Molecular Characterization of Hydrogen Sulfide Role in Vascular System and Method of Endogenous Production Detections with Common Ion Channels Used to Produce Its Biological Effect
Received: 14-Jul-2017 / Accepted Date: 17-Aug-2017 / Published Date: 24-Aug-2017 DOI: 10.4172/2168-9652.1000222
Abstract
In addition to nitric oxide and carbon monoxide, hydrogen sulfide (H2S) is the third gasotransmitter in mammals. It is synthesized from L-cysteine by cystathionine β-synthase, cystathionine γ-lyase or by sequential action of alanine aminotransferase and 3-mercaptopyruvate sulfur transferase. Although initially it was suggested that in the vascular wall H2S is synthesized only by smooth muscle cells and relaxes them by activating ATP-sensitive potassium channels, more recent studies indicate that H2S is synthesized in endothelial cells as well. The physiological functions of H2S are mediated by different molecular targets, such as different ion channels and signaling proteins. Endogenous H2S is involved in the regulation of many physiological processes in the cardiovascular system including the regulation of vascular tone, blood pressure and inhibits atherogenesis. Many new technologies have been developed to detect endogenous H2S production, and novel H2S-delivery compounds have been invented to aid therapeutic intervention of diseases related to abnormal H2S metabolism. The primary purpose of this review was to provide an overview of the role of H2S in the blood vessel, methods of endogenous production detections and common ion channels used to produce its biological effect describe its beneficial effects.
Keywords: Hydrogen sulfide; Blood vessel; Ion channels
Introduction
Until the last two decades of the 20th century, all known chemical transmitters were liquids that are solids in their pure form [1]. Furchgott and Zawadzki demonstrated that the relaxation of rabbit aorta following acetylcholine administration is dependent on the endothelium, and the substance responsible for the vascular relaxation was determined to be an endothelium derived relaxing factor [2]. Palmer et al. proved that this substance is pharmacologically identical to nitric oxide (NO) [3]. NO was then determined to be one of the most important signaling molecules in biological control systems. Moreover, NO was the first gaseous molecule that fulfilled the criteria of a transmitter [4]. Specifically, gaseous transmitters must be 1) freely membrane permeable; 2) endogenously and enzymatically generated and regulated; 3) have defined functions at physiological concentrations; and 4) have specific cellular and molecular targets, although second messengers are not needed [1-4]. Marks et al. discovered that another simple gaseous molecule, carbon monoxide (CO), operates as a transmitter in the mediation of vasoactivity [5]. Abe and Kimura, who studied neuronal activity, identified a third gaseous transmitter, namely, hydrogen sulfide (H2S), which is the endogenous mediator in mammals [6] and the vasoactivity of this compound was revealed by Hosoki et al. [7]. Since that time this hypothesis was confirmed by many studies and the “H2S field” in biology and medicine is now growing rapidly [8]. H2S thus joined two older counterparts, nitric oxide (NO) and carbon monoxide (CO), to form the family of “gasotransmitters” [4]. Other gasotransmitters, such as ammonia (NH3), methane (CH4) and hydrogen (H2) are suggested to exist as well [8,9]. Epidemiological studies report that a diet rich in organosulfur species is associated with longevity and decreased morbidity [10]. Members of the Allium genus (garlic and onions), which contain organosulfur compounds have a well-documented history of health benefits [11]. Indeed, garlic-derived compounds such as diallyl trisulfide release H2S in the presence of cellular reductants like glutathione (GSH) [12]. Populations that consume garlic regularly have low blood pressure, low cholesterol, and less vascular disease [13]. Additionally, the ancient Greeks, Egyptians, and Romans regularly bathed in natural sulfur springs as treatments for disease [14]. Depending on the microbiota and oxygen content, sulfur springs typically contain H2S concentrations ranging from 1 to 500 mM [15,16].
Hydrogen sulfide (H2S), which is endogenously produced, contributes to numerous physiological functions in mammalian systems [17]. Typically, it participates as a transmitter in the regulation of the cardiovascular system, inflammatory and immune response, gastrointestinal tract, kidney and nervous system functions [7,8,18,19]. Data on the concentration of H2S in the cardiovascular system varies between 10 nmol/l and 300 μmol/l [1]. Interest in the cytoprotective actions of H2S has grown since the discovery that it can induce a hypometabolic state characterized by decreased O2 consumption, heart rate, and body temperature in non-hibernating rodents [20]. The main aim of this review article is to describe the molecular physiology of hydrogen sulfide in the cardiovascular system and method of detection of H2S with common ion channels used to produce its biological effect within human being.
