Metastatic Monophasic Synovial Sarcoma: A challenging Diagnosis on Fine Needle Aspiration with Broad Differential Diagnosis
Received: 26-Apr-2016 / Accepted Date: 27-May-2016 / Published Date: 31-May-2016 DOI: 10.4172/2476-2024.1000114
Abstract
Synovial sarcomas (SS) are rare soft tissue sarcomas that usually arise near large extremity joints. SS may pose difficult diagnostic challenges on cytology when encountered as a monophasic variant. We report a 27-year-old woman diagnosed with metastatic monophasic synovial sarcoma by a CT-scan guided fine needle aspiration (FNA) biopsy of a left lower lung lobe nodule. We reviewed the literature on the epidemiologic, cytohistological spectrum, immunophenotypic, and the molecular findings and discussed the differential diagnosis for this rare entity.
Keywords: FNA; Monophasic; Synovial sarcoma; Metastatic; SS18- SSX1 fusion
6678Introduction
Synovial sarcomas (SS) are malignant mesenchymal spindle cell tumors with high variable epithelial differentiation [1]. The name of “Synovial sarcoma” is a misnomer as the tumor does not arise from or differentiate toward synovium. This entity represents 5 to 10% of overall soft tissue sarcomas and usually arises in young individuals between ages of 15 to 35 years with male predominance [2]. SS presents most usually as a slow-growing deep seated mass, and in half of the cases they are painful [3]. SS could arise from any anatomic site, however, most commonly arise near large extremity joints, especially knee joint [4]. SS have three main histologic patterns: Biphasic synovial sarcoma (BSS); Monophasic synovial sarcoma (MSS); and poorly differentiated synovial sarcoma (PSS) [5]. Proper treatment usually is a wide local excision with neoadjuvant radio-chemotherapy. Local recurrences are common and distant metastasis usually occurs to lungs, bones and lymph nodes [6]. With proper treatment and follow up, the 5-year survival rate ranges from 50 to 85% [7].
Material and Methods
CT-guided FNA biopsy was performed using a 21-gauge needle. Material from the FNA was expelled onto glass slides and smeared. Some of the smears were air-dried and stained with Diff-Quik stain. The remaining smears were immediately wet fixed with 95% ethyl alcohol and stained with Papanicolaou stain. Material for cell block was rinsed from the needle in 10% neutral buffered formalin. Paraffinembedded sections from the cell block were stained with hematoxylin and eosin (H&E). Immunohistochemistry stains were performed on unstained sections of formalin fixed, paraffin embedded cell block by the standard avidin–biotin technique. The panel of antibodies used included pancytokeratin (CK AE1/AE3), epithelial membrane antigen (EMA), BCL-2, S-100, CD99, CD34, actin, desmin, melan A, and vimentin.
RT-PCR study of SYT-SSX genes 1 and 2 fusions was performed on unstained sections of cell block.
