Modified Graded Motor Imagery Programme Containing "Fekos Mirror Therapy method": A Novel Therapeutic Method for the Treatment of Shoulder Dysfunctions - a Pilot Study
Received: 18-Dec-2017 / Accepted Date: 26-Dec-2017 / Published Date: 31-Dec-2017 DOI: 10.4172/2165-7025.1000375
Abstract
Objective: Mirror therapy (MT) is an important technique in the rehabilitation of patients who experience pain and decreased function. However, there are limited research studies on its application and effectiveness in shoulder pathologies, especially when it is applied as a part of graded motor imagery (GMI). The present study aims to highlight a novel therapeutic approach for the treatment of shoulder painful pathologies, using an innovative Mirror Therapy (Fekos Mirror Therapy) method in combination with motor imagery.
Purpose: To investigate the efficacy of a novel MT included in an aggressive GMI protocol regarding pain reduction and range of motion (active shoulder flexion) restoration in patients with painful shoulder conditions.
Methods: A novel GMI programme (Implicit Motor Imagery, Explicit Motor Imagery, and Fekos Mirror Therapy) was applied in five female patients (age: 18 -77 years) with symptoms of pain and decreased active range of motion for more than three months, for four treatment sessions (one-hour sessions). Variables assessed in each session included the active shoulder flexion (SpineCor®), pain (VAS), accuracy and response time in laterality recognition of the body (Recognise Online™) and the motor imagery ability (the Kinesthetic and Visual Imagery Questionnaire/KVIQ - 10).
Results: KVIQ-10 showed improvement in the motor imagery ability for the two sub-scales. The active shoulder flexion was increased and the pain decreased in all five patients, but the last one with fluctuation. According to Recognise Online™ programme, only one patient scored higher response times and lower accuracy for the affected limb compared to the healthy one.
Conclusions: Central nervous system damage is present in chronic musculoskeletal patients, and GMI protocol containing Fekos Mirror Therapy technique may have a positive effect on the recovery of painful joint functional capacities. This method is in need of further implementation in randomized control studies for the confirmation of its efficacy.
Keywords: Mirror therapy; Shoulder; Graded motor imagery; Motor imagery; Pain; Active range of motion; Implicit motor imagery
Introduction
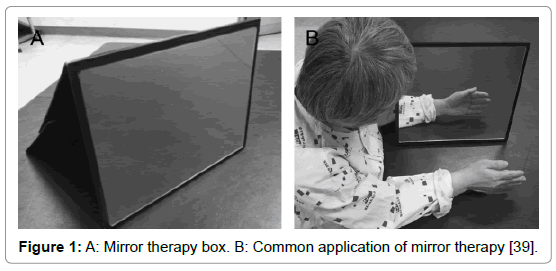
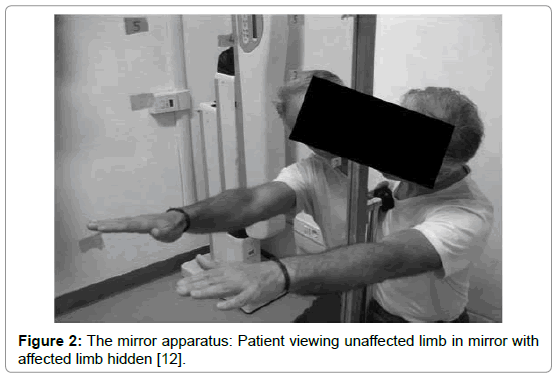
Mirror therapy (MT) is a therapeutical technique in which a mirror box, is utilized in the treatment of several musculoskeletal and neurological injuries and diseases. During MT, the patient stands in front of a mirror that is placed in the sagittal plane of his body, blocking the view of the (affected) limb, which is positioned behind the mirror. With this setting, the patient sees the reflection of the unaffected extremity placed as the affected one and a visual illusion was created in which a movement of the healthy extremity can be perceived as affecting the painful (or paretic) extremity (Figures 1 and 2) [1].
Figure 1: A: Mirror therapy box. B: Common application of mirror therapy [39].
Figure 2: The mirror apparatus: Patient viewing unaffected limb in mirror with affected limb hidden [12].
MT has been reported that it can improve sensory and motor deficits in stroke patients [2,3], reduce phantom limb pain in patients who had undergone amputation of lower limbs [4] and improve complex regional pain syndrome [5,6] and peripheral nerve injury [7]. The exercise protocol that the patient follows in MT consists of either synchronized or asynchronous exercises for both limbs [8].
MT is used in combination with the motor imagery technique for painful motor conditions, as a part of a technique called Graded Motor Imagery (GMI) [4]. By using this method, the therapist is given the possibility to guide patients with the aim of progressive and selective activation of brain cells. This therapeutic method comprises three progressive stages Implicit Motor Imagery (IMI), Explicit Motor Imagery (EMI) and Mirror Therapy [2,6,9-26]. The design of the process promotes a better cortical organization and brain function [16,27]. It is believed that each stage allows an increase in firing the brain regions, which are related to the desired activity, and aims to ignore the “explosion” of pain neurotag [27]. In clinical practice, the patient uses the IMI performing variable image orientation recognition exercises, in which the right or the left side of the body is represented [16,27,28]. The next stage of GMI includes EMI, which requires the patient to mentally represent specific positions of his body member [16,27]. This is a broader term of Mental Practice, which is defined as the widely repeated, conscious and systematic mental exercise, which improves performance [27,29]. The third stage involves the use of a mirror, which has been used for years to treat phantom limb pain and it has proven that there are clear beneficial effects of complex, painful situations [27,30]. In its usual clinical application, the affected limb is placed behind the mirror and the unaffected in front of it. This constitutes the major problem of this application in the shoulder region.
There are only few research efforts which aim to treat the region of the shoulder by the use of the MT, and most of them are based on the use of technology [16,31,32] or use uncomfortable positions for the average shoulder flexion [33]. Therefore, finding a new way of implementing MT would help in the treatment of the shoulder pathologies. The purpose of this research effort is the pilot implementation of a therapeutic protocol using a mirror for the treatment of shoulder, in combination with motor imagery techniques.
Methods
Participants
Five female patients (age range: 18-77 - the average age of approximately 50.3 years) participated in the study. These patients were suffering from diseases of the shoulder girdle (Table 1), which are accompanied by pain and limited range of motion. All of them a) were out-clinic patients, b) had a medical diagnosis of their pathology and c) were referred for physiotherapy. Before their participation in the survey, participants were informed about the goals of the study and signed an informed consent.
| Patients | Medical diagnosis |
|---|---|
| Patient 1 | Supraspinatus muscle tendinitis |
| Patient 2 | Frozen shoulder, after partial rupture of the supraspinatus muscle tendon |
| Patient 3 | Supraspinatus muscle tendinitis |
| Patient 4 | Ankylosing spondylitis, with stiffness and pain in the shoulder |
| Patient 5 | Fracture of the clavicle and shoulder pain |
Table 1: Medical diagnosis of participants.
Inclusion/exclusion criteria
Inclusion criteria for this research included the reduced active range of shoulder flexion and pain in the shoulder area, for longer than three months (> 3 months). Patient who experienced additional pain, spread in the shoulder area, were not excluded. Exclusion criteria included a) neurological disease, b) psychiatric pathologies, c) all kinds of cancer d) acquired immunodeficiency syndrome and e) rare pathologies. The first two exclusion criteria were set because the patients should fully understand and be able to be focused on the treatment. The decision to exclude patients who met one or more of the following criteria (c, d, e) obtained due to insufficient knowledge about the human body’s reactions after application of this treatment programme.
Experimental procedure
The research process was characterized by eight days duration and the repetitions of treatment were about twenty; the interventions and measurements took place in the same room of a private physiotherapy clinic. The interventions and evaluations conducted in a room (3.60 m × 2.40 m × 2.45 m) in which it was possible to regulate lighting, temperature, noise and the number of present people. The adjustment of the light and the room temperature were done with switches that were on the wall. Additionally, the room lighting set with the help of covering the windows, using a manual mechanism. Regarding the noise and the presence of other people in the room, there was a door which insulates exterior noise, the windows were closed, and the entrance to a third person was strictly prohibited during the treatments. The selection of the treaties was to facilitate the gathering of patients, through a mild temperature and dark environment. The room conditions were fixed for each session and listed in Table 2.
| Temperature | 22°C |
| Lighting | Low, High |
| Present people | Patient and physiotherapist (2 people) |
Table 2: Treatment room conditions.
The temperature and the number and kind of attended persons remained stable. Instead, the lighting changed depending on the requirements of the treatment programme. More specifically, upon completion of the demographic form and general patient assessment, during measuring the range of motion and the execution of Fekos Mirror Therapy method, the room was bright, because the vision was an essential element for their execution. However, during the stages EMI [19] and IMI and during the assessment of motor imagery ability, the lighting remained low, in order to prevent external threatening visual stimuli of light and increase the concentration of the patients.
