Mir-27a: A Regulator of Apaf-1, Modulate Proliferation and Apoptosis in Laryngeal Carcinoma
Received: 07-Jun-2016 / Accepted Date: 21-Jun-2016 / Published Date: 24-Jun-2016 DOI: 10.4172/2476-2024.1000116
Abstract
Purpose: APAF-1, a key mediator in cytochrome C-dependent apoptotic pathway, plays a critical role in many cancers. MicroRNA-27a inhibits hypoxia-induced neuronal apoptosis by targeting APAF-1. Whether microRNA-27a participates in carcinogenesis via regulating APAF-1 is not reported.
Methods: Laryngeal cancer tissues, Hep2 cell line and HEK293 cell line were used in the study. qRT-PCR was used to detect microRNA-27a and APAF-1 mRNA levels. Western blot was to monitor APAF-1 protein level. Cell viability, colony formation and apoptosis assays were applied to evaluate the function of microRNA-27a and APAF-1 in laryngeal cancer. Dual-luciferase reporter assay was to detect the binding ability of microRNA-27a to APAF-1 3'UTR.
Results: In the study, we found that up-regulation of microRNA-27a was negatively correlated with downregulation of APAF-1 in laryngeal cancer. MicroRNA-27a was reconfirmed to directly bind APAF-1 mRNA 3'UTR. Similar to si-APAF-1, ectopic miR-27a significantly promoted laryngeal cancer cell proliferation and colony formation ability and suppressed early apoptosis compared to the controls.
Conclusion: miR-27a acts as a potentially oncogenic role in laryngeal squamous cell carcinoma partly though repressing APAF-1 expression, which enriches regulatory network of APAF-1 mediating apoptotic pathway.
Keywords: Laryngeal cancer; Mir-27a; Apaf-1; Proliferation; Apoptosis
6028Introduction
Balance of apoptosis and proliferation, which is required for maintenance of a steady-state cell number and tissue homeostasis, plays a critical role in cell renewal and organ regeneration [1-4]. Due to dysregulation of apoptosis or/and proliferation, the balance between them is disturbed, leading to carcinogenesis [5-7].
Apoptosis, programmed cell death, is the main type of normal cell death. As for apoptosis pathways .in mammals, the intrinsic and the extrinsic pathways have been concerned [8]. In cytochrome Cdependent apoptotic pathway (intrinsic pathway), APAF-1 is core component forming apoptosome together with Caspase-9 [9]. APAF-1 is also an important tumor suppressor gene and its functional loss is a common event in many cancers [10-14]. In addition to LOH and DNA methylation, microRNA (miRNA) dysregulation is the third mechanism for inactivation of tumor suppressor genes including APAF-1 [10-12].
In the previous study, we found that APAF-1 is significantly hypermethylated and down-regulated in laryngeal cancer, suggesting that APAF-1 is inactivated by DNA methylation in laryngeal cancer [15]. Additionally, we also found that up-regulation of microRNA-27a (miR-27a) promotes proliferation and represses apoptosis in laryngeal cancer cells [16]. Chen et al found that miR-27a alleviates hypoxiainduced neuronal apoptosis by targeting APAF-1 [17]. We speculate that miR-27a plays the similar roles via APAF-1 in human diseases including cancer.
In this study, we explored the correlation between miR-27a and APAF-1 and their roles in laryngeal cancer occurrence. We also analyzed the relationship between miR-27a level and clinicpathological characteristics in laryngeal cancer patients. Meanwhile, we reconfirmed the binding of miR-27a to APAF-1 mRNA 3'UTR and compared function of miR-27a and APAF-1 in regulation of laryngeal cancer cell proliferation and apoptosis (Table 1).
| Name | Sequence |
|---|---|
| miR-27a mimics | 5'- UUCACAGUGGCUAAGUUCCGC -3' |
| miR-27a inhibitor | 5'- GCGGAACUUAGCCACUGUGAA -3' |
| mimics NC | 5'-UUCUCCGAACGUGUCACGUTT-3' |
| inhibitor NC | 5'-CAGUACUUUUGUGUAGUACAA-3' |
| NC | 5'-GGCUACGUCCAGGAGCGCACC-3' |
| miRNA-27a | 5'- TTCACAGTGGCTAAGTTCCGC -3' |
| U6F | 5'-CTCGCTTCGGCAGCACA-3' |
| U6R | 5'-AACGCTTCACGAATTTGCGT-3' |
| APAF-1F | 5'-CCTCTCATTTGCTGATGTCG-3' |
| APAF-1R | 5'-TCACTGCAGATTTTCACCAGA-3' |
| GAPDH F | 5'-ATCATCAGCAATGCCTCC-3' |
| GAPDH R | 5'-CATCACGCCACAGTTTCC-3' |
Table 1: General information of the nucleotide sequences used in the study.
Materials And Methods
Patients and tissue samples
This study was approved by the Research Ethics Committee of China Medical University (Shenyang, China) and the 463th Hospital of PLA (Shenyang, China). Written informed consent was obtained from all patients. All specimens were handled and made anonymous according to the ethical and legal standards.