Case Report
Subject
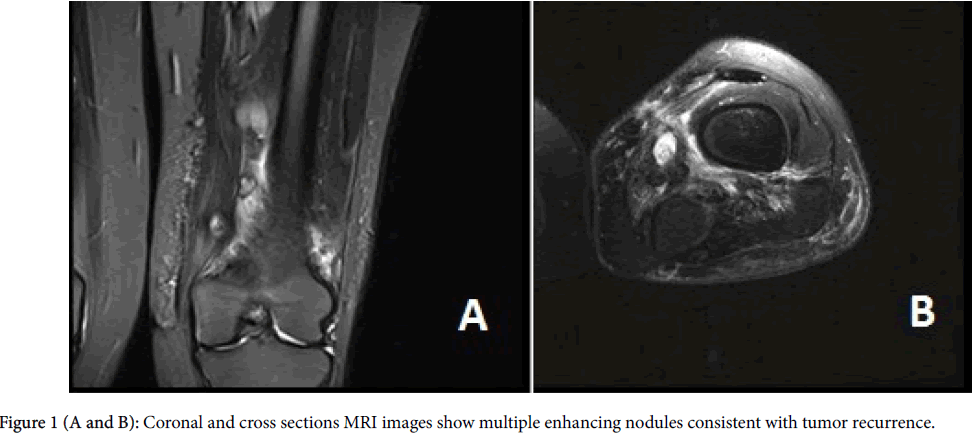
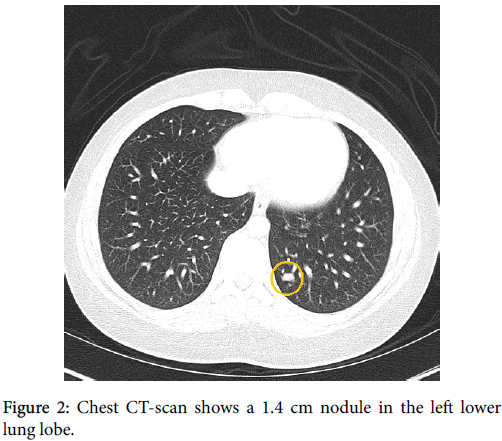
The patient is a 27-year old Puerto Rican female with a history of local recurrent “spindle cell sarcoma” of the left thigh. She first noticed a painless lump in her left thigh 5 years ago; however, as the lump was not painful, she did not seek any medical attention. Two years later, the patient got pregnant and her obstetrician recommended evaluating the lump as it was growing. CT scan of the lower extremities showed a 4.8 cm lobulated mass in the medial aspect of the left thigh. The mass was biopsied and diagnosed as “Grade I spindle cell sarcoma with no necrosis, suggestive of low grade fibrosarcoma”. Few weeks after the diagnosis, the patient had a wide local resection with clear margins. The patient did not receive any neoadjuvant treatment at that time. One year after the initial surgery, the patient noticed a lump in the same area. She had an MRI which showed multiple small nodules adjacent to the prior surgery site (Figures 1A and 1B). Chest CT-scan was negative at that time. Patient had another resection which showed “Grade II sarcoma with no necrosis”. No neoadjuvant chemotherapy was given at that time either. One year after the second resection, the follow up MRI showed multiple enhancing soft tissue masses consistent with local recurrence. The follow-up chest CT-scan showed a 1.4 cmx1.0 cm nodule in the left lower lung lobe (Figure 2). Infectious or inflammatory etiologies were considered. A CT-scan guided fine needle aspiration (FNA) biopsy on the lung nodule showed malignant neoplasm confirmed to be metastatic monophasic synovial sarcoma with proper immunohistochemical and molecular studies. Patient is planned to have an aggressive preoperative combined radioand chemotherapy to be followed by limp-salvage wide surgical resection.
Radiology
The initial left thigh CT-scan showed a 4.8 cm × 3.5 cm × 2.9 cm lobulated mass located at the medial aspect of the left thigh. One year following the first surgical resection, the patient had an MRI which showed at least 4 distinct and enhancing soft tissue masses within the mid to distal and medial left thigh (Figures 1A and 1B). The superior dominant mass is centered approximately 23 cm above the knee joint and measures 2.7 cm × 3.1 cm × 6.2 cm. This lesion was intimately associated with the superficial femoral artery and vein. A second dominant lesion measuring 2.8 cm × 3.2cm × 4.4 cm was seen just inferior to this lesion and was also closely associated with the superficial femoral vessels.
Several smaller nodular masses were seen within the subcutaneous tissues along the muscular fascia. An 8 mm × 8 mm enhancing nodule/ lymph node was seen in the subcutaneous tissues of the mid medial thigh. Underlying the surgical scar there was ill-defined signal abnormality and enhancement which extended from the skin surface to the popliteal vessels. One year after the second resection, the follow up MRI showed multiple enhancing soft tissue masses consistent with local recurrence. Follow up chest CT-scan showed a 1.4 × 1.0 cm left lower lung nodule (Figure 2). No pleural or pericardial effusion or lymph adenopathy were identified.