During the research process, the patients were placed in a sitting position to complete the demographic form and general patient assessment, the measurement of range of motion, and the performance of IMI and EMI. Dickstein and Deutsch emphasize that the sitting position is particularly important to promote the relaxation of the necessary concentration of EMI [34], as required. For the implementation of innovative Fekos Mirror Therapy method programme, the participants were upright. The evaluation of motor imagery ability was done with the patients placed in sitting position for the points A, B and D, while in standing position for the points C and E. During the sitting position, a chair without arm support used, while during the performance of IMI, a physiotherapy bed was used for placing the computer.
The patients were informed for the first time, for the therapeutic intervention, the first day they came for treatment. The information included the number of sessions, the duration of each session, a report of the three stages of the treatment programme, by name, and the type and number of measurements. All the interventions and measurements were made by the same physiotherapist to secure homogeneity of intervention because this therapeutic procedure is characterized by high–level guidance and it is highly dependent on the therapist. Completion of demographic data and the pain measurement was done in collaboration between the patient and the physiotherapist, and he had the role of explaining them the points which had to be filled. The patients received four therapeutic interventions in total. They were asked to come consecutive days, to decrease the unpleasant situations of additional external injuries, which would alter the results of the research process. Each intervention lasted one hour, approximately, and was based on the following techniques: motor imagery and MT. The therapeutic programme also was based on the principle of progressivity and consisted of three parts [16,27]. These were applied with the following series: IMI, EMI and Fekos Mirror Therapy method.
Treatment method
Implicit motor imagery: The left/right judgment exercises [21,35] were applied to the shoulder area and the data were collected through the Recognise Online™ programme [27,36]. Tests were applied to the following image categories basic, vanilla, context, and abstract, based on the principle of progressivity.
a) Procedure: The patient was sitting in front of a laptop (Acer Extensa 5230E). The instructions they received were to place the right finger on the right button and the left finger on the left button of the keyboard, to choose as quickly and correctly as possible if presented a right or left shoulder image (Table 3).
| Implicit Motor Imagery | |||
|---|---|---|---|
| 1: Recognize | 2: Test | 3: category | 4: options |
| Left & Right | Basic | Shoulders | show 20,30,40 |
| Vanilla | display for 10 sec | ||
| Context | |||
| Abstract | |||
Left & Right, basic, shoulders, show 20 + display for 10 sec
Left & Right, basic, shoulders, show 20 + display for 10 sec
Left & Right, basic, shoulders, show 30 + display for 10 sec
Left & Right, basic, shoulders, show 30 + display for 10 sec
2nd session:
Left & Right, basic, shoulders, show 20 + display for 10 sec
Left & Right, vanilla, shoulders, show 20 + display for 10 sec
Left & Right, vanilla, shoulders, show 30 + display for 10 sec
Left & Right, vanilla, shoulders, show 40 + display for 10 sec
3rd session:
Left & Right, vanilla, shoulders, show 20 + display for 10 sec
Left & Right, context, shoulders, show 20 + display for 10 sec
Left & Right, context, shoulders, show 30 + display for 10 sec
Left & Right, context, shoulders, show 40 + display for 10 sec
4th session:
Left & Right, context, shoulders, show 20 + display for 10 sec
Left & Right, abstract, shoulders, show 20 + display for 10 sec
Left & Right, abstract, shoulders, show 30 + display for 10 sec
Left & Right, abstract, shoulders, show 40 + display for 10 sec
Table 3: Implicit motor imagery programmeme.
Explicit motor imagery: A series of voluntary movements was applied mentally in the next step of the GMI programme. The aim was that the patients to represent anatomically and functionally the shoulder flexion, through a considerably guiding programme. The main body of the programme ‘Explicit Motor Imagery” was based on the research of Frenkel et al. [37] and it was partially modified.
a) Procedure: Patients were seated in a chair with their eyes closed, during most time of the programme (Tables 4 and 5).
| Explicit Motor Imagery |
| Level 1: movement of the joint A) Select photos of the affected limb. B) List of images of the affected (treating) area. C) Design the range of motion, and level and direction of movement. Design: • C1) initial, comfortable / neutral 0 position of the limb, • C2) final position of the limb, • C3) restoring position / initial position of the limb, • C4) range of motion with arrows. |
| Level 2: description of the movement. A) The therapist moves his or her limb (the same limb with the patient’s affected side) to teach him (x 3). They are shown: • A1) initial, comfortable / neutral 0 position of the limb, • A2) final position of the limb, • A3) returning position / initial position of the limb, • A4) trajectory of the movement. B) The therapist moves the patient's healthy limb to teach him about the movement: a) with open eyes and b) with closed eyes (x 3) • B1) initial, comfortable / neutral 0 position of the limb, • B2) final position of the limb, • B3) returning position / initial position of the limb, • B4) trajectory of movement. C) The therapist asks the patient to express the movement verbally (x 1). |
| Level 3: guidance of the movement, key points identification A) Key points: • A1) Up and slowly: controlled flexion, • A2) Down and slowly: controlled extension (return). B) Linking the key points to the kinesthetic concept: • B1) pilot execution with the healthy limb. |
| Level 4: Mental Practice and movement with the healthy limb of the body (Motor Imagery teaching) A) Active movement with the healthy limb (3 x open eyes) and observation of the limb. B) "Visual Motor imagery" (2 x healthy, 2 x affected): mental representation with closed eyes of static positions (initial position, middle position, final position). C) Active movement with the healthy side (3 x closed eyes). D) Kinaesthetic Motor Imagery (2 x healthy, 2 x affected): mental representation of the movement with closed eyes and the ability to see and feel herself moving. |
| Level 5: Mental Practice programme execution A) Active movement with the healthy limb (3 x open eyes) and observation of the limb B) Kinaesthetic Motor Imagery: • 1 x healthy, 1 x affected, • 1 x healthy, 3 x affected, • 1 x healthy, 5 x affected, • break for 1 minute • - , 10 x affected Notes: The patient opens the eyes in every limb switch. The patient reports the word "stop" whenever a motor imagery process ends. C) Kinaesthetic Motor Imagery of a functional activity (integration of memory with functional activity through "Motor Imagery"): • C1: Question about activity, • C2: Execution (1x healthy, 3 x affected) |
| Level 6: Active physical execution of the movement. Execution of the movement once (highest level: 3 in Oxford scale). |
Table 4: Explicit motor imagery programme.
| 1st session | 2ndsession | 3rdsession | 4thsession | |
|---|---|---|---|---|
| Patient 1 | reach and grasp the book of the tall shelf | reach and grasp the book of the tall shelf | reach and grasp the plate of the tall shelf | reach and grasp the plate of the tall shelf |
| Patient 2 | reach and grasp the glass of the tall shelf | reach and grasp the glass of the tall shelf | reach and grasp the vase of the tall shelf | reach and grasp the vase of the tall shelf |
| Patient 3 | clean the highly located shelf with a cloth | clean the highly located shelf with a cloth | clean the ceiling with a cloth | clean the ceiling with a cloth |
| Patient 4 | clean the highly located shelf with a cloth | clean the highly located shelf with a cloth | paint the ceiling with a cloth | paint the ceiling with a cloth |
| Patient 5 | reach and grasp the cup of the tall shelf | reach and grasp the cup of the tall shelf | paint the ceiling with a cloth | paint the ceiling with a cloth |
Table 5: The functional tasks, which were executed for the level 5 (C2).
Fekos mirror therapy method: During the third and final stage of GMI, the patients performed a series of specially designed exercises using a mirror.
a) Procedure: The patients were placed just in front of a mirror (dimensions: 104 cm × 50 cm) (Figure 3). The mirror was made of sharp glass, which does not deform the reflected body. Before the programme implementation, the factor of similarity of the two body sides was tested, the lack of which would reduce the quality of illusion, and so the objects from the upper limbs of patients were removed. The aim of the process was to create the impression that the patients see the suffered shoulder in the mirror through the illusion, when in fact the reflection of the healthy side is depicted. In order to achieve this, the therapist explained in detail such complex procedure, during the first appointment.
The discussion included the following:
- Physiotherapist: Look at the mirror and shake the fingers of your right hand. Which hand do you see in the mirror?
- Patient: I see the right one.
- Physiotherapist: Look again and think of yourself appearing in the mirror.
- Patient: Yes
- Physiotherapist: Now observe which of the two hands moves.
- Patient: The left!!!
- Physiotherapist: Are you sure that it is the left?
- Patient: Yes, I see my left hand moving!!!