Forty-two laryngeal cancer patients who did not receive radio- or chemotherapy prior to surgery during 1999 and 2011 were enrolled in the study. All patients suffered from primary laryngeal cancer were selected randomly in the Otolaryngology Department of the 463th Hospital of PLA (Shenyang, China) and met the inclusion criteria during the period. Laryngeal carcinoma tissue and the paired normal tissue from each patient were stored at −80°C immediately after surgical resection. Evaluation of laryngeal cancer tissues in all patients was performed by a pathologist. Twenty-eight patients had received complete follow-up after surgery and median follow up period was 56.5 (range 30 to 111) months. Fourteen patients did not enter our follow-up cohort study, because six patients died within a year after surgery and eight patients did not respond to our first interview when six months after the operation.
Cells and cell culture
Human laryngeal cancer cells Hep-2 and human embryonic kidney cells HEK293 were bought from the Cell Biology Institute of Shanghai, Chinese Academy of Science. Cells were maintained in RPMI 1640 (GIBCO, Los Angeles, CA) with 100 units/ml penicillin, 100 μg/ml streptomycin and 10% fetal bovine serum (Hyclone, Logan, USA) in a humidified condition at 37°C with 5% CO2.
qRT-PCR analysis
Trizol reagent (Takara, Dalian, China) was used in total RNA extraction. miRcute miRNA isolation kit (tiangen, Bejing, China) was applied in microRNA separation. Small and total RNA concentrations were monitored according to the absorbance at OD260/280 nm.
qRT-PCR was performed to detect miR-27a and APAF-1 mRNA expression in LSCC tissues and cells by using the ABI 7500 Real Time PCR system (Applied Biosystems, Foster City, USA). miR-27a reverse transcription was performed using the One Step PrimeScript miRNA cDNA Synthesis Kit (Takara, Dalian, China) following the manufacturer’s instructions and quantitative PCR was carried out by using SYBR® Premix Ex Taq™ II (Takara, Dalian, China). U6 small nuclear RNA (snRNA) was used as control. The thermal cycling conditions for miR-27a and U6 snRNA included 95°C for 30 s, 40 cycles of 95°C for 5 s and 60°C for 34 s. In APAF-1 mRNA detection, reverse transcription was performed using the cDNA Synthesis Kit (Takara, Dalian, China) and quantitative PCR was carried out by using SYBR® Premix Ex Taq™ II (Takara, Dalian, China) and GAPDH was used as control. The PCR conditions in APAF-1 and GAPDH mRNA synthesis were 95°C for 30 min, 40 cycles of 95°C for 5 s and 60°C for 34 s. miR-27a and APAF-1 mRNA levels were normalized to the internal U6 and GAPDH mRNA levels, respectively. Equation 2-ΔΔCt was used to evaluate the fold change in miR-23a or APAF-1 mRNA level. Fold change ≤ 2 or >2 indicate low or high miR-27a expression level, respectively [18].
Western blot analysis
Protein extraction reagent (Beyotime, Shanghai, China) was used to isolate proteins from laryngeal cancer tissues and cells and BCA Protein Assay kit (Beyotime, Shanghai, China) was applied to measure protein concentration. Fifty μg of protein from each sample was separated on 8% SDS-PAGE and transferred to a PVDF membrane. Membrane was then blocked with 5% non-fat milk and incubated with anti-APAF-1 antibody (1:500 dilution; Abcam, Cambridge, USA) followed by horseradish peroxidase-conjugated antibody (1:2000 dilution; ZhongShan, China). Membrane was stained with ECL Plus (Beytime, Nantong, China) and exposed to a film (Fuji, Japan). β-actin was used for normalization.
Cell viability detection
Two to three thousands of Hep-2 cells tansfected with different small RNAs were plated into 96-well plates in triplicate and cultured for 24 h, 48 h, 72 h, 96 h and 120 h, respectively. The cells were then incubated with 100 μl sterile MTT dye (0.5 mg/ml, Sigma) for 4 h at 37°C and 150 μl DMSO for 15 min. Cell growth curve was constructed by using OD 490 nm as ordinate axis.
Colony formation detection
Hep-2 cells tansfected with different small RNAs for 12 h were seeded at a density of 3000-5000 cells in a 60-mm Petri dishes and cultured in RPMI 1640 (GIBCO, Los Angeles, CA) with 10% fetal bovine serum. Fourteen days later, cell colonies were fixed with methanol for 30 min, stained with hematoxylin for 20 min, and scored under a microscope. The mock and the scramble-treated cells were used as controls.
Cell apoptosis assay
Cells grown in 96-well plates to about 60% confluence were transiently transfected with the desired miRNAs and siAPAF-1 at a final concentration of 50 pmol. Cells were collected after transfection for 48 h, and washed with PBS. Apoptosis was then detected by Annexin V-EGFP Apoptosis Detection Kit (KeyGEN, Nanjing, China).