Cytology
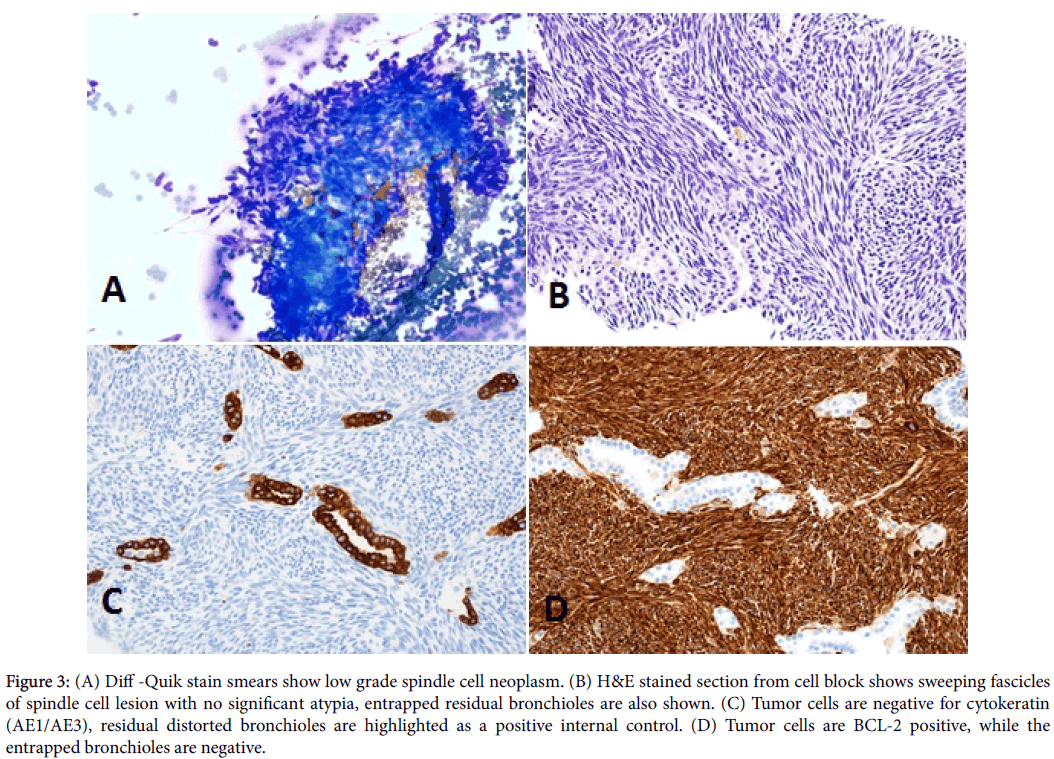
Moderate to hypercellular smears composed of monotonous population of spindle cells. The cells had a fascicular arrangement with focal storiform pattern with no mitosis or significant atypia (Figures 3A and 3B). No necrosis, calcifications, ossification, or epithelial component was identified. Most of the nuclei of the lesional cells are oval- to fusiform, with finely granular and evenly distributed chromatin. Previous resections slides were reviewed in conjunction with the current case. The histomorphologic features were identical.
Immunohistochemical findings
A properly controlled immunostain panel was performed on the cell block to determine the nature of the tumor. Differential diagnoses of this spindle cell tumor include metastatic fibrosarcoma, sarcomatoid squamous cell carcinoma, malignant spindle cell melanoma, malignant solitary fibrous tumor, atypical carcinoid tumor, monophasic synovial sarcoma, malignant peripheral sheath, Ewing Sarcoma/PNET, and leiomyosarcoma. The current case tumor cells were positive for vimentin, CD99, and Bcl-2. Tumor cells were negative for cytokeratin AE1/AE3, EMA, actin, desmin, CD34, S-100, and melan A (Figures 3C and 3D).
Molecular findings
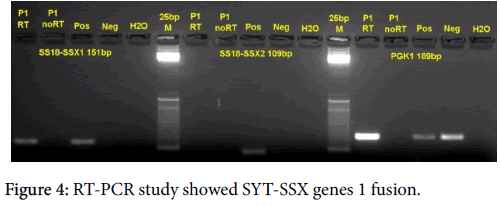
RT-PCR study of SYT-SSX genes 1 and 2 fusions was performed on unstained sections of cell block by Dr. Sarah Kerr and her group at Mayo Clinic, Rochester, USA. SYT-SSX genes 1 fusion was detected (Figure 4).