After understanding the therapeutic procedure, the therapeutic programme for the shoulder area was launched (Tables 6 and 7). The treatment was based on the suggestive programme [27]. During the programme, the patients focused on their affected limb presented in the mirror, and were asked not to take their eyes from the “reflected and affected” area of the shoulder. Unlike the fundamental view about the simultaneous movements of the two limbs [5,27,30], in this research, the exercises are performed separately for the affected and non-affected limb. The execution of movements to the “real affected” side was away from the mirror, 90 degrees shift of the patient, to look on the wall. Such choice can be justified by two reasons (Figure 4). The first reason is the existence of the possibility to display worsening pain and functional impairment due to the simultaneous mobilization of the hidden and the visible limb. The second explanation is related to avoid causing any visual confusion between the affected and non-affected side of the body, which will lead to the disturbance of the acquired illusion. Perhaps, this means that the illusion might be kept after the absence of the mirror where the mind is focused on the illusory activity. To make the illusion last longer, the therapist was guiding the patient in the following way:
- Physiotherapist: All those times you saw yourself lifting your arm (showing the affected upper extremity, but not called the word affected when in fact the patient lifts the healthy member) without being hurt. Is it true?
- Patient: Yes, it is true.
- Physiotherapist: Are you sure?
- Patient: Yes.
- Therapist: Okay, then raise it again to see it (the guidance was continuous, and it was the following: “up, up ... up, up and down, down ... down and make it come close to the body).
- Patient: ...executes the movement...
- Therapist: continue the movement for x times.
| Fekos Mirror Therapy | |
|---|---|
| Healthy Limb | Affected Limb |
| comfortable limb position – observing its reflection for 10-15 sec | relaxed limb |
| shoulder circle movements (10 forward, 10 backward) | relaxed limb |
| Shoulder flexion by 90ο and slow return × 10 | relaxed limb |
| shoulder flexion (full range of motion) × 10 | shoulder flexion by the limit of pain × 5 |
| shoulder flexion with holding an object (1kgr) × 5 | shoulder flexion with feeling some pain × 5 |
| dangerous task* which contains shoulder flexion × 5 | dangerous task* which contains shoulder flexion × 5 |
Table 6: Fekos Mirror Therapy programmeme.
| 1stsession | 2ndsession | 3rdsession | 4thsession | |
|---|---|---|---|---|
| Patient 1 | lift an opened scissor with minimally dangerous handle | lift a plastic cup filled up to 5cm with water | lift the air conditioner remote control as an expensive item | lift the air conditioner remote control as an expensive item |
| Patient 2 | lift an opened scissor with minimally dangerous handle | lift a plastic cup filled up to 5cm with water | lift the air conditioner remote control as an expensive item | lift the air conditioner remote control as an expensive item |
| Patient 3 | lift an opened scissor with minimally dangerous handle | lift a plastic cup filled up to 5cm with water | lift the air conditioner remote control as an expensive item | lift the air conditioner remote control as an expensive item |
| Patient 4 | lift a plastic cup filled up to 5cm with water | lift a plastic cup filled up to 5cm with water | lift the air conditioner remote control as an expensive item | lift the air conditioner remote control as an expensive item |
| Patient 5 | lift a plastic cup filled up to 5cm with water | lift a plastic cup filled up to 5cm with water | lift the air conditioner remote control as an expensive item | lift the air conditioner remote control as an expensive item |
Table 7: The dangerous tasks, which were executed by the patients.
The therapeutic programme should highlight that the last two series of exercises performed equally in regard to the number of repetitions. This decision was taken to inhibit the transmission of disparate messages to the brain, which may activate the pain neurotag [27] , as Moseley et al. report that the above proposal is valid for the simultaneous movement of the two body extremities [27], but without giving the reason for this validation. This might happens because of the existing illusion of the action created by the mirror at the time of observation, which is either lost when the upper limb stops moving or still exists but the brain creates an image of a stationary member. However, the changes of the body position and the vision in a region away from the mirror, combined with verbal guidance maintain an active illusion of previously driven member, providing a degree of similarity in the movements of the two sides of the body and preventing stimulation of the pain neurotag.
Methods for the evaluation of patients
Assessment of motor imagery ability: The sample of patients in regard to their ability they had to run the «motor imagery» programme was assessed by the use of the Kinesthetic and Visual Motor Imagery Questionnaire - 10 (KVIQ - 10) [38]. The choice of this questionnaire was done for specific reasons. Initially, the main concern was the economy of time because the patients were evaluated every time before implementing the programme. Therefore, it is taken into account the avoidance of mental fatigue of the patients, since the treatment programme was quite large. Secondly, according to the authors’ opinion, the questionnaire should be guided by the therapist, taking into account that the patients lack the familiarity with the procedure. Thirdly, this questionnaire considers the kinesthetic and visual dimension of mental representations, which were used in the treatment programme. Fourthly, it uses the first-person perspective, which was a feature point of the implemented EMI programme.
Range of motion measurements: The range of motion was measured by the manual goniometer SPINECOR®, while the patient was sitting down on a chair. The measurement involved the active flexion of the shoulder, with the upper limb starting from the neutral position. In each session, there were two measurements. The first was done at the beginning of the session and the second at the end of the session.
Pain measurement: The Visual Analog Scale (VAS) [39] numbered from 0 to 10 was utilized for the measurement of pain. Patients were asked to rate their levels of pain for that moment on the initial evaluation paper. The pain assessment was done before the start of the treatment programme, after EMI and after Fekos Mirror Therapy. Moreover, the pain was evaluated during the period of the IMI four times, as required by the programme Recognise Online™ [27].
Measurement and analysis of data of time selection and accuracy of responses by using the Recognise Online™ programme: The measurements of speed and accuracy of responses (correct answers), during practicing Recognise Online™ [27,36], were made by the same programme. The results showed the mean value of each trial on such parameters for each participant individually. Therefore, four values for speed and four for the accuracy of responses corresponding to each patient for each treatment session.
Results
The physical and demographic characteristics of the patients
All patients (100%) completed the treatment sessions. The 4 out of 5 patients (80%) attended the treatment sessions for four consecutive days. One patient completed the sessions within seven days due to personal obligations, which were not related to the condition (Table 8).
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Average | Standard deviation | |
|---|---|---|---|---|---|---|---|
| Sex | Female | Female | Female | Female | Female | - | - |
| Year of birth | 1997 | 1967 | 1938 | 1971 | 1945 | - | - |
| Age (years) | 18 | 48 | 77 | 44 | 69 | 51,2 | 23,2 |
| Height (m) | 1,63 | 1,70 | 1,62 | 1,74 | 1,51 | 1,64 | 0,1 |
| Weight (kgr) | 68 | 70 | 62 | 62 | 57 | 63,8 | 5,2 |
| Job | High school student | Household | Household | Private employee | Household | - | - |
| Prevalent limb | Right | Right | Right | Right | Right | - | - |
| Affected limb | Left | Right | Left | Left | Right | - | - |
| Pain duration | 4 months | 8 months | 11 months | 12 months | 3 months and 20 days | - | - |
| Previous pain | No | No | Yes (left upper limb) | No | Yes (right upper limb | - | - |
| Familiarity with computer use (excellent, medium, no familiarity) | Excellent | Excellent | No familiarity | Excellent | No familiarity | - | - |
Table 8: Physical and demographic characteristics of the patients.
Motor imagery ability
The Kinesthetic and Visual Imagery Questionnaire – 10 (KVIQ – 10) was applied successfully by all the patients (100%). Differences were observed between the sessions, for each patient individually and in general, regarding their ability to represent movements mentally.
For all the participants, the values of the results ranged from 12 to 22 units, for the visual representation of movements, and from 8 to 21 units, for the kinesthetic representation of movements. The average for each patient was higher in the “visual” than in the “kinesthetic” scale. Also, an increase in motor imagery ability (“visual” and “kinesthetic”) was observed, comparing the results of the first to the last session. The results of the assessment by KVIQ - 10 are presented in Table 9.
| KIVQ–10 (VISUAL - KINESTHETIC) | |||||
|---|---|---|---|---|---|
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
| 1stsession | 18 – 19 | 15 -15 | 15 - 17 | 17 - 15 | 12 – 8 |
| 2nd session | 19 – 19 | 22 – 21 | 21 – 19 | 18 -14 | 14 – 10 |
| 3rdsession | 19 – 20 | 20 – 20 | 19 - 19 | 19 – 16 | 18 – 13 |
| 4thsession | 19 – 21 | 20 – 20 | 19 - 18 | 19 - 15 | 15 – 14 |
| Average (±S.D.) | 18,75 (± 0,5) - 19,75 (± 0,96) | 19,25 (±2,99) - 19 (±2,7) | 18,5 (±2,5 ) - 18,25 (± 0,96) | 18,25 (±2,5) - 15 (± 0,81) | 14,75 (± 0,96) - 11,25 (± 2,75) |
Table 9: Kinesthetic and Visual Imagery Questionnaire –10 results.