APAF-1-3'-UTR activation detection
miRanda (http://cbio.mskcc.org/cgi-bin/mirnaviewer/mirnaviewer.pl?type=miRanda), pictar (http://pictar.mdc-berlin.de/) and TargetScan (http://www.targetscan.org/) were applied to predict the potential binding of miR-27a to APAF-1-3'UTR. GV272- APAF-1-3'UTR and GV272-APAF-1-3'UTR-mut vectors were constructed by GENECHEM (Shanghai, China). GV272- APAF-1-3'UTR or GV272-APAF-1-3'UTR -mut and related miRNAs (NC duplex; GenePharma) were cotransfected into HEK293 cells in 96-well plates. pRL-TK (Promega Corporation) was used as control. Dual-luciferase reporter assay kit (Promega Corporation, Madison, USA) was used to detect luciferase activity of the cells transfected for 24 h. Luciferase activity was read by a Chemiluminescence Meter (Promega Corporation, Madison, USA).
Statistical analysis
Data were presented as means ± standard deviation (SD) and analyzed by SPSS 16.0 software. miR-27a expression difference in laryngeal cancer was analyzed by a paired-samples T-test. Analytic correlation between miR-27a expression and clinic-pathological characteristics of laryngeal cancer patients was performed by Independent-Sample Test. Correlation between APAF-1 and miR-27a levels in laryngeal cancer was assessed by Pearson's product-moment correlation coefficient. Independent samples T-test and one-way ANOVA were applied to analyze the data from cell-based experiments. Survival differences were estimated by the log-rank test. Prognostic factors were evaluated by Univariate analyses (Cox proportional hazards regression model). Each experiment was performed in triplicate. Statistical significance is indicated as P< 0.05.
Results
MiR-27a overexpression and APAF-1 protein downregulation are negatively correlated in LSCC
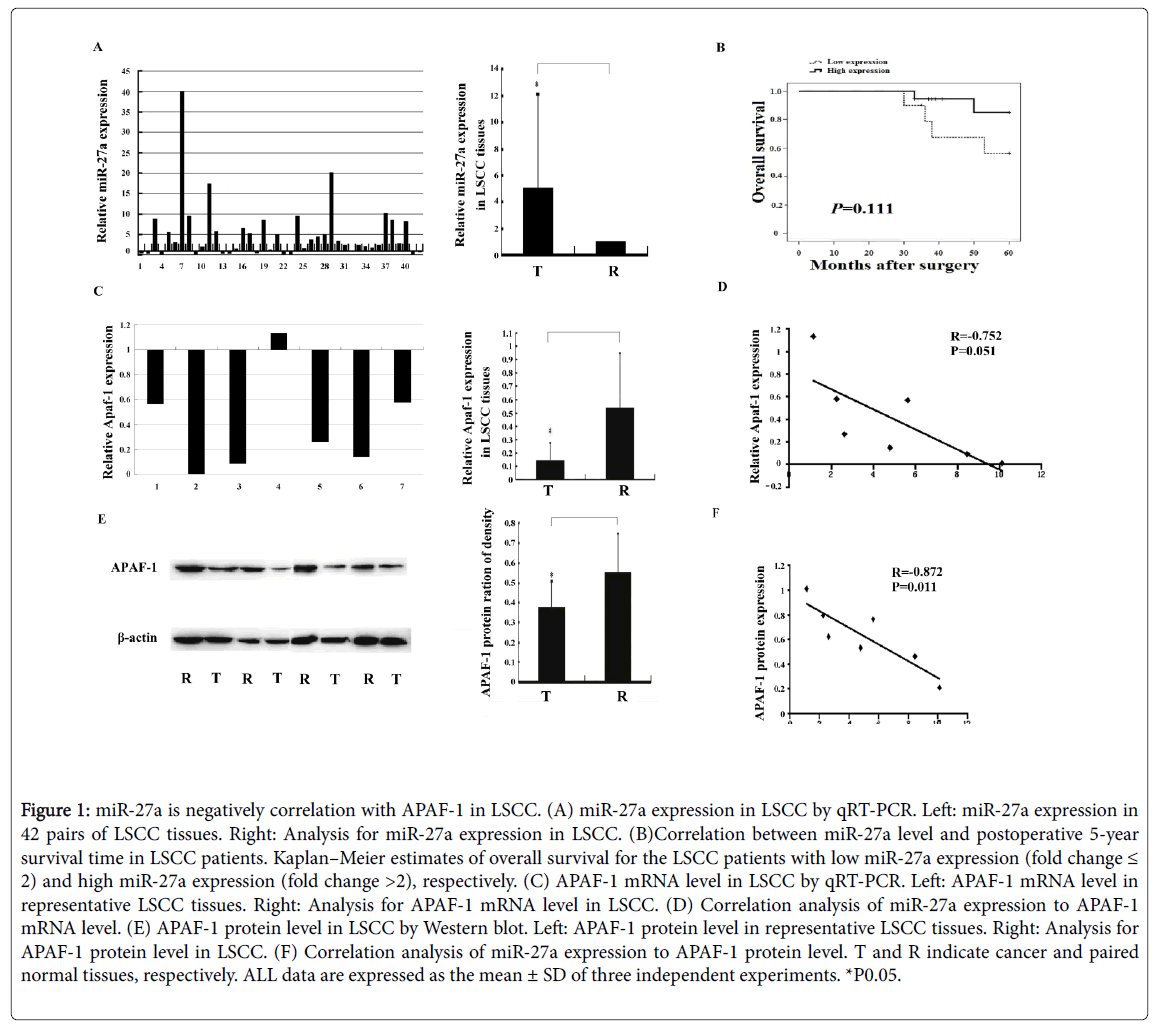
miR-27a upregulation was detected in 30 out of 42 cancer tissues (Figure 1A). In general, miR-27a was significantly overexpressed in cancer tissue compared to the normal control (Figure 1A). One-way ANOVO analysis results displayed that miR-27a expression was not significantly associated with patients' survival and other clinicpathological features including differentiation, clinical stage, lymph node metastasis and tumor depth (Figure 1B and Table 2).