Discussion
Synovial sarcomas (SS) are rare soft tissue sarcomas that usually arise near large extremity joints, especially knee joint [4]. SS may pose difficult diagnostic challenges on cytology when encountered as a monophasic variant. SS mainly affects the deep soft tissues of the extremities in adolescents and young adults with male predominance, but can occur in almost any part of the body [8]. SS represented 5%–10% of all soft tissue sarcomas, with about 800 cases per year in the United States [9]. SS are divided mainly into three histological types: biphasic (BSS), monophasic (MSS), and poorly differentiated (PSS) [10]. The biphasic type consists of epithelial and spindle cell components, whereas the monophasic type has uniform spindle cells and are difficult to distinguish from other spindle cell neoplasms such as fibrosarcoma, sarcomatoid squamous cell carcinoma, malignant spindle cell melanoma, malignant solitary fibrous tumor, atypical carcinoid tumor, malignant peripheral sheath, Ewing Sarcoma/PNET, and leiomyosarcoma [11]. In either type of synovial sarcomas, epithelial markers, such as EMA and cytokeratin, may be reactive, however the golden diagnostic tool in about 90% of cases is the t(X;18) (p11;q11) translocation [12]. This is a rearrangement of the SS18 gene (formerly known as SYT) in the 18q11 region and one of the SSX1, SSX2, or SSX4 genes in Xp11 [13]. Currently, surgical excision is the main treatment modality for SS. Radiotherapy decreases the rate of local recurrence and adjuvant chemotherapy shown to increase time to local or distal recurrence, but not necessarily improve overall survival [13]. Spillane et al. [14], studied 150 cases of synovial sarcoma which showed a 5 year survival rate of 57%. The poor prognostic factors for SS include tumor size >5 cm, age >20 years at diagnosis, and the SS18 (SYT)-SSX1 translocation fusion gene [15].
The differential diagnosis of SS, especially the monophasic variant, is very challenging on cytology because the cytologic features are usually bland and do not direct the pathologist toward a diagnosis of an aggressive nature. The nuclei are uniform with evenly distributed chromatin; necrosis is uncommon and mitoses are infrequent [16]. This case illustrates the misleading bland cytologic appearance of SS with insignificant mitotic activity and absence of necrosis. Therefore, the use of ancillary studies including immunocytochemistry and molecular pathology is invaluable. EMA is the most sensitive marker to detect the epithelial component. Cytokeratin AE1/AE3 immunostain illustrates the epithelial component in the BSS [17]. The use of EMA, CK AE1/AE3 and CK7 appears to yield the best chance for detecting epithelial differentiation in SS [18]. CD34, SMA, S100, and desmin immunostains are usually negative. The spindle cells in SS are usually positive for Bcl-2 and CD99 [19].
Spindle cell squamous cell carcinoma is one of the main differential diagnosis, however, this entity are often shows a high degree of atypia and pleomorphism, and tumor cells are positive for CK AE1/AE3, p63, or p40 and negative for CD99 or BCL-2. The other differential diagnosis is leiomyosarcoma which have cigar-shaped nuclei with perinuclear vacuoles, however, they are almost always CK AE1/AE3 and EMA negative and desmin and actin positive [20]. Malignant solitary fibrous tumor shows stag-horn shaped vasculature with ropy collage, and usually positive for CD34 and BCL-2; however, it is usually negative for CD99 and CK AE1/AE3. Fibrosarcoma is also considered an important differential diagnosis for the MSS. This entity is vimentin positive, however, is BL-2, and CD99 negative [21]. Atypical carcinoid tumor could pose similar tumor morphology with more spindle features; however, they are CK AE1/AE3, synaptophysin and chromogranin positive and both CD99 and CD34 negative. Malignant melanoma could mimic the cytologic features of MSS. However, malignant melanoma is positive for S100, Melan A, HMB45, and negative for CK AE1/AE3, CD99 and CD34. Table 1 shows the differential diagnosis of MSS with the different immunophenotypic characteristics.