Visual analog scale results (VAS, 0 – 10)
Pain measurements were performed for all patients as predetermined. The values of the visual analog scale scores of pain were reduced from the first to the last session, which indicates the improvement of the patient’s pain experience. However, the progress made by the patients was not continuous, because in some sessions there was a change in pain levels. This was attributed to the aggressiveness of the programme. The analytical results of pain assessment with the VAS are presented in Table 10.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| 1st session | |||||
| Before the session | 5/10 | 8/10 | 8/10 | 8/10 | 7/10 |
| After the session | 6/10 | 7/10 | 4/10 | 7/10 | 6/10 |
| 2nd session | |||||
| Before the session | 4/10 | 8/10 | 6/10 | 9/10 | 8/10 |
| After the session | 4/10 | 7/10 | 4/10 | 6/10 | 8/10 |
| 3rd session | |||||
| Before the session | 4/10 | 8/10 | 3/10 | 7/10 | 6/10 |
| After the session | 3/10 | 7/10 | 2/10 | 6/10 | 4/10 |
| 4th session | |||||
| Before the session | 1/10 | 6/10 | 5/10 | 5/10 | 5/10 |
| After the session | 1/10 | 6/10 | 1/10 | 5/10 | 5/10 |
Table 10: Visual analog scale results (VAS, 0-10).
Time response, accuracy and pain level results by the Recognise Online™ programme
Pain levels were measured during IMI for all patients (100%). The results of pain measurements were consistently presented in all treatment sessions for all patients. The values of the results were the same as the visual analog scale values of the pain at the beginning of the sessions. The specific values along with those of the times and the accuracy of the given answers are shown in the Table 11 and Figure 5.
| Response time (RT) (Left limb) | Response time (RT) (Right limb) | Accuracy (Leftlimb) | Accuracy(Rightlimb) | Pain | |
|---|---|---|---|---|---|
| Patient 1 | |||||
| 1st session | |||||
| 1.6 sec | 1.2 sec | 90% | 100% | 5/10 | |
| 1.0 sec | 1.3 sec | 100% | 100% | ||
| 1.3 sec | 1.0 sec | 100% | 93% | ||
| 1.3 sec | 1.2 sec | 93% | 100% | ||
| 2nd session | |||||
| 1.4 sec | 1.1 sec | 100% | 100% | 4/10 | |
| 1.7 sec | 1.5 sec | 90% | 100% | ||
| 1.6 sec | 1.4 sec | 100% | 100% | ||
| 1.7 sec | 1.4 sec | 90% | 100% | ||
| 3rd session | |||||
| 1.9 sec | 1.4 sec | 100% | 90% | 4/10 | |
| 1.5 sec | 1.4 sec | 100% | 100% | ||
| 2.0 sec | 2.1 sec | 100% | 93% | ||
| 2.0 sec | 1.8 sec | 100% | 90% | ||
| 4th session | |||||
| 1.7 sec | 1.5 sec | 100% | 100% | 1/10 | |
| 1.7 sec | 2.7 sec | 100% | 100% | ||
| 1.7 sec | 1.5 sec | 87% | 93% | ||
| 1.4 sec | 1.3sec | 100% | 100% | ||
| Patient 2 | |||||
| 1st session | |||||
| 1.4 sec | 1.2 sec | 100% | 100% | 8/10 | |
| 1.2 sec | 0.9 sec | 100% | 100% | ||
| 1.1 sec | 1.2 sec | 100% | 93% | ||
| 1.5 sec | 1.0 sec | 87% | 100% | ||
| 2nd session | |||||
| 1.1 sec | 1.1 sec | 100% | 100% | 8/10 | |
| 1.5 sec | 1.2 sec | 100% | 100% | ||
| 1.6 sec | 1.5 sec | 93% | 93% | ||
| 1.3 sec | 1.4 sec | 100% | 100% | ||
| 3rd session | |||||
| 1.9 sec | 1.5 sec | 100% | 100% | 8/10 | |
| 1.7 sec | 1.5 sec | 90% | 100% | ||
| 2.1 sec | 1.6 sec | 93% | 100% | ||
| 1.4 sec | 1.5 sec | 95% | 100% | ||
| 4th session | |||||
| 2.1 sec | 1.7 sec | 90% | 100% | 6/10 | |
| 1.8 sec | 1.5 sec | 100% | 90% | ||
| 1.5 sec | 1.4 sec | 100% | 93% | ||
| 1.6 sec | 1.4 sec | 90% | 100% | ||
| Patient 3 | |||||
| 1st session | |||||
| 4.3sec | 3.7 sec | 40% | 40% | 8/10 | |
| 1.9 sec | 1.9 sec | 40% | 40% | ||
| 1.8 sec | 1.9 sec | 53% | 53% | ||
| 1.2 sec | 1.2 sec | 40% | 40% | ||
| 2nd session | |||||
| 2.7sec | 2.8 sec | 60% | 60% | 6/10 | |
| 1.2 sec | 1.8 sec | 40% | 40% | ||
| 1.4 sec | 1.3 sec | 40% | 40% | ||
| 1.3 sec | 1.4 sec | 50% | 50% | ||
| 3rd session | |||||
| 1.3sec | 1.3sec | 50% | 50% | 3/10 | |
| 1.2 sec | 1.1 sec | 60% | 60% | ||
| 1.1 sec | 1.1 sec | 53% | 53% | ||
| 1.8 sec | 1.8 sec | 45% | 45% | ||
| 4th session | |||||
| 1.3sec | 1.3 sec | 50% | 50% | 5/10 | |
| 1.6 sec | 1.9 sec | 50% | 50% | ||
| 1.4 sec | 1.5 sec | 60% | 60% | ||
| 1.2 sec | 1.2 sec | 60% | 60% | ||
| Patient 4 | |||||
| 1st session | |||||
| 2.1 sec | 2.6 sec | 60% | 60% | 8/10 | |
| 3.5 sec | 3.9 sec | 90% | 90% | ||
| 3.2 sec | 4.7 sec | 87% | 93% | ||
| 2.3 sec | 2.9 sec | 93% | 93% | ||
| 2nd session | |||||
| 2.1 sec | 2.7 sec | 100% | 90% | 9/10 | |
| 4.1 sec | 2.6 sec | 80% | 80% | ||
| 3.7 sec | 2.5 sec | 80% | 100% | ||
| 4.0 sec | 2.5 sec | 80% | 85% | ||
| 3rd session | |||||
| 3.9 sec | 2.5 sec | 80% | 80% | 7/10 | |
| 4.6 sec | 3.5 sec | 80% | 80% | ||
| 4.5 sec | 3.2 sec | 80% | 93% | ||
| 3.3 sec | 3.2 sec | 95% | 100% | ||
| 4th session | |||||
| 2.0sec | 3.4 sec | 100% | 100% | 5/10 | |
| 3.8 sec | 3.7 sec | 80% | 90% | ||
| 3.2 sec | 3.3 sec | 73% | 100% | ||
| 2.9 sec | 2.9 sec | 85% | 85% | ||
| Patient 5 | |||||
| 1st session | |||||
| 2.1 sec | 2.6 sec | 60% | 60% | 7/10 | |
| 3.5 sec | 3.9 sec | 90% | 90% | ||
| 3.2 sec | 4.7 sec | 87% | 93% | ||
| 2.3 sec | 2.9 sec | 93% | 93% | ||
| 2nd session | |||||
| 2.1 sec | 2.7 sec | 100% | 90% | 8/10 | |
| 4.1 sec | 2.6 sec | 80% | 80% | ||
| 3.7 sec | 2.5 sec | 80% | 100% | ||
| 4.0 sec | 2.5 sec | 80% | 85% | ||
| 3rd session | |||||
| 3.9 sec | 2.5 sec | 80% | 80% | 6/10 | |
| 4.6sec | 3.5 sec | 80% | 80% | ||
| 4.5 sec | 3.2 sec | 80% | 93% | ||
| 3.3sec | 3.2 sec | 95% | 100% | ||
| 4th session | |||||
| 2.0sec | 3.4 sec | 100% | 100% | 5/10 | |
| 3.8 sec | 3.7 sec | 80% | 90% | ||
| 3.2 sec | 3.3 sec | 73% | 100% | ||
| 2.9 sec | 2.9 sec | 85% | 85% |
Table 11: Response time, accuracy and pain level results according to the “Recognise OnlineTM” programme.