| Features | No.of cases | miR-27a expression | P-value |
|---|---|---|---|
| Age | |||
| <60 | 23 | 5.52 ± 8.76 | 0.611 |
| ≥ 60 | 19 | 4.38 ± 4.56 | |
| Gender | |||
| male | 35 | 5.20 ± 7.63 | 0.684 |
| female | 7 | 3.99 ± 3.76 | |
| Differentiation | |||
| Moderate-well | 36 | 5.00 ± 7.48 | 0.981 |
| Poor | 6 | 5.07 ± 4.76 | |
| Tumor Depth | |||
| T1T2 | 18 | 4.61 ± 4.34 | 0.76 |
| T3T4 | 24 | 5.30 ± 8.72 | |
| Lymph node | |||
| Negative | 31 | 4.22 ± 4.89 | 0.236 |
| Positive | 11 | 7.21 ± 11.33 | |
| Stage | |||
| 15 | 4.43 ± 4.75 | 0.703 | |
| 27 | 5.32 ± 8.20 | ||
Independent-Sample Test was used to analyze the correlation between miR-27a expression and clinicopathological features.
Table 2: Association between miR-27a and clinicopathological characteristics in LSCC patients.
Figure 1: miR-27a is negatively correlation with APAF-1 in LSCC. (A) miR-27a expression in LSCC by qRT-PCR. Left: miR-27a expression in 42 pairs of LSCC tissues. Right: Analysis for miR-27a expression in LSCC. (B)Correlation between miR-27a level and postoperative 5-year survival time in LSCC patients. Kaplan–Meier estimates of overall survival for the LSCC patients with low miR-27a expression (fold change ≤ 2) and high miR-27a expression (fold change >2), respectively. (C) APAF-1 mRNA level in LSCC by qRT-PCR. Left: APAF-1 mRNA level in representative LSCC tissues. Right: Analysis for APAF-1 mRNA level in LSCC. (D) Correlation analysis of miR-27a expression to APAF-1 mRNA level. (E) APAF-1 protein level in LSCC by Western blot. Left: APAF-1 protein level in representative LSCC tissues. Right: Analysis for APAF-1 protein level in LSCC. (F) Correlation analysis of miR-27a expression to APAF-1 protein level. T and R indicate cancer and paired normal tissues, respectively. ALL data are expressed as the mean ± SD of three independent experiments. *P0.05.
Significant differences in lymph node metastasis and clinical stage were found in laryngeal carcinoma (Table 3), suggesting that lymph node metastasis and clinical stage are risk factors in progression of laryngeal cancer. Both APAF-1 mRNA and protein levels decreased significantly in laryngeal cancer tissue compared to normal control, respectively (Figures 1C and 1E). Statistically, miR-27a level was negatively correlated with APAF-1 protein level but not mRNA level in laryngeal cancer, respectively (Figures 1D and 1F).
| Univariate analysis hazard ratio (95% confidence interval) |
P value | |
|---|---|---|
| Gender | ||
| Male vs female | 1.294 (0.397-4.219) | 0.669 |
| Age | ||
| ≥ 60 vs <60 | 1.027 (0.981-1.076) | 0.256 |
| Smoking | ||
| Smoker vs Nonsmoker | 1.544 (0.198-12.054) | 0.678 |
| Drinking | ||
| Drinker vs nondrinker | 1.418 (0.43-4.679) | 0.566 |
| Differentiation | ||
| Poor vs moderate vs well | 0.777 (0.374-1.617) | 0.501 |
| Tumor depth (PT) | ||
| T4vs T3 vs T2 vs T1 | 1.727 (0.986-3.023) | 0.056 |
| Lymph node metastasis | ||
| Positive vs negative | 3.966 (1.588-9.9) | 0.003* |
| Clinical stage | ||
| vs vs vs | 4.674 (1.373-15.909) | 0.014* |
| miR-27a expression | ||
| High vs low | 1.169 (0.375-3.643) | 0.788 |
| *P<0.05. | ||
Table 3: Univariate Cox hazard regression analysis for prognostic factors in LSCC.