Chemical properties, synthesis and metabolism of H2S
H2S is the colorless flammable gas with a strong odor of rotten eggs, soluble in both water and organic solvents [21]. Like NO and CO, H2S is also toxic at high concentrations and shares with them the main mechanism of toxicity - inhibition of cytochrome c oxidase (mitochondrial complex IV) [21-23]. Some studies state that the concentration of free H2S in blood and tissues is only 14-15 nmol/L [8,24,25]. In aqueous solutions, H2S dissociates into H+, HS- and S2- with a pKa of 6.76 [21]. At physiological pH (7.4), such as in the blood and other physiological solutions, approximately 14% of the free sulphides are present as undissociated (gaseous) H2S, more than 80% is present as HS-, and the rest is S2- (1). Similarly to O2 and CO2, undissociated H2S is lipophilic and easily permeates plasma membranes [26,27]. The main sources of endogenous H2S are the amino acids cysteine and methionine, which are present in food [1] and there are three pathways for its endogenous production [8,21,28]:
a. Desulfhydration of L-cysteine by cystathionine β-synthase (CBS, EC 4.2.1.22),
b. Desulfhydration of L-cysteine by cystathionine γ-lyase (CSE, EC 4.4.1.1), and
c. Transamination reaction between L-cysteine and α-ketobutyrate catalyzed in mitochondria by cysteine aminotransferase (identical with aspartate aminotransferase) to form aspartate and 3-mercaptopyruvate, followed by decomposition of the latter to pyruvate and H2S by 3-mercaptopyruvate sulfurtransferase (3-MST).
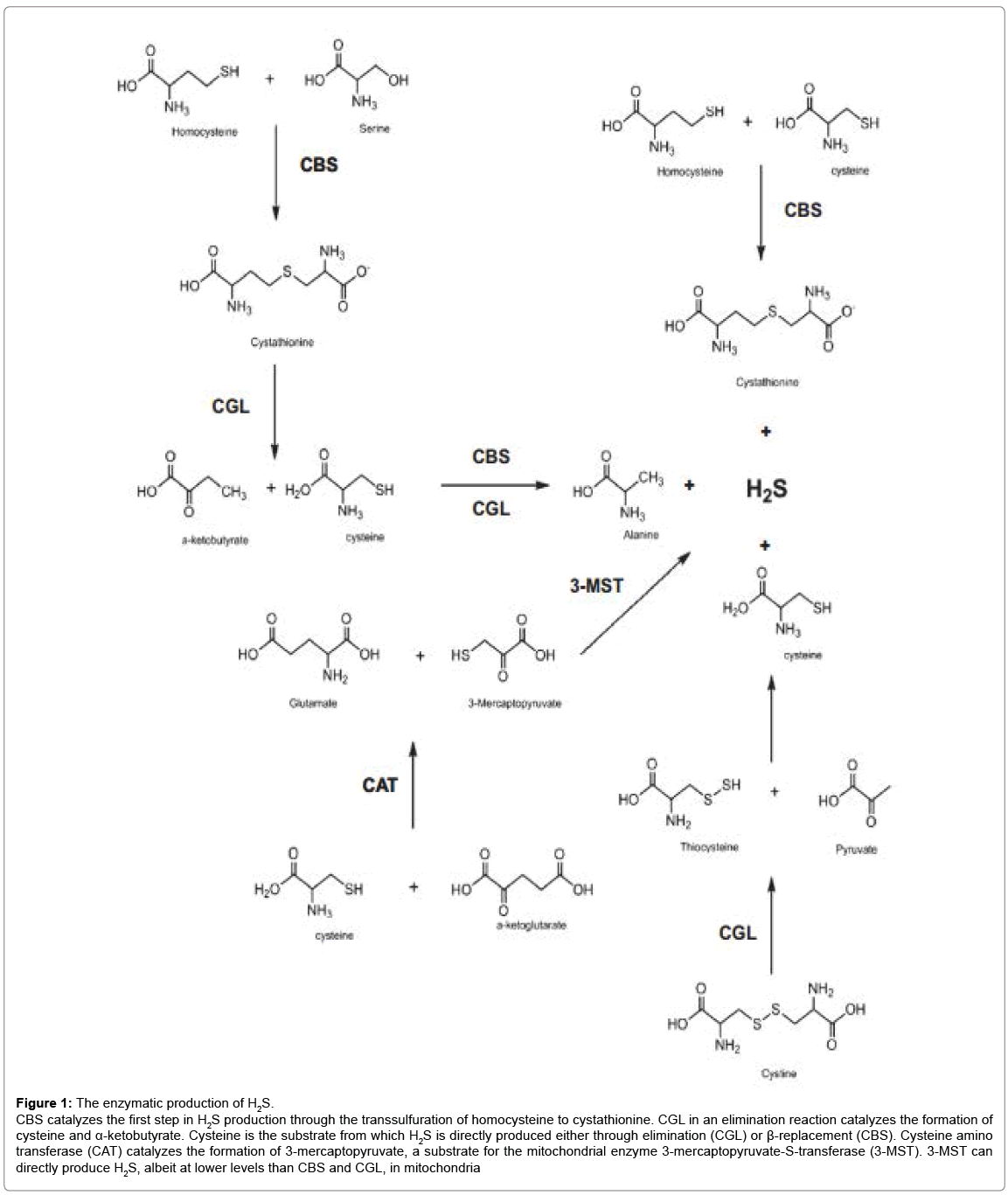
The first two enzymes, CBS and CSE, determine amount H2S production than 3-mercaptopyruvate sulfur transferase and are pyridoxal 5’-phosphate (vitamin B6)-dependent enzymes. Additionally, they are found in the cytosol and act sequentially in the transsulfuration pathway to convert L-homocysteine to L-cysteine with L-cystathionine as the intermediate [8,29,30]. CBS catalyzes the reaction between homocysteine and serine to form cystathionine and H2O, whereas CSE breaks down cystathionine to cysteine, ammonia and 2-ketobutyrate [8]. H2S may be synthesized by these enzymes in alternative reactions [30,31]. In particular, in the reaction catalyzed by CBS serine may be replaced by cysteine with cystathionine and H2S being the products. CSE may catalyze β-elimination of cysteine to pyruvate, H2S and NH4 +, γ-elimination of homocysteine to 2-ketobutyrate, H2S and NH4 + and β- or γ-replacement reaction between two cysteine or two homocysteine molecules, with lanthionine or homolanthionine, respectively, as the co-products [30,31]. At physiological concentrations of these amino acids, about 70% of H2S is synthesized from cysteine and the remaining 30% from homocysteine; the contribution of homocysteine increases in hyperhomocysteinemia [30]. The third, 3-MST-dependent pathway, was until now observed only in vitro in the nervous system [32] and in endothelial cells of some species (e.g. rat and human but not mouse) [33] and its contribution to overall H2S formation is unknown [21]. The enzymatic mechanisms of H2S production are shown in Figure 1.