Active range of motion results for shoulder flexion
The patients were successfully tested on the active range of motion measurements for shoulder flexion. The results of the measurements showed, generally, the improvement of the patients. In detail, the values of the active shoulder flexion after each measurement for each patient are shown in Table 12.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| 1stsession | |||||
| Before“ΙΜΙ” | 85° | 75° | 70° | 107° | 127° |
| After“ΕΜΙ” | 60° | 80° | 148° | 118° | 132° |
| After“ΜΤ” | 85° | 81° | 163° | 167° | 154° |
| 2ndsession | |||||
| Before“ΙΜΙ” | 85° | 82° | 142° | 152° | 121° |
| After“ΕΜΙ” | 95° | 87° | 161° | 159° | 133° |
| After“ΜΤ” | 110° | 90° | 173° | 166° | 149° |
| 3rdsession | |||||
| Before“ΙΜΙ” | 110° | 86° | 152° | 155° | 158° |
| After“ΕΜI” | 130° | 90° | 167° | 159° | 172° |
| After“ΜΤ” | 150° | 94° | 169° | 172° | 176° |
| 4th session | |||||
| Before“ΙΜΙ” | 170° | 90° | 166° | 156° | 130° |
| After“ΕΜΙ” | 176° | 92° | 168° | 169° | 149° |
| After“ΜΤ” | 186° | 96° | 170° | 181° | 158° |
Table 12: Active range of motion results for shoulder flexion by SpineCor® goniometer.
Discussion
Research procedure and environmental conditions
Globally, there are a few studies examining the application and effectiveness of GMI [27]. No research has been found so far, to investigate the efficacy of GMI in any shoulder pathology. In this direction, the novelty of the present pilot study is that it assessed the implementation of an innovative Mirror Therapy (Fekos Mirror Therapy) method in combination with the motor imagery technique for the rehabilitation of shoulder pathologies on the basis of the theoretical hypothesis that all musculoskeletal injuries or pathologies also have a significant neuromuscular component.
The applied programme was based on the research studies of Moseley, Johnson et al. and Lagueux et al. and it is a modified version of these [6,15,17,20]. The modifications were made to all three parts of the programme with the aim of a) the more aggressive nature of the programmes so far, which would enable it to be implemented successfully within a short period and b) its adaptation to the shoulder area. As it was expected, the patients’ pain levels were initially increased (on the Visual Analog Scale), due to the aggressive character of this programme, but the pain was decreased soon.
Although the aggressive character of the programme has initially increased the pain levels, the patients ended up with overall improvement.
According to the IMI implementation principles [27], the progress has been done only in one parameter for every series during the left/ right judgment exercises. Then, by keeping the number of repetitions stable, the images changed from basic to vanilla. Finally, for the next series, the type of images was kept the same, and only the repetitions increased (20, 30, 40), except for the first session to avoid the excessive exercise load (20, 30, 30).
Considering the importance of patients’ familiarization with the programme prior to its implementation each session at the IMI stage was smoothly introduced; that is the reason why the first series was consisted of 20 attempts of the last type of images of the previous session (sessions: 2, 3, 4), while for the first series two attempts of 20 sessions were applied. It is also assumed that at the brain level, cells in the premotor region are pre-fired in this way before they receive the new and more difficult stimulus, with the absence of patients being surprised and feeling pain. Regarding the second stage of motor imagery movements, the therapy aimed to the mental representation of movements by the kinesthetic way, because it is most likely to contribute to beneficial results [40,41].
Furthermore, each part of the stage was based on the progressive activation of the brain structures. The ways of achieving the gradual activation of the brain were likely to be, in order: visual, verbal, visually mental and kinesthetically mental. The performance of mental movements (level 5: B and C) with the contralateral limb, both in the teaching and the main part of the programme, was based on the information that the physical movement of the contralateral limb provides additional feedback [37,40]. Based on a similar model of neuronal activation between mental and physical movements [16,42], it was assumed that the mentally represented movements are based on the same principle. Also, the fact that physical and mental functional activities, such as those in the third (C) part of level 5, are characterized as functionally equivalent [43,44], is a serious evidence for the above case.
Fekos Mirror Therapy programme was based on the book of Moseley et al. [27], and the progressive activation of the brain structures and workload of peripheral structures were present as repetitions, difficulty, and involvement of the affected limb gradually increased.
A key point for the applications of EMI and Fekos Mirror Therapy method is the type of exercises performed. It seems that these programmes start with simple anatomic shoulder flexion exercises and end with performing the flexion movement through a functional activity. This fact is especially important for the rapid introduction of the brain into daily basis and the progressive activation of brain structures.
Implicit motor imagery effectiveness
It appears to be a technique that promotes analgesia in patients, and it is, thus, the first part of the GMI [16,27]. Our research findings show that the pain score does not change after the lateralization exercises of the shoulder region. Considering that Lagueux et al. and Johnson et al. did not assess the progression of pain, following the use of IMI in a GMI programme [15,17], and due to its effect on cellular level, according to other authors [16,27], it can be claimed that the decrease of pain sensation is estimated to come later.
According to the results of response time and accuracy, it appears that only the first patient had a higher response time and lower accuracy on the affected limb than the other. Mainly, it is known that IMI is a tool for assessing the condition of the brain in painful conditions of peripheral body parts. Coslett et al. found that the ability to recognize the laterality of shoulder/arm could be an essential additional clinical tool in assessing patients with chronic pain in these areas. The results showed that patients with chronic musculoskeletal or radicular shoulder/arm pain achieved worse results, in accuracy and response time than the group of people with pain in areas that did not include the shoulder and healthy group, and these were related to the images’ rotation [45]. The same researchers [46], also, assessed the relationship between sustained pain and foot laterality recognition, comparing similar, in quality, groups to their previous research. The worst performance, for the accuracy and response times, and the significantly greater reduction in this performance depending on the wider rotation range of the images displayed for the group with the painful lower limb, suggest that the Implicit Motor Imagery gives important information about the nature of the pain experience. Similar results have also been reported in patients with chronic pain, who have been assigned to recognize the different facially expressed emotions, which were shown in some photographs, and the eye, tongue, brow, or jaw movements with direction to the left or right side of the face (left/right judgment) [47].
A present painful condition is not the only criterion of difficulty in its treatment. From the results of the current study, it seems that the existence of past pain in the same body area (patients 3 and 5) may be the cause of stronger cortical changes. This conclusion is reinforced by the findings of a study of 1008 individuals showing that, in patients with current pain and history of back pain, the performance in the lateral recognition of the trunk is significantly reduced compared to patients experiencing current pain and without a history of pain. The data supported the view of the disruption of cortical proprioceptive representation of the trunk in patients with past and current back pain [48].
However, the fact that all people are not familiar with using computers should not be neglected. The correlation between the ability to use a computer and performance in the Recognise Online™ programme appeared to be present in all five patients. More specifically, the patients with “excellent” computer knowledge had better performance than those who had never used it before. Only for the patient 5 it cannot be sure of the grade of better results if she would be more familiar with computers, since her condition did not lead to an improvement similar to patient 3, who had the same rating for computer use. That is why the coexistence of past pain in the upper limb and low computer use leads to such confusion.
It is quite important to note that the fluctuations in the results by the change in quality and rotation level of the observed images are a logical phenomenon. Researchers reported increased response times [46,49] and decreased accuracy [46] when the rotation angle of the depicted body members and objects changed.
In fact, recognizing the laterality of a human body part can give us valuable information about the perception of pain. Only for patients in the shoulder/arm pain group, there was a correlation between the degree of reduced response time and the degree of severity of pain during movement [45].
Explicit motor imagery effectiveness
The application of EMI to improve the mobility of the upper limb is mainly based on the research by Gentili et al. who examined the relationship between mentally represented and physically executed upper limb movements. Researchers showed that the duration of the mentally represented movements reflected the duration of body movements with additional resistance or without, in each direction of movement. The findings suggest that the brain represents the inertial properties of the arm and uses them for sensorimotor control and the generation of mental images [50].
In this study, the application of EMI led to an improvement in the active range of motion after its completion for each patient at each session, except for the first session of the patient 1. These data on shoulder mobility are consistent with other researches for different body areas, containing the shoulder.
The effect of the EMI was investigated quite extensively in neurological patients for the upper extremity. Ang et al. and Várkuti et al. reported that the combined use of EMI with MANUS (= upper - extremity robot-assisted rehabilitation) device resulted in improved upper limb performance in patients with stroke [51,52]. Such a combined application was not associated with adverse effects [51], and the utility of adding EMI to MANUS is evidenced by the existing functional changes in brain-related connectivity, in comparison to the implementation of only MANUS application [52].