miR-27a is reconfirmed to target APAF-1-3’UTR
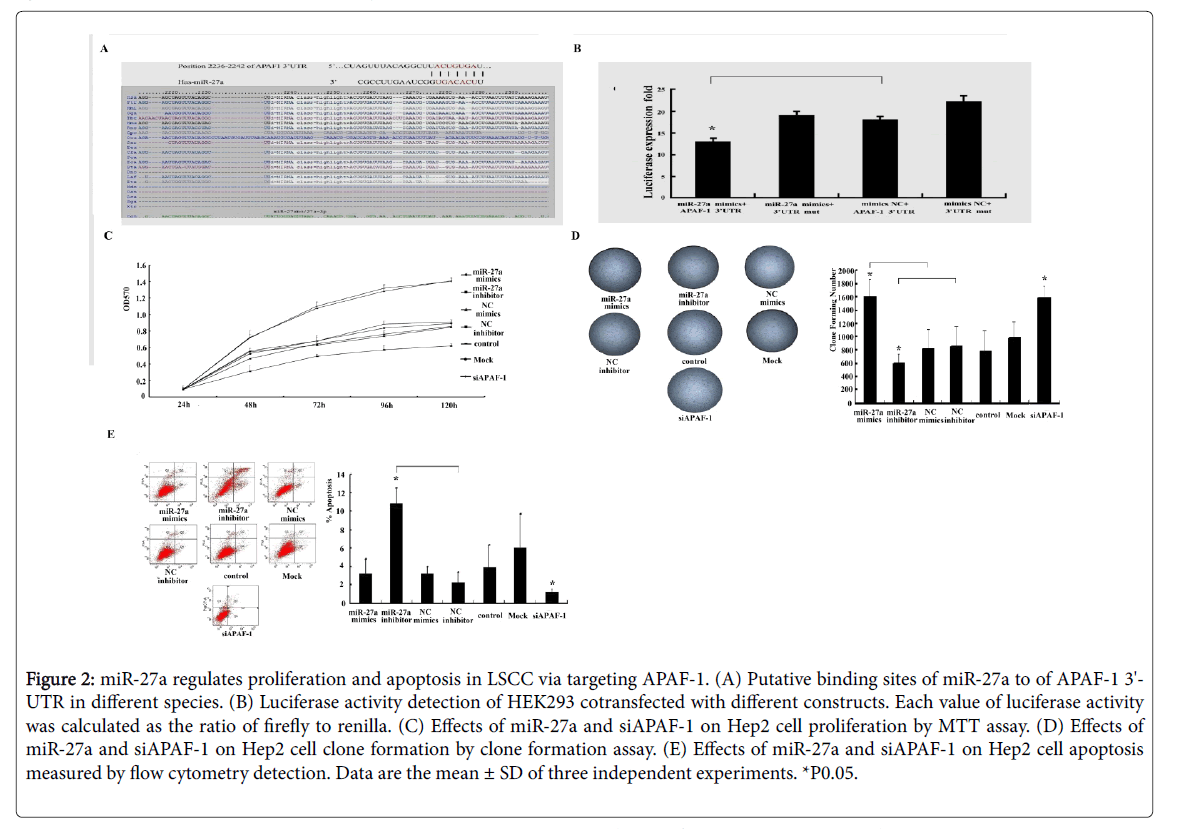
By prediction, we found a highly-conserved miR-27a binding sequence in APAF-1 3'-untranslated region (3'-UTR), suggesting that APAF-1 is a candidate target of miR-27a (Figure 2A). We also discovered that luciferase activity significantly decreased in the presence of miR-27a in HEK293 cells cotransfected with GV272- APAF-1 3’UTR but not with GV272-3’UTR-mut (Figure 2B). Taken together with the evidence that miR-27a expression is negatively correlated with APAF-1 expression in LSCC (Figure 1F), we speculate that miR-27a directly regulates APAF-1 expression in laryngeal carcinoma.
MiR-27a overexpression and APAF-1 knockdown promote proliferation and suppress apoptosis in Hep-2 cells
As shown in Figures 2C and 2D, miR-27a mimics and inhibitor significantly increased and decreased cell viability and colony formation compared to the controls, respectively. There were significant increase and decrease in the early apoptosis in the Hep-2 cells transfected with miR-27a inhibitor and mimics compared to the controls, respectively (Figure 2E). Similar to miR-27a mimics, siAPAF-1 significantly promoted cell viability and colony formation and repressed early apoptosis in laryngeal cancer cells, respectively (Figures 2C-2E).
Figure 2: miR-27a regulates proliferation and apoptosis in LSCC via targeting APAF-1. (A) Putative binding sites of miR-27a to of APAF-1 3'- UTR in different species. (B) Luciferase activity detection of HEK293 cotransfected with different constructs. Each value of luciferase activity was calculated as the ratio of firefly to renilla. (C) Effects of miR-27a and siAPAF-1 on Hep2 cell proliferation by MTT assay. (D) Effects of miR-27a and siAPAF-1 on Hep2 cell clone formation by clone formation assay. (E) Effects of miR-27a and siAPAF-1 on Hep2 cell apoptosis measured by flow cytometry detection. Data are the mean ± SD of three independent experiments. *P0.05.