Figure 1: The enzymatic production of H2S.
CBS catalyzes the first step in H2S production through the transsulfuration of homocysteine to cystathionine. CGL in an elimination reaction catalyzes the formation of cysteine and a-ketobutyrate. Cysteine is the substrate from which H2S is directly produced either through elimination (CGL) or ß-replacement (CBS). Cysteine amino transferase (CAT) catalyzes the formation of 3-mercaptopyruvate, a substrate for the mitochondrial enzyme 3-mercaptopyruvate-S-transferase (3-MST). 3-MST can directly produce H2S, albeit at lower levels than CBS and CGL, in mitochondria
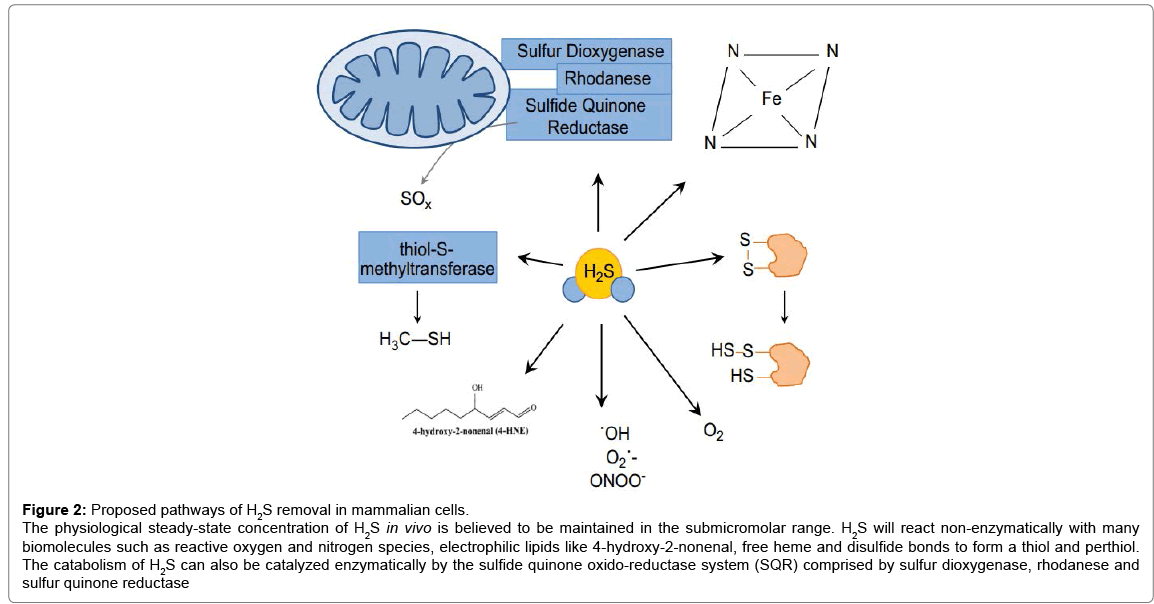
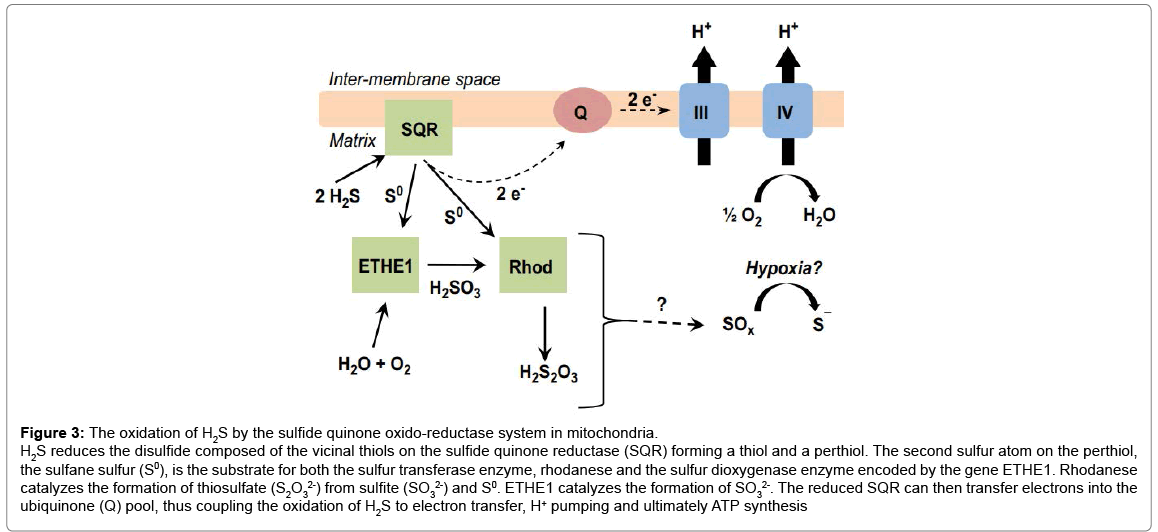
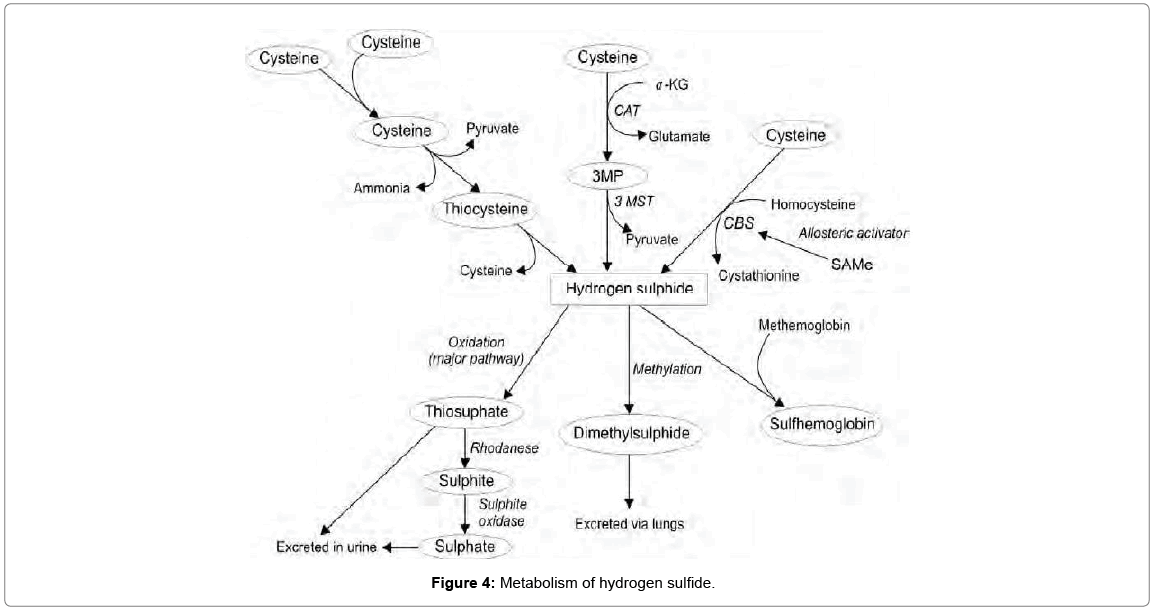
The gene expression of CBS and CSE has been detected in various cell types, including the liver, kidney, lymphatic system, vascular wall, cardiomyocytes and fibroblasts. These enzymes contribute equally to the local production of H2S in the liver and kidney [34]; however, one of the enzymes could be dominant in other organs [1]. The key enzyme for H2S synthesis in the central and peripheral nervous system is CBS [6]. The source of H2S in brain could also be the CAT/3-MST complex [35]. In contrast, there is a prevalence of CSE in cardiovascular system, although CSE expression is 24% higher in the myocardium in comparison to the thoracic aorta [36]. Relatively high concentration of CSE is observed in arteries, and H2S is produced by both endothelial cells [37] and smooth muscle cells of the vessel wall [38]. The expression of CAT and 3-MST was also observed in the endothelium [33]. Although the concentration of free sulphides in the blood and other tissues/physiological solutions of mammals is very low (<100 nmol/l), it can be increased in the parts of the body where increased concentrations of H2S synthesizing enzymes are present [39]. In specific intracellular spaces (microspaces), the concentration of free H2S can be increased several fold, whereupon it immediately diffuses, binds or oxidizes. For example, a much higher concentration of H2S (1 μmol/l) is observed in the aorta of mice. This concentration is 20-200 times higher in comparison with other tissues [40]. It is suggested that endogenously produced H2S is rapidly oxidized to sulphates or incorporated into proteins [41]. In order to maintain in vivo H2S concentrations, most likely, in the nM to low μM range, there are several enzymatic and nonenzymatic processes participate in H2S catabolism (Figures 2-4) [17]. Even though all cell are able to oxidize H2S, it is primarily degraded in liver [25,42] and mitochondria are very active site in sulphide oxidation [25]. Rhodanese, a mitochondrial sulfur transferase enzyme, catalyzes the oxidation of H2S [43]. It is one part of three enzymatic activities characterized as a major pathway for H2S catabolism. This pathway consists of a sulfide quinone oxido-reductase (SQR), a sulfur dioxygenase, and the sulfur transferase enzyme rhodanese (Figures 2 and 3) [17]. H2S reduces the external disulfide on the SQR to form a thiol (RSH) and a perthiol (RSSH) [8,44]. This two electron oxidation of H2S reduces the FAD prosthetic group, which uses ubiquinone (Q) as an electron acceptor [44].