| MSS | SSCC | FS | MM | ACT | LMS | MSFT | MPNST | ES/PNET | |
|---|---|---|---|---|---|---|---|---|---|
| CK AE1/AE3 | +/- | + | - | - | + | - | - | - | - |
| EMA | +/- | + | - | - | + | - | +/- | - | - |
| CD99 | ++ | - | - | - | - | - | ++ | - | ++ |
| CD34 | - | - | - | - | - | - | ++ | +/- | - |
| Actin | - | - | +/- | - | - | + | +/- | - | - |
| Desmin | - | - | +/- | - | - | + | - | - | - |
| BCL-2 | ++ | - | - | - | - | - | ++ | - | - |
| Synaptophysin | - | - | - | - | + | - | - | - | - |
| Chromogranin | - | - | - | - | + | - | - | - | - |
| Melan A | - | - | - | + | - | - | - | - | - |
| S100 | - | - | - | + | - | - | +/- | +/- | +/- |
| HMB 45 | - | - | - | + | - | - | - | - | - |
| Vimentin | + | - | + | - | - | + | ++ | - | - |
| CD56 | + | - | - | - | + | - | - | - | - |
| TTF-1 | - | - | - | - | + | - | - | - | - |
| CK 20 | - | - | - | - | - | - | - | - | - |
| CK 7 | - | - | - | - | +/- | - | - | - | - |
| P63 | - | + | - | - | - | - | - | - | - |
| CK 5/6 | - | +/- | - | - | - | - | - | - | - |
| Molecular | t(X;18) | N/A | N/A | N/A | N/A | N/A | N/A | Complex | t(11;22)(q24; q12) |
MSS: Monophasic Synovial Sarcoma; SSCC: Sarcomatoid Squamous Cell Carcinoma; FS: Fibrosarcoma; MM: Malignant Melanoma; ACT: Atypical Carcinoid Tumor; LMS: Leiomyosarcoma; MSFT: Malignant Solitary Fibrous Tumor; MPNST: Malignant Nerve Sheath Tumor; ES/PNET: Ewing’s Sarcoma/Primitive Neuroectodermal Tumor.
Table 1: IPOX for the differential diagnosis of synovial sarcoma.
The golden standard diagnostic tool is the demonstration of the characteristic translocation (X;18). SS consistently harbors t (X;18) resulting in SS18-SSX1, SS18-SSX2, and rarely SS18-SSX4 fusion transcripts [22].
References
- Spillane AJ, A'Hern R, Judson IR, Fisher C, Thomas JM (2000) Synovial sarcoma: a clinicopathologic, staging, and prognostic assessment. J ClinOncol 18: 3794-3803.
- Arnold MA, Arnold CA, Li G, Chae U, El-Etriby R, et al. (2013) A unique pattern of INI1 immunohistochemistry distinguishes synovial sarcoma from its histologic mimics. Hum Pathol 44: 881-887.
- Tanas MR, Rubin BP, Tubbs RR, Billings SD, Downs-Kelly E, et al. (2010) Utilization of fluorescence in situ hybridization in the diagnosis of 230 mesenchymal neoplasms: an institutional experience. Arch Pathol Lab Med 134: 1797-1803.
- Srinivasan R, Gautam U, Gupta R, Rajwanshi A, Vasistha RK (2009) Synovial sarcoma: diagnosis on fine-needle aspiration by morphology and molecular analysis. Cancer 117: 128-136.
- Tomlins SA, Palanisamy N, Brenner JC, Stall JN, Siddiqui J, et al. (2013) Usefulness of a monoclonal ERG/FLI1 antibody for immunohistochemical discrimination of Ewing family tumors. Am J ClinPathol 139: 771-779.
- Kosemehmetoglu K, Vrana JA, Folpe AL (2009) TLE1 expression is not specific for synovial sarcoma: a whole section study of 163 soft tissue and bone neoplasms. Mod Pathol 22: 872-878.