Discussion
Laryngeal cancer, commonly found in the upper aerodigestive tract, severely affects life quality of the patients [19]. Presently, searching for molecular biomarkers seems to be the best way on laryngeal cancer diagnosis and treatment study.
microRNA (miRNA) is a kind of small noncoding RNAs and has been described as a "fine-tuner" in various cellular events [20]. miR-122 has been considered as a unique molecule with great potential in diagnosis, therapy and prognosis of liver disease [21]. Importantly, miRNA is detectable in lots of human fluids such as blood, stool, bile, saliva and urine, indicating that miRNA is the most useful biomarker of human diseases at presence [22].
miR-27a can target different genes and regulate a series of pathological processes including osteoarthitis, viral infections, sepsis, cardiomyocyte hypertrophy and cancer, implying that miR-27a act as an important regulator in human diseases [23-28]. However, different groups have discovered that miR-27a plays suppressive or oncogenic role in different cancer. For examples, miR-27a is a tumor suppressor in glioblastoma, esophageal squamous cell carcinoma and acute leukemia [29-31]. However, oncogenic role of miR-27a is found in most cancer such as breast cancer, chonic myeloid leukemia, renal cancer, hepatocellular cancer, colon cancer, ovarian cancer, prostate cancer and pancreatic cancer, respectively, suggesting that miR-27a has tissue specificity [32-39]. In laryngeal cancer, only our group found that miR-27a is a potential oncogene and plays its role through targeting PLK2 [16]. Therefore, further investigation of molecular mechanism of miR-27a in laryngeal cancer is necessary.
During development in mouse cortex, miR-27a expression is reversely correlated with APAF-1 expression [17]. In the present study, we found that there is negative correlation between miR-27a and APAF-1 protein levels in laryngeal cancer. However, we did not found a significant correlation between miR-27a and APAF-1 mRNA levels. We speculate that there are two reasons. One is that miR-27a induces APAF-1 protein degradation via targeting APAF-1 and another one dues to small size of samples, which will be investigated in our future work.
We also reconfirmed that miR-27a can target APAF-1 directly. Furthermore, miR-27a overexpression and APAF-1 knockdown have the similar function in regulation of laryngeal cancer cell viability and apoptosis. These results present a novel opinion that miR-27a might play its roles in laryngeal cancer though APAF-1-mediated apoptotic pathway. In addition to miR-27a, APAF-1 is also the target of several other miRNAs such as miR-17, miR-221, miR-155, miR-23a and miR-24a [40-44]. Therefore, our study enriches APAF-1-associated miRNA regulatory network, which helps us better understand cytochrome C-dependent apoptotic pathway.
In the study, we found that lymph node metastasis and clinical stage are risk factors in laryngeal cancer progression, which is also confirmed in other studies [45-47]. Moreover, we did not find significant correlation between miR-27a expression and survival time and other clinic-pathological features of laryngeal cancer, which implies that the sample size is relatively small in the study. Thus, we will collect and analyze many more samples in the future study.
In conclusion, up-regulation of miR-27a directly inhibits APAF-1 expression, leading to increase of proliferation and decrease of apoptosis in LSCC, which provides a novel regulator in the intrinsic apoptotic pathway.
Acknowledgments and Funding
This work was supported by the National Natural Science Foundations of China (81172577 and 81372876). We also appreciate Professor Hong-Bo Liu (Department of Health Statistics, School of Public Health, China Medical University) for some good advice about statistical analysis.
References
- Cinquin A, Chiang M, Paz A, Hallman S, Yuan O, et al. (2016) Intermittent Stem Cell Cycling Balances Self-Renewal and Senescence of the C. elegans Germ Line.PLoS Genet 12: e1005985.
- Zhou Y, Xu JC, Jia YF, Xu CS (2015) Role of death receptors in the regulation of hepatocyte proliferation and apoptosis during rat liver regeneration.Genet Mol Res 14: 14066-14075.
- Bortner CD1, Cidlowski JA (2014) Ion channels and apoptosis in cancer.Philos Trans R SocLond B BiolSci 369: 20130104.
- Mommers EC, van Diest PJ, Leonhart AM, Meijer CJ, Baak JP (1999) Balance of cell proliferation and apoptosis in breast carcinogenesis.Breast Cancer Res Treat 58: 163-169.
- Kutanzi KR, Koturbash I, Bronson RT, Pogribny IP, Kovalchuk O (2010) Imbalance between apoptosis and cell proliferation in female genital tract malignacies. Mutat Res 694: 1-6.
- Fu C, Wan Y, Shi H, Gong Y, Wu Q, et al. (2016) Expression and regulation of CacyBP/SIP in chronic lymphocytic leukemia cell balances of cell proliferation with apoptosis.J Cancer Res ClinOncol 142: 741-748.