Figure 2: Proposed pathways of H2S removal in mammalian cells.
The physiological steady-state concentration of H2S in vivo is believed to be maintained in the submicromolar range. H2S will react non-enzymatically with many biomolecules such as reactive oxygen and nitrogen species, electrophilic lipids like 4-hydroxy-2-nonenal, free heme and disulfide bonds to form a thiol and perthiol. The catabolism of H2S can also be catalyzed enzymatically by the sulfide quinone oxido-reductase system (SQR) comprised by sulfur dioxygenase, rhodanese and sulfur quinone reductase
Figure 3: The oxidation of H2S by the sulfide quinone oxido-reductase system in mitochondria.
H2S reduces the disulfide composed of the vicinal thiols on the sulfide quinone reductase (SQR) forming a thiol and a perthiol. The second sulfur atom on the perthiol, the sulfane sulfur (S0), is the substrate for both the sulfur transferase enzyme, rhodanese and the sulfur dioxygenase enzyme encoded by the gene ETHE1. Rhodanese catalyzes the formation of thiosulfate (S2O32-) from sulfite (SO32-) and S0. ETHE1 catalyzes the formation of SO32-. The reduced SQR can then transfer electrons into the ubiquinone (Q) pool, thus coupling the oxidation of H2S to electron transfer, H+ pumping and ultimately ATP synthesis
The second sulphur atom on the perthiol is a reactive sulfane (S0), which is oxidized by a sulfur dioxygenase enzyme (persulfide dioxygenase) encoded by the gene ETHE1, consuming O2 and H2O to form sulfite (SO32-) [17]. While the protein responsible for this enzymatic activity is not known, the ETHE1 gene encoding the protein has been identified. Mutations in this gene cause a buildup of H2S leading to ethylmalonic encephalopathy [45,46]. Rhodanese then transfers sulfane sulfur to sulfite to form thiosulfate (SO32-) [47]. This proposed oxidation pathway, in close proximity to CcO, functions as a major clearance pathway of cellular H2S.
In addition to liver and kidney, even though in healthy conditions, the amount of H2S excreted by expiration is negligible, lung is also involved in oxidization of H2S (Figure 4) [48]. H2S can also be oxidized by non-mitochondrial heme proteins such as hemoglobin (Hb) and myoglobin [49]. H2S will reduce the ferric iron in met-Hb, restoring the oxygen binding abilities of the protein [50]. At high concentrations of H2S, sulf-Hb can also be formed from oxy-Hb [51]. While displaying very weak affinity for O2, sulf-Hb can still deliver O2, albeit with no cooperativity [52]. As a result, the bioavailability of H2S, whether in the context of steady state in vivo concentrations or exogenously administered, is dictated by the O2 concentration.
Therefore, O2 can be considered an H2S antagonist, accelerating its oxidation and attenuating its biological actions [17]. The effect of O2 on H2S concentration is both direct and indirect. The spontaneous reaction of H2S with O2, while slow, can cause an appreciable decrease in the H2S concentration. Thus, tissues with relatively high O2 concentrations (e.g. alveolar epithelium) may have less H2S compared to tissues that are in a lower O2 environment (e.g. centrilobular region of liver). This has implications in pathological states of hypoxia such as ischemia reperfusion, where the availability, and thus the signaling effects of H2S may be augmented. Furthermore, O2 concentration can indirectly affect H2S concentration through changes in the redox state of heme proteins. Proteins such as Hb will react with H2S at different rates depending on the redox status of the hemes. For example, H2S will react more rapidly with met-Hb (Fe3+) than with deoxy-Hb (Fe2+) [50]. Because H2S is a nucleophile, it can also react with electrophilic lipids [53]; and the thiolate anion, HS-, can also reduce disulfide bonds (Figure 2) [54]. Indeed, the exfoliation of skin cells in hot sulfur springs is due to H2S reducing the structural disulfide bonds of cellular junctions in keratinocytes [16]. While this can be harmful at high concentrations, the reduction of external disulfide bonds by H2S may, in some instances, reverse a deleterious posttranslational protein modification. Although still contentious, the S-sulfhydration of cysteine residues may represent an important sink for free H2S [55]. In theory, H2S can also reduce higher thiol oxidation states such as S-nitrosothiols and sulfenic acids [56]. H2S can also be methylated by the cytosolic enzyme thiol-S methyltransferase to form methane thiol [17]. As with virtually all molecules, H2S can react with other free radical species, as well as, a number of non-radical reactive oxygen (ROS) and nitrogen (RNS) species (Figure 2) [57]. Many of the oxidized sulfur species as well as sulfur-centered radicals formed are less reactive than their oxygencontaining counterparts [58]. One of the most important oxidants responsible for the catabolism of H2S is O2. In the presence of molecular O2 and redox active metals, H2S will spontaneously oxidize [59]. In an oxygenated biological medium, metalloproteins catalyze H2S oxidation. This makes O2 tension a critical methodological consideration when conducting biologically relevant experiments.