Assis et al. demonstrated the effectiveness of the NeuroR system in upper limb motor function in patients with stroke, by inducing a significant increase in ROM [53]. Hewett et al. observed that the active ROM in shoulder flexion and elbow extension was increased after reach and grasp movement execution in the shoulder and elbow level, which were performed with the help of mentally represented movements [54]. In another category of neurological patients with high-level spinal cord injuries, it has been shown that a Brain-Computer Interface system in conjunction with EMI can help them gain control of a neuroprosthesis for the upper limb [55], but this is disadvantageous to the fact that the initial patient performance in this situation cannot be improved with more practice [56]. Additionally, it is evident that research has been directed to the lower extremity. In patients with post-stroke hemiparesis, the Explicit Motor Imagery was beneficial for functional gait, increasing stride length, cadence, and single-support time of the affected lower limb, whereas double-support time was decreased [57]. Similar results were found in the case study by Dickstein et al., in which, in a 69-year-old patient with left post-stroke hemiparesis, the application of the Explicit Motor Imagery for six weeks had positive effects on the knee range of motion, gait speed and double-support time [34]. However, in the same category of patients the effect of voluntary mental movements on the speed of upper limb movement is not clear, as according to Assis et al. increases [53], whereas Hewett et al. have shown that the linear speed of performing hands-on activities seems to be unaffected [54].
Recent data suggest that the central nervous system is affected and suffers from various musculoskeletal disorders. In the research of Zangrando et al. following the application of the EMI to patients suffering from chronic shoulder pain after impingement syndrome, the pain was greatly reduced, while the Constant-Murley Score and Shoulder Rating Questionnaire scores showed improvement in shoulder and upper limb movement, and overall shoulder functionality, respectively [39]. Also, by incorporating it into a treatment programme of physically performed exercises in patients with second-degree shoulder impingement had positive results in Constant-Murley Score, shoulder movement and pain, in comparison with neutral movements, and possibly in the prevention or delay of the third stage, which needs surgical intervention [58].
Although previous studies have shown encouraging results in the treatment of pain levels in the shoulder area, it has been remarked that the patients in the current research had experienced a worsening of pain initially before their pain reduction. It is a fact that the mentally represented movements can force the body to work very hard [27]. To sum up, it is not strange that patients may experience pain at the beginning of performing mentally represented exercises. Moseley et al. highlight the event as normal, as the brain is tested on sensitive activities [27]. However, the pain can be reduced when the brain feels happier and familiar with exercises [27], which also happened in this research.
In addition to the effect of the motor imagery shoulder exercises, it has been identified that there are other studies which deal with the lower and the other part of the upper limbs. A pilot study reported that by applying mentally represented movements to healthy people whose radiocarpal joint was immobilized with a splint for three weeks, the adverse effect of a reduced range of motion was prevented to a greater extent than in those who did not receive any physiotherapeutic intervention [37]. The above findings are enhanced by Einsiedel et al. who also report the improved motor performance of the hand in healthy people or patients, simulating or suffering from distal radial fracture after immobilization, who mentally trained in comparison to those who did not. They also point to the beneficial effect of the EMI on the forearm muscular atrophy, which is enhanced by the activation of the various brain regions, which are responsible for the movements of the radiocarpal joint [59].
EMI has gained a prominent position in orthopedic conditions after surgery, as it appears to improve central nervous system function on the preparation time of the hand movement [60].
It is known that unrestricted painless range of motion is necessary to perform everyday functional skills and working activities [61]. Therefore, stretching exercises are a powerful tool in the hands of physiotherapists, which solve many problems, so the best way to achieve them is a necessary challenge. According to Guillot et al. the EMI can increase the flexibility of the lower limb muscles, but this does not apply to the shoulder in healthy swimming athletes [62]. According to other results, it appears that the EMI can be combined with passive stretching for the upper limb in neurologic patients with arm spasticity, but without being more effective than the combination of progressive relaxation and passive stretching [63].
In terms of lower limb injuries, Lebon et al. found that the EMI, as an additional technique in a traditional physiotherapy programme (Passive joint mobilization, massage and strength training) does not benefit more from the knee range of motion and pain in patients with surgical repair of the anterior cruciate ligament. However, central neuronal activation was the explanation for increased muscle activation, which makes the mentally represented movements an important part of the restoration of motor functions in the above category of patients [64].
Certainly, the research efforts that have been made on healthy people have led to useful conclusions on the sector of rehabilitation, according to the outcomes of the EMI on a structural and functional level. Using an electromyogram, Creelman argued that mental movements can increase the active range of motion of long toe’s abduction [65].
One of the most important trials of combining mental movements with another physiotherapeutic method was that of Williams et al. [66]. The researchers assessed the beneficial effect of the EMI in combination with the Proprioceptive Neuromuscular Facilitation (PNF) method on healthy students. For people undergoing motor rehabilitation, the results highlighted the usefulness of adding EMI to the PNF method due to the short-term benefit in the range of motion compared to the PNF method and control group.
Mirror Therapy effectiveness
One part of the application of Fekos Mirror Therapy was based on Lee et al. A comparison between the asymmetric and the symmetrical application of MT with a virtual reality tool, combined with traditional physiotherapy, was done in stroke patients. The improvement in the “Fugl - Meyer Motor Assessment” and range of motion has led to the conclusion that the asymmetric execution is equally functionally effective for upper limb [35].
The results of this research showed a beneficial effect both on the range of motion and the pain levels for the shoulder area. Lin et al. showed that the combination of MT and afferent stimulation is more effective than MT, which reduces the motor damage and muscle synergy during the shoulder movements [67]. In a large study of 208 individuals [33], the results showed that MT, compared to placebo, reduces pain and enhances upper limb motor function in patients with stroke and complex regional pain syndrome type 1 of the upper limb, in short- and long-term.
It has been emphasized that the mirror can be a very cheap clinical tool [68]. We have received tremendous information from two recent research efforts, which combined the MT technique with expensive therapeutic devices. It has been shown that the use of MT and electromyographic triggered neuromuscular stimulation (ETMS) has positive results for patients after acute stroke and these results are different depending on the time of their application during recovery, but without unpleasant complications. In particular, the ETMS - MT direct intervention team showed a significantly greater improvement in the Fugl - Meyer Motor Assessment when it was applied for the first 4 weeks, while the delayed duration of similar intervention showed a significantly greater improvement in the active range of motion during the next 4 weeks [43]. One year later, Kim and Lee utilized Functional Electrical Stimulation (FES) and MT with or without the help of biofeedback, aiming to motor recovery of the wrist in patients with stroke (longer that six months). It turned out that the combination of FES, MT and biofeedback increased the wrist extension and improved the quality of patients’ life [69]. In patients with chronic stroke (>12 months), the hand function, according to the Functional Independence Measure (FIM), improved after the combined use of MT and conventional rehabilitation treatment compared to the control group after four weeks of the treatment and six months follow-up, while MT did not affect spasticity [70]. An interesting attempt is the integration of MT into classical rehabilitation treatment. Lee et al. found that if MT is added to a motor rehabilitation programme can enhance motor recovery of the upper limb in six-month stroke patients [1].
In addition to the motor gains offered by MT for the shoulder and the entire upper extremity, the sensory improvement is remarkable. In the research of Wu et al. hemiplegic patients in the intervention group, following the application of MT with bilateral simultaneous movements, showed better results in the Fugl Meyer Assessment for the entire and the distal part of the upper limb and showed shorter reaction times. Also, the sensory assessment with the help of Revised Nottingham Sensory Assessment, showed significantly greater temperature improvement for the intervention group than for the control group [71]. Consequently, it is assumed from the context of the above investigations and brain activation that the progress of the rehabilitation in patients, using a mirror, may be explained by the recovery of both motor and somatosensory brain regions to normal levels.
The above hypothesis is not different for orthopedic patients. Rostami et al. evaluated the effect of MT on orthopedic patients characterized by reduction in the hand active range of motion. The total active range of motion and Disability of Arm, Shoulder and Hand (DASH) score improved significantly after the intervention in both groups, with the improvement being continued in the follow-up period. In addition, patients in the combined conventional physiotherapy with MT group experienced significantly greater changes in total active movement scores and the DASH questionnaire compared to the conventional physiotherapy group, either immediately after the intervention, or during the follow-up [72]. Also, in a 39-year-old woman who suffered from a distal radius fracture, MT combined with electrical stimulation for the extension muscles improved the active wrist extension within approximately two and a half months [68].
In an article which includes three case studies of complex pain regional syndrome type 1, because of musculoskeletal injuries [73] the application of combined Mirror and cognitive resulted in pain decline during three conditions: pain at rest, pain after measuring allodynia/ hyperalgesia and pain after measuring strength. The range of motion and strength improved in two and one individuals, respectively. The area of hyperalgesia was increased for all patients, while the allodynia area remained stable in two patients while it was decreased in one [73].
Graded motor imagery effectiveness
Finally, the applied GMI programme led to improvement in active shoulder flexion, pain during shoulder flexion, and accuracy and response time parameters for body laterality recognition exercises. Similar results were shown by Lagueux et al. for pain levels, which modified the GMI programme in the part of MT, for pain levels in patients with complex regional pain syndrome of the upper limb, following orthopedic injuries [17]. Surprisingly, research by Johnson et al. has contrasted with the estimated results in reducing pain in patients after orthopedic injuries [15].
