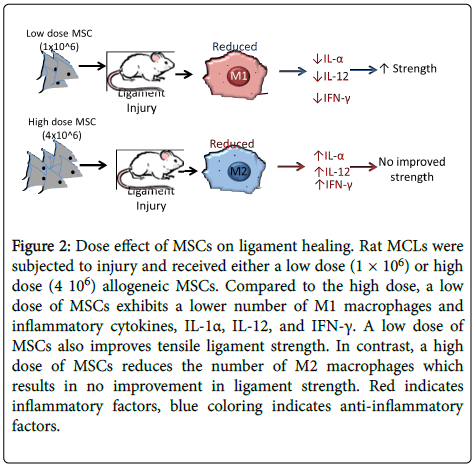
Mesenchymal stem/stromal cell (MSC) therapy has developed as a treatment to improve soft tissue and bone healing. MSCs are multipotent cells that have the potential to differentiate into multiple cell lineages including fibroblasts, tenocytes, osteoblasts, adipocytes, chondrocytes, and myocytes. MSCs were originally thought to improve healing through this ability to differentiate into lineage appropriate cell types. It is now believed that MSCs provide more of a therapeutic benefit via paracrine actions including, immunomodulation, anti-apoptosis, angiogenesis, support of stem progenitor cells, anti-scarring, and chemo-attraction [5,6]. Examination of the immune mediated response by MSCs during healing indicates that MSCs reprogram macrophages from a pro-inflammatory M1 phenotype to an anti-inflammatory M2 phenotype (Figure 1) [7,8]. In vitro studies of macrophages co-cultured with bone marrow-derived MSCs produced high levels of the anti-inflammatory factors CD206 (a marker for M2 macrophages) and IL-10, as well as low levels of inflammatory cytokines IL-12 and TNF-α [8]. In vivo studies from our lab examined MSC-mediated immune modulation during ligament healing from a dose-dependent perspective [9]. Specifically, injured rat medial collateral ligaments (MCLs) were treated with either 1 × 106 (low dose) or 4 × 106 (high dose) allogeneic MSCs [9]. Within the early healing ligament, fewer M1 macrophages localized within the low dose group resulting in lower amounts of inflammatory cytokines (IL-1α, IL-12 and IFN-γ) than the high dose MSC group (Figure 2) [9]. The low dose group also increased failure strength and stiffness of the healing MCL, improving functional behavior. Based on these findings, MSC treatment can improve ligament healing by reducing the inflammatory macrophages and their associated cytokines. Importantly, ligament healing was dependent on the applied MSC dose. The increased inflammatory response by the higher MSC-dosed tendons may be due to the use of allogeneic cells and the potential mismatching of major histocompatibility complex (MHC) class I and II. To support this concept, a previous study implanted allogeneic C57Bl/6 mouse MSCs into an MHC mismatched Balb/c mouse [10]. Allogeneic implants resulted in increased inflammation and immune rejection compared to the syngeneic controls [10]. Taken together evidence suggests that the high dose of MSCs in our study may have initiated an inflammatory response that negated improved healing as seen in the low dose group.
Figure 1: Polarization of macrophages. Macrophages polarize into 2 broadly classified phenotypes based on their cytokine environment exposure. Macrophages exposed to inflammatory cytokines (IFN-γ and TNF-α) polarize to an M1 phenotype. The M1 macrophages are inflammatory in nature and produce additional inflammatory cytokines (e.g.TNF-α, IL-1, IL-12, IFN-γ). These cells are thought to be responsible for scarring and fibrosis. In contrast, exposure to anti-inflammatory cytokines (IL-4, IL-13) will polarize macrophages to an anti-inflammatory M2 phenotype. The M2 macrophages produce additional anti-inflammatory factors (IL-10, IL-1RA, arginase, TGF-β) and in turn are thought to be responsible for regeneration, rather than scarring and fibrosis. Red indicates inflammatory factors, blue coloring indicates anti-inflammatory factors; mac: macrophages; M1: M1 macrophages; M2: M2 macrophages.
Figure 2: Dose effect of MSCs on ligament healing. Rat MCLs were subjected to injury and received either a low dose (1 × 106) or high dose (4 106) allogeneic MSCs. Compared to the high dose, a low dose of MSCs exhibits a lower number of M1 macrophages and inflammatory cytokines, IL-1α, IL-12, and IFN-γ. A low dose of MSCs also improves tensile ligament strength. In contrast, a high dose of MSCs reduces the number of M2 macrophages which results in no improvement in ligament strength. Red indicates inflammatory factors, blue coloring indicates anti-inflammatory factors.