Method of Detection of Endogenous H2S Production
Through determination of physiological level of endogenous H2S
The determination of the physiological concentrations of H2S in circulation and in specific tissues is pivotal for determining the impact of H2S on a given physiological function; correlating H2S levels with the specific pathophysiological changes; examining physiological roles of H2S under in vitro conditions at organ, tissue, and cellular levels; and guiding pharmacological and therapeutic administrations of H2S donors not always the measurement of H2S gives the consistent values [27]. The physiological range of H2S in circulation has been estimated at 10-100 μM in health animals and humans [38,60-62]. Aging appears to have no effect on circulating H2S. A study revealed no change in serum H2S concentration among three age groups of humans spanning 50-80 years (34-36 μM) [63].
Rat serum contains 46 μM H2S [38] and it is 34 μM in mouse serum [64]. In New Zealand rabbits, a quantitative assay detects a plasma H2S level around 16.5 μM [65]. Plasma H2S at micromolar ranges has also been reported in many other vertebrates [66]. Endogenous levels of H2S in rat brain homogenates are 50-160 μM [6,67-69]. Similar H2S levels were reported in the liver, kidney and pancreas [68-70]. H2S production was clearly measured in the cardiovascular system [7,38]. Not always the measurement of H2S gives the consistent values [27]. Using HPLC analysis, Sparatore et al. [71] reported a plasma sulfide level below 0.55 μM. Another study could not detect H2S levels in lamprey, trout, mouse, rat, pig, and cow blood samples using a special house-made polarographic H2S sensor that can detect 14 nM H2S [39]. One explanation for these low values of H2S is the rapid decay of H2S concentration from micromolar concentration to undetectable level within 30 min in vitro. Whether H2S would disappear that fast in vivo is unknown. Regardless, even 30 min would be far more than sufficient to regulate a specific physiological function [24]. A quick decay may actually indicate a homeostatic mechanism to trigger and to end H2S signaling. Another related concern is the measurement technologies themselves. The real-time polarographic sensor was initially developed by Doeller et al. in 2005 [24]. Using the same kind of sensor, Benavides et al. [12] demonstrated that red blood cells produced H2S. In two other studies using polarographic sensors, free H2S concentrations in whole rat blood have been detected at > 5 μM [72]. As the polarographic sensors are housemade in the study by Whitfield et al. [39], whether the failure to detect H2S in animal blood was due to some intrinsic factors with the sensor itself cannot be commented on. Availability of these house-made sensors to other research teams would have helped replicate these results or allowed for a better comparison. The simultaneous employment of the polarographic sensor and other detection methods for H2S detection would also help validate the actual blood levels of H2S. Finally, in contrast to the sulfur ion selective electrode which detects total sulfur in the blood including its acid labile, bound or free H2S forms, the polarographic sensor is sensitive only to freely dissolved H2S gas [27]. It is possible that a significant amount of H2S in circulation may not be in a free form as a dissolved gas, offering the rationale for the fact that our blood is not so smelly and the possibility that a polarographic sensor may potentially report a low value. A sensitive “nose” can smell “rotten eggs” in the blood if these eggs are broken, releasing free H2S gas.
Whereas whether H2S is a circulating gasotransmitter for both its generation and transportation is still being debated, the paracrine or autocrine effects of H2S may nevertheless be more critical for regulating the functions of the cells, tissues, and organs where H2S is produced in the proximity [27]. Using gas chromatography technique, Furne et al. [25] found very low tissue production of H2S at nanomolar range in homogenized mouse brain and liver. An interesting comparison for this observation is that Hyspler et al. [61] also used gas chromatographymass spectrometry (GC-MS) analysis and detected human whole blood H2S levels at 35-80 μM. Even using a polarographic sensor, others have detected significant tissue production of H2S from the brain and liver [27]. The detection of the volatile gasotransmitter is already difficult to ascertain and what adds to the challenge is the fact that the safety zone to separate toxicological level and physiological level of H2S is very narrow [27]. The toxic level of H2S reported by Warenycia et al. [69] is less than twofold higher than its endogenous level in rat brain tissues. At the time of death of mice who exposed to NaHS (60 μg/g), the sulfide concentration in brain, liver, and kidney only elevated from the baseline by 57, 18 and 64%, respectively [73]. Comparison between healthy human subjects and age matched patients with COPD only told a 49.4% increase in serum levels of H2S with stable COPD [63]. This percentage change translates to a H2S concentration difference of <20 μM. This narrowness of the transition zone between physiological/biological and toxicological levels of H2S can also be found in pharmacological studies where the dose-response relationship of H2S is relatively steep before a given function change occurred and can quickly cause the opposite effect when H2S concentration further increased [38]. As such, an ideal measurement method for detecting H2S in mammalians should be sensitive, specific, accurate, noninvasive, on real-time and require a small quantity of samples. Many of the current H2S measurement techniques, such as spectrophotometry, chromatography, and ionselective electrode, were originally invented to meet the industrial demand for monitoring H2S pollution in the environment [27]. These techniques are usually invasive and require a bulky quantity of samples. They also do not take account of the conditions for biological studies, such as the existence of H2S scavenging molecules, interference of hemoglobins or other pigment compounds, redox balance, pH changes, etc.