There is great attention to the research of patients’ functional progress. In the first study, no difference was observed between the functional improvements of the upper limb from the initial to the final measurement, according to the DASH questionnaire [17]. The other researchers managed to lead patients with complex regional pain syndrome type 1 to improve functionality, which was not the same for patients with complex pain regional syndrome type 2, according to the Brief Pain Inventory [15]. Moreover, it was noticed that, especially, from the first to the second session, our programme led to increased mobility, but with a negative impact on the pain levels. For the time being two substantial questions arise: a) Does the higher motor improvement promote the risk of pain worsening and b) if yes, which are the contributing factors?
The findings of this pilot study, if finalized with more studies (with better methodological design), are significant as they will contribute to the reinforcement of traditional musculoskeletal rehabilitation programmes. Through this pilot study, the importance of novel and specialized neurological and neuromuscular intervention for the treatment of painful musculoskeletal disorders is also emerging. Physiotherapists treating musculoskeletal pathologies should consider them as an integral part of all musculoskeletal rehabilitation programs aiming at decreasing pain and improving neuromuscular function.
Research Limitations
Several limitations of this study should be noted. First, our findings cannot be generalized to all chronic musculoskeletal patients. Second, only the short-term effects of the intervention were examined as the study was limited to 4 sessions and had no follow-up assessment. Third, there is no a clear conclusion about the results of Recognise Online™ programme, as not all the patients had the same computer knowledge and familiarization with its use.
Conclusion
The present pilot study has shown that a novel Mirror Therapy procedure in combination with the application of an aggressive GMI protocol can be useful in reducing pain and restoring the range of motion (active shoulder flexion) and functionality in patients with painful shoulder conditions. Based on the findings of this study it can be concluded that various chronic musculoskeletal disorders may have another significant variable to be taken into account when planning rehabilitation programmes, which is sensitization of the central nervous. Randomized control studies are needed to reinforce the findings of the present pilot study. These surveys should use the present or other modifications of GMI method and evaluate their effectiveness in the rehabilitation of various human joints painful syndromes.
References
- Lee MM, Cho H, Song CH (2012) The mirror therapy programme enhances upper – limb motor recovery and motor function in acute stroke patients. Am J Phys Med Rehabil 91: 689-696.
- Diers M (1999) Neuroprosthesis and sensorimotor training. In: Â Ramachandran VS, Blakeslee S (1999) Phantoms in the brain: Probing the mysteries of the human mind. Harper Collins 164.
- Lin KC, Chen YT, Huang PC, Wu CY, Huang WL, et al. (2014) Effect of mirror therapy combined with somatosensory stimulation on motor recovery and daily function in stroke patients: A pilot study. J Formos Med Assoc 113: 422-428.
- Beams T (2012) Treatment Through Graded Motor Imagery. In: Moseley GL, Butler DS, Beames T, Giles T (2012) The graded motor imagery handbook. Adelaide, Australia: Noigroup Publications.
- McCabe CS, Haigh RC, Ring EFR, Halligan PW, Blake DR (2003) A controlled pilot study of the utility of mirror visual feedback in the treatment of complex regional pain syndrome (type 1). Rheumatology 42: 97-101.
- Moseley GL (2004) Graded motor imagery is effective for long – standing complex regional pain syndrome: a randomized controlled trial. Pain 108: 192-198.
- Rosén B, Lundborg G (2005) Training with a mirror in rehabilitation of the hand. Scand J Plast Reconstr Surg Hand Surg 39: 104-108.
- Walsh N, Jones L, McCabe C (2015) The mechanisms and actions of motor imagery within the clinical setting. In: Knotkova H, Rasche D (2015) Textbook of neuromodulation: principles, methods and clinical applications. Springer, New York, USA.
- Bowering KJ, O'Connell NE, Tabor A, Catley MJ, Leake HB, et al. (2013) The effects of graded motor imagery and its components on chronic pain: a systematic review and meta-analysis. J Pain 14: 3-13.
- Birklein F, O'Neill D, Schlereth T (2015) Complex regional pain syndrome: An optimistic perspective. Neurology 84: 89-96.
- Cossins L, Okell RW, Cameron H, Simpson B, Poole HM, et al. (2000-2012) Treatment of complex regional pain syndrome in adults: a systematic review of randomized controlled trials published from June 2000 to February 2012.
- Daffada PJ, Walsh N, McCabe CS, Palmer S (2015) The impact of cortical remapping interventions on pain and disability in chronic low back pain: a systematic review. Physiotherapy 101: 25-33.
- Daly AE, Bialocerkowski AE (2009) Does evidence support physiotherapy management of adult complex regional pain syndrome type one? A systematic review. Eur J Pain 13: 339-353.
- Johnson S, Hall J, Barnett S, Draper M, Derbyshire G, et al. (2012) Using graded motor imagery for complex regional pain syndrome in clinical practice: Failure to improve pain. Eur J Pain 16: 550-561.
- Knotkova H, Rasche D (2015) Textbook of neuromodulation: Principles, methods and clinical applications. Springer, New York, USA.
- Lagueux EE, Charest J, Lefrancois Caron E, Mauger M, Mercier E, et al. (2012) Modified graded motor imagery for complex regional pain syndrome type 1 of the upper extremity in the acute phase: a patient series. Int J Rehabil Res 35: 138-145.
- Maihöfner C, Speck V (2012) Graded motor imagery for complex regional pain syndrome: where are we now? Eur J Pain 16: 461-462.
- Meyer P, Matthes C, Kusche KE, Maurer K (2012) Imaginative resonance training (IRT) achieves elimination of amputees' phantom pain (PLP) coupled with a spontaneous in-depth proprioception of a restored limb as a marker for permanence and supported by pre-post functional magnetic resonance imaging (fMRI). Psychiatry Res 202: 175-179.
- Moseley GL (2006) Graded motor imagery for pathologic pain: a randomized controlled trial. Neurology 67: 2129-2134.
- Oberfeld E, Ammann B, Vögelin E (2014) [Therapeutic modalities influencing neuropathic pain in hand surgery patients]. Ther Umsch 71: 423-429.
- Oral A, Ilieva EM, Küçükdeveci AA, Varela E, Valero R, et al. (2013) Generalised and regional soft tissue pain syndromes. The role of physical and rehabilitation medicine physicians. The European perspective based on the best evidence. A paper by the UEMS-PRM Section Professional Practice Committee. Eur J Phys Rehabil Med 49: 535-549.
- O'Connell NE, Wand BM, McAuley J, Marston L, Moseley GL (2013) Interventions for treating pain and disability in adults with complex regional pain syndrome. Cochrane Database Syst Rev 30: CD009416.
- Priganc VW, Stralka SW (2011) Graded motor imagery. J Hand Therapy 24: 164-169.
- von Piekartz H, Mohr G (2014) Reduction of head and face pain by challenging lateralization and basic emotions: a proposal for future assessment and rehabilitation strategies. J Man Manip Ther 22: 24-35.
- Walz AD, Usichenko T, Moseley GL, Lotze M (2013) Graded motor imagery and the impact on pain processing in a case of CRPS. Clin J Pain 29: 276-279.
- Moseley GL, Butler DS, Beames T, Giles T (2012) The graded motor imagery handbook. Noigroup Publications, Adelaide, Australia.
- Parsons LM (2001) Integrating cognitive psychology, neurology and neuroimaging. Acta Psychologica 107: 155-181.
- Jackson PL, Lafleur MF, Malouin F, Richards C, Doyon J (2001) Potential role of mental practice using motor imagery in neurologic rehabilitation. Arch Phys Med Rehabil 82: 1133-1141.
- Ramachandran VS, Blakeslee S (1998) Phantoms in the brain. First edtn William Morrow, New York.
- Bach F, Buschmann J, Schmitz B, Maaß HC¸ Akmak H, et al. (2010) Using interactive immersive VR/AR for the therapy of phantom limb pain. 13th International conference on humans and computers. University of Aizu Press.
- Bach F, Cakmak H, Maass H (2012) Vision-based hand representation and intuitive virtual object manipulation in mixed reality. Biomed Tech 57: 462-465.
- Cacchio A, De Blasis E, De Blasis V, Santilli V, Spacca G (2009) Mirror therapy in complex regional pain syndrome type 1 of the upper limb in stroke patients. Neurorehabil Neural Repair 23: 792-799.
- Dickstein R, Deutsch JE (2007) Motor imagery in physical therapist practice. Phys Ther 87: 942-953.
- Lee D, Lee M, Lee K, Song CH (2012) Asymmetric training using virtual reality reflection equipment and the enhancement of upper limb function in stroke patients: A randomized controlled trial. J Stroke Cerebrovasc Dis 6: 1319-1326.