- Kannan N, Eaves CJ2 (2014) Tipping the balance: MTDH-SND1 curbs oncogene-induced apoptosis and promotes tumorigenesis.Cell Stem Cell 15: 118-120.
- Hassan M, Watari H, AbuAlmaaty A, Ohba Y, Sakuragi N (2014) Apoptosis and molecular targeting therapy in cancer.Biomed Res Int 2014: 150845.
- Bratton SB, Salvesen GS (2010) Regulation of the Apaf-1-caspase-9 apoptosome.J Cell Sci 123: 3209-3214.
- Zhang XW, Liu N, Chen S, Wang YE, Sun KL, et al. (2015) Upregulation of microRNA-23a regulates proliferation and apoptosis by targeting APAF-1 in laryngeal carcinoma. Oncol Lett 10: 410-416.
- Liu N, Sun YY, Zhang XW, Chen S, Wang Y, et al. (2015) Oncogenic miR-23a in Pancreatic Ductal Adenocarcinogenesis Via Inhibiting APAF1.Dig Dis Sci 60: 2000-2008.
- Yong FL, Wang CW, Roslani AC, Law CW (2014) The involvement of miR-23a/APAF1 regulation axis in colorectal cancer.Int J MolSci 15: 11713-11729.
- Tanase C, Albulescu R, Codrici E, Calenic B, Popescu ID, et al. (2014) Decreased expression of APAF-1 and increased expression of cathepsin B in invasive pituitary adenoma.Onco Targets Ther 8: 81-90.
- Paik SS, Jang KS, Song YS, Jang SH, Min KW, et al. (2007) Reduced expression of Apaf-1 in colorectal adenocarcinoma correlates with tumor progression and aggressive phenotype. Ann SurgOncol 14: 3453-3459.
- Huang DF, Fu WN, Shang C, Xu ZM, Li ZG, et al. (2004) Expression and promoter methylation of Apaf-1 gene in laryngeal squamous cell carcinoma. Yi ChuanXueBao 31: 1327-1331.
- Tian Y, Fu S, Qiu GB, Xu ZM, Liu N, et al. (2014) MicroRNA-27a promotes proliferation and suppresses apoptosis by targeting PLK2 in laryngeal carcinoma.BMC Cancer 14: 678.
- Chen Q, Xu J, Li L, Li H, Mao S, et al. (2014) MicroRNA-23a/b and microRNA-27a/b suppress Apaf-1 protein and alleviate hypoxia-induced neuronal apoptosis.Cell Death Dis 5: e1132.
- Li X, Shi Y, Yin Z, Xue X, Zhou B (2014) An eight-miRNA signature as a potential biomarker for predicting survival in lung adenocarcinoma.J Transl Med 12: 159.
- Du L, Li H, Zhu C, Zheng R, Zhang S, et al. (2015) Incidence and mortality of laryngeal cancer in China, 2011.Chin J Cancer Res 27: 52-58.
- Naito Y, Tanaka Y, Ochiya T (2015) microRNAs and Hepatitis B.AdvExp Med Biol 888: 389-399.
- Thakral S, Ghoshal K (2015) miR-122 is a unique molecule with great potential in diagnosis, prognosis of liver disease, and therapy both as miRNA mimic and antimir.Curr Gene Ther 15: 142-150.
- Igaz I1, Igaz P (2015) Diagnostic Relevance of microRNAs in Other Body Fluids Including Urine, Feces, and Saliva.EXS 106: 245-252.
- Tardif G, Hum D, Pelletier JP, Duval N, Martel-Pelletier J (2009) Regulation of the IGFBP-5 and MMP-13 genes by the microRNAs miR-140 and miR-27a in human osteoarthritic chondrocytes.BMC MusculoskeletDisord 10: 148.
- Buck AH, Perot J, Chisholm MA, Kumar DS, Tuddenham L, et al. (2010) Post-transcriptional regulation of miR-27 in murine cytomegalovirus infection.RNA 16: 307-315.
- Wang Z, Ruan Z, Mao Y, Dong W, Zhang Y, et al. (2014) miR-27a is up regulated and promotes inflammatory response in sepsis.Cell Immunol 290: 190-195.
- Ye H, Ling S, Castillo AC, Thomas B, Long B, et al. (2013) Nebivolol induces distinct changes in profibrosis microRNA expression compared with atenolol, in salt-sensitive hypertensive rats. Hypertension 61: 1008-1013.
- Gottardo F, Liu CG, Ferracin M, Calin GA, Fassan M, et al. (2007) Micro-RNA profiling in kidney and bladder cancers.UrolOncol 25: 387-392.
- Liu T, Tang H, Lang Y, Liu M, Li X (2009) MicroRNA-27a functions as an oncogene in gastric adenocarcinoma by targeting prohibitin.Cancer Lett 273: 233-242.
- Li WQ, Yu HY, Zhong NZ, Hou LJ, Li YM, et al. (2015) miR‑27a suppresses the clonogenic growth and migration of human glioblastoma multiforme cells by targeting BTG2.Int J Oncol 46: 1601-1608.