Through usage of spectrophotometry
The use of spectrophotometry, also known as the methyleneblue method, to measure trace amounts of H2S can be traced back to Fischer’s study in 1883 for its principle [74] and to the work by Fogo and Popowsky in 1949 for the refining of the technique with the adaption of spectrophotometry [75]. This assay is based on the formation of the dye methylene blue when H2S reacts with ferric chloride (FeCl3) and N,N-dimethyl-p-phenylenediamine (NDPA). Absorbance of the dye in the reaction milieu can be detected by the spectrophotometer. The quantitative relationship (Beer’s law) between H2S concentration and the intensity of the transmitted monochromatic light can then be determined. The minimum detectable concentration of H2S is determined by the sensitivity of the spectrophotometer to the optical density changes. Photoacoustic spectroscopy of H2S converted to methylene blue has greater sensitivity than standard spectrophotometric methods. As the acidification is an important component of the methylene blue method, the incorporation of acid-labile sulfide may impact on the interpretation of the actual H2S level [66]. For animal tissue samples or cells, the methylene blue method has been used often but usually is for detecting the H2S generation capacity of the samples. In other words, the activity of H2S-generation enzymes in term of H2S production rate is assayed, rather than the absolute H2S concentration. All variations in this application of the methylene blue method are derived from the original 1982 method of Stipanuk and Beck [76]. Tissue or cell samples are homogenized and incubated in a reaction mixture. The contents of the mixture are important because including L-cysteine is critical should CSE activity be assayed, but homocysteine should be a component if CBS activity is the goal to examine [27]. This first step is to generate H2S from samples. Step 2 is to transform H2S to methylene blue. The generated H2S at 37°C is trapped with an alkaline zinc acetate solution in an apparatus. Zinc sulfide is formed, precipitated, and subsequently dissolved in a hydrochloric acid solution of p-aminodimethylaniline (N,N-dimethyl-p-phenylenediamine). In the presence of ferric chloride, methylene blue is formed. The emitted blue color can be stable for hours and measured at 670 or 650 nm [38,77]. This method can also be adapted to detect sulfate level in water or biological solutions by first reducing sulfate to H2S with hydriodic and hypophosphorous acids [78].
The application of the methylene blue method to cell-free plasma or other cell-free biological fluids will detect the H2S already existent, rather than to be generated, since H2S generating enzymes are not in the fluid. Therefore, step 1 as described above to maximally activate H2Sgenerating enzymes is no longer needed [27]. The fluid sample can be agitated by adding acid to release H2S into the gas phase, which then interacts with zinc acetate and NPDA to form methylene blue [76]. Alternatively, the acid release of H2S gas and trapping processes are omitted by directly adding NPDA and trichloracetic acid (TCA) to the plasma to directly form methylene blue [79]. ForH2S in air samples, the methylene blue method can be modified to use an alkaline solution of cadmium hydroxide to absorb H2S [80].
Through usage of nanotube-based sensors
Electrochemical detection is the most commonly used technology incorporated in compact and portable H2S gas monitors [81]. The principle behind it is the conductivity changes of thin films upon exposure to H2S gas. Relying on solid state sensors made of semiconducting metal oxides or metals, these portable apparatuses are expensive and suitable for industry utilization. Their drawbacks include high power consumption as found in metal oxide sensors that require high operating temperatures, low sensitivity, short lifetime of often less than 1 year and interference by other gases, such as NH3 and NOx [82]. More popular electrochemical sensors nowadays are based on one-dimensional nanostructures such as bare or functionalized semiconducting single-walled carbon nanotubes (SWNTs) [83,84], metal oxides, and conducting polymer nanowires [85,86]. Potentially, these sensors may be used to monitor gases with high sensitivity, low sample volume requirement, low power consumption and low cost [82]. A catalytic chemiluminescence sensor made of R-Fe2O3 nanotubes has been developed, which can specifically detect H2S gas as low as at 10 ppm. The problem with this sensor is that high temperature over 110°C is required for catalytic oxidation of H2S to occur. It is also not suitable for measuring H2S in liquid [87]. Other sensors based on SnO2 nanowire [88], In2O3 nanowire [89] and ZnO nanowires [90] with increased sensitivity have been reported. The challenges with these onedimension structures are the difficulties in making the nanostructures and in obtaining large quantities as well as their application under in vivo physiological conditions. CuO-SnO2 and ZnSb2O6 have been shown to detect H2S at concentrations below 1 ppm at 300°C [91]. Using singlewall carbon nanotubes (SWNT) [92] as an H2S sensor as well as an H2S carrier has attracted a great attention in recent years. This is because of the adsorption of H2S by activated carbon and the realization of the structural advantages of the carbon nanotubes, which are the uniform pore size distribution, high surface area, and excellent electronic properties. High surface area will result in an increased amount of irreversibly adsorbed H2S. The activated carbon facilitates H2S reaction with oxygen at low temperatures, leading to the production of sulfur and water [93]. SWNT-based H2S biosensor will also potentially reduce the sample volume to nanoscale. The initial attempt of using multi-wall carbon nanotubes to measure H2S in solution was made by Wu et al. [94]. After carbon nanotubes are immersed in a H2S solution, on the contact interface between carbon nanotubes and H2S solution formed is a thin water film. Oxygen molecule is also dissolved in the film and adsorbed by the carbon nanotubes. Carbon nanotubes also absorb H2S by the van der Waals force. The interaction of H2S (hydrosulfide ions and protons) and oxygen on the nanotubes forms hydroxyl ions and sulfur. The protons neutralize the hydroxyl ions and produce water. But the spectra of fluorescence of sulfur on carbon nanotubes can be assayed with either a Raman or a confocal laser scanning microscope [95]. It was found that fluorescence intensity was increased, closely correlated with the increased concentrations of H2S in the solution. In this preliminary study, 10 μM H2S in water was successfully measured [94]. To take one step further toward the biological application of the carbon nanotubebased H2S biosensor, Wu et al. [96] applied this carbon nanotube fluorescence technique to measure H2S level in serum and reported that the binding of H2S to nanotubes was not affected by the presence of proteins in rat serum. After removing endogenous H2S in the serum with hemoglobin, exogenous H2S added to the serum was successfully detected with a linear relationship between H2S concentrations (20, 50 and 100 μM) and fluorescence intensities. The mechanism for using carbon nanotubes to detect H2S, even in the presence of proteins, is believed to be due to a continuous serum albumin film formed on the surface of carbon nanotubes. Other proteins or large molecules cannot pass the albumin film, but H2S can easily move and pass through this film to the surface of carbon nanotubes. What Wu and co-workers [94-96] did is the combination of carbon nanotube adsorption with the fluorescence emission detection, a chemical approach. A different strategy by detecting the conductance change of carbon nanotubes after binding with H2S was taken, an electrical approach [82].