Immunosuppressive Effects of Primed MSCs on Musculoskeletal Healing
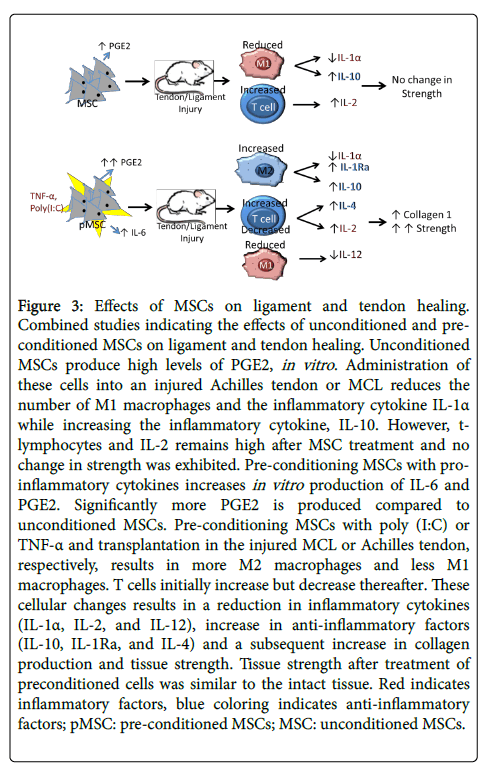
Because acute tissue damage initiates an inflammatory response and the upregulation of macrophages, an inflammatory environment may be an important prerequisite for MSCs to stimulate a reparative response. This logic suggests that MSC-mediated immunosuppression is induced by inflammatory cytokines. Previous research demonstrated that injected MSCs preferentially migrate to inflammatory sites due to increased immune cell-stimulated cytokines and chemokines [11]. Furthermore, MSC immunosuppression required stimulation by inflammatory factors such as interferon (IFN)-γ, and TNF-α [12-14]. Although in vivo administration of MSCs into an injury may provide adequate inflammatory signaling, studies suggest that preconditioning MSCs with inflammatory cytokines prior to application can further enhance their immunosuppressive ability, engraftment, and survival at injury sites, thereby increasing their therapeutic benefit [15,16]. Recent studies from our group elucidated the effects of preconditioned MSCs on tendon and ligament healing (Figure 3) [17,18]. Injured rat MCLs administered preconditioned polyinosinic acid and polycytidylic acid (Poly(I:C)) MSCs increased the number of M2 macrophages and anti-inflammatory cytokine IL-1Ra [17]. These preconditioned MSCs also increased type I procollagen production and failure strength compared to treatment with unconditioned MSCs. Similar anti-inflammatory effects were observed with rat Achilles tendon segmental defects treated with TNF-α primed MSCs [18]. Both the number of M1 macrophages and inflammatory IL-12 cytokine were decreased by TNF-α primed MSCs while anti-inflammatory factors, such as IL-1Ra, IL-10, and IL-4 were increased (Figure 3) [18]. Preconditioned MSCs also increased type I procollagen production and failure stress of the tendon such that injured tendons were similar to intact tendons. Unlike our previous studies using unconditioned cells, MSC preconditioning increased the M2 macrophages while decreasing the M1 macrophages. Taken together, these results suggest that preconditioned MSCs better modulate macrophage polarization and cytokine production than unconditioned MSCs and thereby promote a more regenerative healing response.
Figure 3: Effects of MSCs on ligament and tendon healing. Combined studies indicating the effects of unconditioned and preconditioned MSCs on ligament and tendon healing. Unconditioned MSCs produce high levels of PGE2, in vitro. Administration of these cells into an injured Achilles tendon or MCL reduces the number of M1 macrophages and the inflammatory cytokine IL-1α while increasing the inflammatory cytokine, IL-10. However, tlymphocytes and IL-2 remains high after MSC treatment and no change in strength was exhibited. Pre-conditioning MSCs with proinflammatory cytokines increases in vitro production of IL-6 and PGE2. Significantly more PGE2 is produced compared to unconditioned MSCs. Pre-conditioning MSCs with poly (I:C) or TNF-α and transplantation in the injured MCL or Achilles tendon, respectively, results in more M2 macrophages and less M1 macrophages. T cells initially increase but decrease thereafter. These cellular changes results in a reduction in inflammatory cytokines (IL-1α, IL-2, and IL-12), increase in anti-inflammatory factors (IL-10, IL-1Ra, and IL-4) and a subsequent increase in collagen production and tissue strength. Tissue strength after treatment of preconditioned cells was similar to the intact tissue. Red indicates inflammatory factors, blue coloring indicates anti-inflammatory factors; pMSC: pre-conditioned MSCs; MSC: unconditioned MSCs.