- Zhu L, Wang Z, Fan Q, Wang R, Sun Y (2014) microRNA-27a functions as a tumor suppressor in esophageal squamous cell carcinoma by targeting KRAS.Oncol Rep 31: 280-286.
- Scheibner KA, Teaboldt B, Hauer MC, Chen X, Cherukuri S, et al. (2012) MiR-27a functions as a tumor suppressor in acute leukemia by regulating 14-3-3θ.PLoS One 7: e50895.
- Zhang S, Ma C, Pang H, Zeng F, Cheng L, et al. (2016) Arsenic trioxide suppresses cell growth and migration via inhibition of miR-27a in breast cancer cells.BiochemBiophys Res Commun 469: 55-61.
- Jurkovicova D, Lukackova R, Magyerkova M, Kulcsar L, Krivjanska M, et al. (2015) microRNA expression profiling as supportive diagnostic and therapy prediction tool in chronic myeloid leukemia. Neoplasma 62: 949-958.
- Li W, Liu M, Xu YF, Feng Y, Che JP, et al. (2014) Combination of quercetin and hyperoside has anticancer effects on renal cancer cells through inhibition of oncogenic microRNA-27a. Oncol Rep 31:117-124.
- Wu XJ, Li Y, Liu D, Zhao LD, Bai B, et al. (2013) miR-27a as an oncogenic microRNA of hepatitis B virus- related hepatocellular carcinoma.Asian Pac J Cancer Prev 14: 885-889.
- Del Follo-Martinez A, Banerjee N, Li X, Safe S, Mertens-Talcott S (2013) Resveratrol and quercetin in combination have anticancer activity in colon cancer cells and repress oncogenic microRNA-27a.Nutr Cancer 65: 494-504.
- Xu L, Xiang J, Shen J, Zou X, Zhai S, et al. (2013) Oncogenic MicroRNA-27a is a target for genistein in ovarian cancer cells.Anticancer Agents Med Chem 13: 1126-1132.
- Fletcher CE, Dart DA, Sita-Lumsden A, Cheng H, Rennie PS, et al. (2012) Androgen-regulated processing of the oncomir miR-27a, which targets Prohibitin in prostate cancer. Hum Mol Genet 21: 3112-3127.
- Ma Y, Yu S, Zhao W, Lu Z, Chen J (2010) miR-27a regulates the growth, colony formation and migration of pancreatic cancer cells by targeting Sprouty2. Cancer Lett 298: 150-158.
- Song S, Seo HH, Lee SY, Lee CY, Lee J, et al. (2015) MicroRNA-17-mediated down-regulation of apoptotic protease activating factor 1 attenuates apoptosome formation and subsequent apoptosis of cardiomyocytes. BiochemBiophys Res Commun 465: 299-304.
- Sun X, Liu B, Zhao XD, Wang LY, Ji WY (2015) MicroRNA-221 accelerates the proliferation of laryngeal cancer cell line Hep-2 by suppressing Apaf-1.Oncol Rep 33: 1221-1226.
- Zang YS, Zhong YF, Fang Z, Li B, An J (2012) MiR-155 inhibits the sensitivity of lung cancer cells to cisplatin via negative regulation of Apaf-1 expression.Cancer Gene Ther 19: 773-778.
- Lian S, Shi R, Bai T, Liu Y, Miao W, et al. (2013) Anti-miRNA-23a oligonucleotide suppresses glioma cells growth by targeting apoptotic protease activating factor-1. Curr Pharm Des 19: 6382-6389.
- Walker JC, Harland RM (2009) microRNA-24a is required to repress apoptosis in the developing neural retina.Genes Dev 23: 1046-1051.
- Huang Y, Li Z, Zhong Q, Li G, Zhang Y, et al. (2014) Association of TBX2 and P21 expression with clinicopathological features and survival of laryngeal squamous cell carcinoma.Int J ClinExp Med 7: 5394-5402.
- Ma H, Lian M, Feng L, Li P, Hou L, et al. (2014) Factors contributing to lymph node occult metastasis in supraglottic laryngeal carcinoma cT2-T4 N0M0 and metastasis predictive equation. Chin J Cancer Res 26: 685-691.
- Ahmed RA, ShawkyAel-A, Hamed RH (2014) Prognostic significance of cyclin D1 and E-cadherin expression in laryngeal squamous cell carcinoma.PatholOncol Res 20: 625-633.
Citation: Ji X, Zhang XW, Liu N, Qiu GB, Xu ZM, et al. (2016) Mir-27a: A Regulator of Apaf-1, Modulate Proliferation and Apoptosis in Laryngeal Carcinoma. Diagn Pathol Open 1: 116. DOI: 10.4172/2476-2024.1000116
Copyright: ©2016 Ji X. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 12259
- [From(publication date): 6-2016 - Aug 30, 2025]
- Breakdown by view type
- HTML page views: 11183
- PDF downloads: 1076