- Cheng Y, Sheng W, Zhou X, Wang J (2012) Pericardial synovial sarcoma, a potential for misdiagnosis: clinicopathologic and molecular cytogenetic analysis of three cases with literature review. Am J Clin Pathol 137: 142-149.
- Karamchandani JR, Nielsen TO, van de Rijn M, West RB (2012) Sox10 and S100 in the diagnosis of soft-tissue neoplasms. Appl Immunohistochem Mol Morphol 20: 445-450.
- Varma T, Adegboyega P (2012) Primary cardiac synovial sarcoma.Arch Pathol Lab Med 136: 454-458.
- Foo WC, Cruise MW, Wick MR, Hornick JL (2011) Immunohistochemical staining for TLE1 distinguishes synovial sarcoma from histologic mimics. Am J ClinPathol 135: 839-844.
- Kusakabe T, Watanabe K, Nomura-Tanaka M, Ishida T, Munakata M, et al. (2010) Primary pulmonary synovial sarcoma - transbronchial needle aspiration is a diagnostic approach: a case report with cytological features. Cytopathology 21: 52-55
- Miura Y, Keira Y, Ogino J, Nakanishi K, Noguchi H, et al. (2012) Detection of specific genetic abnormalities by fluorescence in situ hybridization in soft tissue tumors. Pathol Int 62: 16-27.
- Fisher C (2010) Soft tissue sarcomas with non-EWS translocations: molecular genetic features and pathologic and clinical correlations. Virchows Arch 456: 153-166.
- Matsuyama A, Hisaoka M, Iwasaki M, Iwashita M, Hisanaga S, et al. (2010) TLE1 expression in malignant mesothelioma. Virchows Arch 457: 577-583.
- Ouansafi I, Klein M, Sugrue C, Morgenstern N, Frank D, et al. (2010) Monophasic parapharyngeal synovial sarcoma diagnosed by cytology, immunocytochemistry, and molecular pathology: case report and review of the literature. Diagn Cytopathol 38: 822-827.
- Collini P, Sorensen PH, Patel S, Blay JY, Issels RD, et al. (2009) Sarcomas with spindle cell morphology. Semin Oncol 36: 324-337.
- Gulley ML, Kaiser-Rogers KA (2009) A rational approach to genetic testing for sarcoma. Diagn Mol Pathol 18: 1-10.
- Ferrari A, Gronchi A, Casanova M, Meazza C, Gandola L, et al. (2004) Synovial sarcoma: a retrospective analysis of 271 patients of all ages treated at a single institution. Cancer 101: 627-634.
- Ladanyi M, Antonescu CR, Leung DH, Woodruff JM, Kawai A, et al. (2002) Impact of SYT-SSX fusion type on the clinical behavior of synovial sarcoma: a multi-institutional retrospective study of 243 patients. Cancer Res 62: 135-140.
- Chan JA, McMenamin ME, Fletcher CD (2003) Synovial sarcoma in older patients: clinicopathological analysis of 32 cases with emphasis on unusual histological features. Histopathology 43: 72-83.
- Coindre JM, Pelmus M, Hostein I, Lussan C, Bui BN, et al. (2003) Should molecular testing be required for diagnosing synovial sarcoma? A prospective study of 204 cases. Cancer 98: 2700-2707.
- Pelmus M, Guillou L, Hostein I, Sierankowski G, Lussan C (2002) Monophasic fibrous and poorly differentiated synovial sarcoma: immunohistochemical reassessment of 60 t (X;18) (SYT-SSX)-positive cases. Am J SurgPathol 26: 1434-1440.
Citation: Tranesh G, Kerr S, Nassar A (2016) Metastatic Monophasic Synovial Sarcoma: A challenging Diagnosis on Fine Needle Aspiration with Broad Differential Diagnosis. Diagn Pathol Open 1:114. DOI: 10.4172/2476-2024.1000114
Copyright: ©2016 Tranesh G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 14799
- [From(publication date): 6-2016 - Jun 24, 2025]
- Breakdown by view type
- HTML page views: 13774
- PDF downloads: 1025