In conclusion, ligament and tendon injuries are a prevalent problem, frequently resulting in scar formation and compromised function. MSC treatment can attenuate some of these issues. It improves ligament and tendon strength and provides a more favorable type I collagen composition, indicating a beneficial therapeutic response to these cells. After preconditioning MSCs to accentuate their anti-inflammatory response, results were even better. Taken together, our experiments indicate that the primary mechanism of action by the MSCs in these models is due to their paracrine-mediated immunosuppressive effect. MSC therapy modulates macrophage phenotypes, which creates a more anti-inflammatory environment. In a broader sense, our results suggest that MSC treatment and its immunomodulatory effects should not be limited to wound healing. They also hold great promise for a variety of other conditions and pathologies where inflammation is problematic.
Acknowledgement
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number AR059916.
References
- Chamberlain CS, Crowley E, Vanderby R (2009) The spatio-temporal dynamics of ligament healing. Wound Repair Regen 17: 206-215.
- Chamberlain CS, Leiferman EM, Frisch KE, Wang S, Yang X, et al. (2011) The influence of interleukin-4 on ligament healing. Wound Repair Regen 19: 426-435.
- Chamberlain CS, Leiferman EM, Frisch KE, Duenwald-Kuehl SE, Brickson SL, et al. Interleukin-1 receptor antagonist modulates inflammation and scarring after ligament injury. Connect Tissue Res 55: 177-186.
- Chamberlain CS, Leiferman EM, Frisch KE, Brickson SL, Murphy WL, et al. (2013) Interleukin expression after injury and the effects of interleukin-1 receptor antagonist. PLoS One 8:Â e71631.
- Meirelles LD, Fontes AM, Covas DT, Caplan AI(2009) Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev 20: 419-427.
- Singer NG,Caplan AI (2011)Mesenchymal stem cells: Mechanisms of inflammation. Ann Rev Pathol 6: 457-478.
- Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmalee A, et al. (2009) Bone marrow stromal cells attenuate sepsis via prostaglandin e-2-dependent reprogramming of host macrophages to increase their interleukin-10 production.Nat Med 15: 462-462.
- Kim J, Hematti P (2009) Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol 37: 1445-1453.
- Saether EE, Chamberlain CS, Leiferman EM, Kondratko-Mittnacht JR, Li WJ, et al. (2014) Enhanced medial collateral ligament healing using mesenchymal stem cells: Dosage effects on cellular response and cytokine profile. Stem Cell Rev 10: 86-96.
- Eliopoulos N, Stagg J, Lejeune L, Pommey S, Galipeau J(2005) Allogeneic marrow stromal cells are immune rejected by mhc class i- and class ii-mismatched recipient mice. Blood 106: 4057-4065.
- Ponte AL, Marais E, Gallay N, Langonné A, Delorme B, et al. (2007) The in vitro migration capacity of human bone marrow mesenchymal stem cells: Comparison of chemokine and growth factor chemotactic activities. Stem Cells 25: 1737-1745.
- Ren G, Zhang L, Zhao X, Xu G, Zhang Y, et al. (2008) Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2: 141-150.
- Bartosh TJ, Ylostalo, JH, Mohammadipoor A, Bazhanov N, Coble K, et al. (2010) Aggregation of human mesenchymal stromal cells (mscs) into 3d spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci USA 107: 13724-13729.
- Roddy GW, Oh JY, Lee RH, Bartosh TJ, Ylostalo J, et al. (2011) Action at a distance: Systemically administered adult stem/progenitor cells (mscs) reduce inflammatory damage to the cornea without engraftment and primarily by secretion of tnf-alpha stimulated gene/protein 6. Stem Cells 29: 1572-1579.
- Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM (2010) A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One 5: e10088.
- Lombardo E, DelaRosa O, Mancheno-Corvo P, Menta R, RamÃrez C, et al. (2009) Toll-like receptor-mediated signaling in human adipose-derived stem cells: Implications for immunogenicity and immunosuppressive potential. Tissue Eng Part A. 15: 1579-1589.
- Saether EE, Chamberlain CS, Aktas E, Leiferman EM, Brickson SL, et al. (2016) Primed mesenchymal stem cells alter and improve rat medial collateral ligament healing. Stem Cell Rev 12: 42-53.
- Aktas E, Chamberlain CS, Saether EE, Duenwald-Kuehl SE, Kondratko-Mittnacht J, et al. (2016) Immune modulation with primed mesenchymal stem cells delivered via biodegradable scaffold to repair an achilles tendon segmental defect. J Orthop Res 2016.
Citation: Chamberlain CS, Saether EE, Aktas E, Vanderby R (2017) Mesenchymal Stem Cell Therapy on Tendon/Ligament Healing. J Cytokine Biol 2: 112. DOI: 10.4172/2576-3881.1000112
Copyright: © 2017 Chambarlain CS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4702
- [From(publication date): 0-2017 - Aug 30, 2025]
- Breakdown by view type
- HTML page views: 3654
- PDF downloads: 1048