- Richter HO, Roijezon U, Bjorklund M, Djupsjobacka M (2010) Long – term adaptation to neck/shoulder pain and perceptual performance in a hand laterality motor imagery test. Perception 39: 119-130.
- Frenkel MO, Herzig DS, Gebhard F, Mayer J, Becker C, et al. (2014) Mental practice maintains range of motion despite forearm immobilization: a pilot study in healthy persons. J Rehabil Med 46: 225-232.
- Malouin F, Richards CL, Jackson PL, Lafleur M, Durand A, et al. (2007) The kinesthetic and visual imagery questionnaire (KVIQ) for assessing motor imagery in persons with physical disabilities: a reliability and construct validity study. J Neurol Phys Ther 31: 20-29.
- Zangrando F, Paolucci T, Vulpiani MC, Lamaro M, Isidori R, et al. (2014) Chronic pain and motor imagery: a rehabilitative experience in a case report. Eur J Phys Rehabil Med 50: 67-72.
- Schott N, Korbus H (2014) Preventing functional loss during immobilization after osteoporotic wrist fractures in elderly patients: a randomized clinical trial. BMC Musculoskelet Disord 15: 287.
- Yao WX, Ranganathan VK, Allexandre D, Siemionow V, Yue GH (2013) Kinesthetic imagery training of forceful muscle contractions increases brain signal and muscle strength. Front Hum Neurosci 7: 561.
- Dominey P, Decety J, Broussolle E, Chazot G, Jeannerod (1995) Motor imagery of a lateralized sequential task is asymmetrically slowed in hemi – Parkinson’s patients. Neuropsychologia 33: 727-741.
- Kojima K, Ikuno K, Morii Y, Tokuhisa K, Morimot S, et al. (2014) Feasibility study of a combined treatment of electromyography – triggered neuromuscular stimulation and mirror therapy in stroke patients: a randomized crossover trial. NeuroRehabilitation 34: 235-244.
- Mulder T, de Vries S, Zijlstra S (2005) Observation, imagination and execution of an effortful movement: more evidence for a central explanation of motor imagery. Exp Brain Res 163: 344-351.
- Coslett HB, Medina J, Kliot D, Burkey A (2010) Mental motor imagery indexes pain: the hand laterality task. Eur J Pain. 14: 1007-1013.
- Coslett HB, Medina J, Kliot D, Burkey A (2010) Mental motor imagery and chronic pain: the foot laterality task. J Int Neuropsychol Soc 16: 603-612.
- von Piekartz H, Wallwork SB, Mohr G, Butler DS, Moseley GL (2015) People with chronic facial pain perform worse than controls at a facial emotion recognition task, but it is not all about the emotion. J Oral Rehabil 42: 243-250.
- Bowering KJ, Butler DS, Fulton IJ, Moseley GL (2014) Motor imagery in people with a history of back pain, current back pain, both, or neither. Clin J Pain 30: 1070-1075.
- Tomasino B, Budai R, Mondani M, Skrap M, Rumiati RI (2005) Mental rotation in a patient with an implanted electrode grid in the motor cortex. Neuroreport 16: 1795-1800.
- Gentili R, Cahouet V, Ballay Y, Papaxanthis C (2004) Inertial properties of the arm are accurately predicted during motor imagery. Behav Brain Res 155: 231-239.
- Ang KK, Chua KS, Phua KS, Wang C, Chin ZY, et al. (2014) A randomized controlled trial of EEG – based motor imagery brain – computer interface robotic rehabilitation for stroke. Clin EEG Neurosci 46: 310-320.
- Várkuti B, Guan C, Pan Y, Phua KS, Ang KK, et al. (2013) Resting state changes in functional connectivity correlate with movement recovery for BCI and robot – assisted upper – extremity training after stroke. Neurorehabil Neural Repair 27: 53-62.
- Assis GA, Corrêa AG, Martins MB, Pedrozo WG, Lopes RD (2014) An augmented reality system for upper – limb post – stroke motor rehabilitation: a feasibility study. Disabil Rehabil Assist Technol 11: 521-528.
- Hewett TE, Ford KR, Levine P, Page SJ (2007) Reaching kinematics to measure motor changes after mental practice in stroke. Top Stroke Rehabil 14: 23-29.
- Muller Putz GR, Scherer R, Pfurtscheller G, Rupp R (2005) EEG - based neuroprosthesis control: A step towards clinical practice. Neuroscience Letters 382: 169-174.
- Rohm M, Schneiders M, Müller C, Kreilinger A, Kaiser V, et al. (2013) Hybrid brain – computer interfaces and hybrid neuroprostheses for restoration of upper limb functions in individuals with high – level spinal cord injury. Artif Intell Med 59: 133-142.
- Dunsky A, Dickstein R, Marcovitz E, Levy S, Deutsch J (2008) Home – based motor imagery training for gait rehabilitation of people with chronic post stroke hemiparesis. Arch Phys Med Rehabil 89: 1580-1588.
- Hoyek N, Di Rienzo F, Collet C, Hoyek F, Guillot A (2014) The therapeutic role of motor imagery on the functional rehabilitation of a stage II shoulder impingement syndrome. Disabil Rehabil 36: 1113-1119.
- Einsiedel T, Herzig D, Grön G, Mayer J, Becker C, et al. (2011) Mental practice has influence on limitation of motion and muscle atrophy following immobilisation of the radiocarpal joint – a prospective randomised experimental study. Z Orthop Unfall 149: 288-295.
- Stenekes MW, Geertzen JH, Nicolai JP, De Jong BM, Mulder T (2009) Effects of motor imagery on hand function during immobilization after flexor tendon repair. Arch Phys Med Rehabil 90: 553-559.
- Kisner C, Colby AL (1996) Therapeutic Exercise, Foundations and Techniques, Third Edition. FA Davis Company, Philadelphia.
- Guillot A, Tolleron C, Collet C (2010) Does motor imagery enhance stretching and flexibility? J Sports Sci 28: 291-298.
- Bovend’Eerdt TJ, Dawes H, Sackley C, Wade DT (2009) Mental techniques during manual stretching in spasticity – a pilot randomized controlled trial. Clin Rehabil 23: 137-145.
- Lebon F, Guillot A, Collet C (2012) Increased muscle activation following motor imagery during the rehabilitation of the anterior cruciate ligament. Appl Psychophysiol Biofeedback 37: 45-51.
- Creelman J (2003) Influence of mental practice on development of voluntary control of a novel motor acquisition task. Percept Mot Skills 97: 319-337.
- Williams JG, Odley JL (2004) Callaghan M. Motor imagery boosts proprioceptive neuromuscular facilitation in the attainment and retention of range – of – motion at the hip joint. J Sports Sci Med 3: 160-166.
- Lin KC, Huang PC, Chen YT, Wu CY, Huang WL (2014) Combining afferent stimulation and mirror therapy for rehabilitating motor function, motor control, ambulation, and daily functions after stroke. Neurorehabil and Neural Repair 28: 153-162.
- Altschuler EL, Hu J (2008) Mirror therapy in a patient with a fractured wrist and no active wrist extension. Scand J Plast Reconstr Surg Hand Surg 42: 110-111.
- Kim JH, Lee BH (2015) Mirror therapy combined with biofeedback functional electrical stimulation for motor recovery of upper extremities after stroke: a pilot randomized controlled trial. Occup Ther Int 22: 51-60.
- Yavuzer G, Selles R, Sezer N, Sütbeyaz S, Bussmann JB, et al. (2008) Mirror therapy improves hand function in subacute stroke: a randomized controlled trial. Arch Phys Med Rehabil 89: 393-398.
- Wu CY, Huang PC, Chen YT, Lin KC, Yang HW (2013) Effects of mirror therapy on motor and sensory recovery in chronic stroke: a randomized controlled trial. Arch Phys Med Rehabil 94: 1023-1030.
- Rostami HR, Tabatabai S, Babadi N (2013) Effects of mirror therapy on hand function in patients with orthopedic injuries. Disabil Rehabil 35: 1647-1651.
- Tichelaar YIV, Geertzen JH, Keizer D, Paul Van Wilgen C (2007) Mirror box therapy added to cognitive behavioural therapy in three chronic complex regional pain syndrome type I patients: a pilot study. Int J Rehabil Res 30: 181-188.
Citation: Fekos C, Kallistratou A, Fousekis K, Iakovidis P, Kottaras S, et al. (2017) Modified Graded Motor Imagery programme containing “Fekos Mirror Therapy method”: Α novel therapeutic method for the treatment of shoulder dysfunctions - a pilot study. J Nov Physiother 7: 375. DOI: 10.4172/2165-7025.1000375
Copyright: © 2017 Fekos C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6389
- [From(publication date): 0-2018 - Nov 18, 2025]
- Breakdown by view type
- HTML page views: 5399
- PDF downloads: 990