The principle for this strategy is to conduct site-specific electrodeposition of gold nanoparticles on SWNT networks. The adsorption of H2S molecules at different concentrations onto the gold nanoparticle surface can change the carbon nanotube conductivity to different degrees. The researcher reported superior sensitivity of these nanostructures toward H2S at room temperature with a detection limit of 3 ppb. The application of these nanostructures for detecting H2S in liquid preparation and biological samples has not been reported.
Through usage of sulfur ion-specific electrodes
Sulfur ion-specific electrodes have been frequently used in detecting H2S level in blood and cell culture media. The method is easy to operate, and the initial setup is of low cost. Typically, the ion-specific electrode has a linear response range of between 0.1M and 10 μM and a detection limit on the order of 1-10μM. The observed detection limit is often affected by the presence of other interfering ions or impurities. With a modified sulfide-specific electrode, Searcy and Peterson [97] reported measurement of very low free sulfide concentration (0.5 μM). This measurement was done with continuous injection of Na2S solution into the sample chamber to maintain a constant concentration. Its application to biological fluids close to physiological conditions is not clear. Sulfur ion-specific electrodes are sensitive only to S2- and as such, free H2S needs to be fully dissociated. This can be achieved under a strong alkali conditions and with a complete lack of oxidation [98]. For both blood (whole blood, serum or plasma) and cell culture media, this alkali and antioxidant condition might cause protein desulfuration and the electrodes may detect S2- dissociated from H2S and released from proteins. Furthermore, using the electrodes still requires bulky samples and is an off-line measurement [27].
Through usage of polarographic H2S sensors
A novel polarographic H2S sensor (PHSS) was developed in 2005 as a voltammetry, which is a method of determining the chemical makeup of an H2S permeable polymer membrane by measuring electrical activity, or the accumulation of chemicals, on electrodes placed in the substance [24]. The application of PHSS has been reported at cellular, tissue and organ levels with the claimed high sensitivity at the nanomolar range and rapid response time to H2S. Real-time measurement of the levels of H2S and O2 in respirometry and vessel tension experiments with PHSS has been achieved [72].
Most of PHSS have the dimensions similar to that of the polarographic oxygen sensor. Recent advance sees the availability of the miniature PHSS for real-time measurement of H2S production in biological samples. It was reported that the miniature PHSS detected H2S production by brain supernatants at ~10.6 pmol·s-1·mg protein-1 [99], which is significantly higher than that in vascular tissues (0.5- 1.1 pmol·s-1·mg protein-1) [7,38]. Just like the real-time polarographic sensors for other gas molecules (O2, NO or CO), however, to have consistent and reliable reading of H2S level with commercially available PHSS is more often than not a daunting challenge and a frustrating experience [27]. Because the polarographic sensor only measures H2S gas, sulfide (HS− and S2−) is estimated indirectly from pH.
Through chromatography analysis
Chromatography includes gas chromatography, liquid chromatography, ion-exchange chromatography, affinity chromatography, and their variations such as HPLC (high-performance liquid chromatography or high-pressure liquid chromatography). The readers are referred to a thorough review by Ubuka [100]
which detailed the application of chromatograph technology in H2S detection. In short, liquid chromatographic determination of sulfide with or without derivatization and ion chromatography of sulfide have been conducted. HPLC analyses of sulfide after conversion to methylene blue, to thionine, or to the monobromobimane derivative or after labeling with o-phthalaldehyde (OPA) have been reported. Gas chromatography has also been employed to analyze sulphur compounds in air, aqueous, and biological samples [100]. For example, the measurement of H2S in air by ion chromatography has the working range of 20-500 μM for a 20 l air sample [101]. Gas chromotographymass spectrometry has been used to detect H2S in animal tissues based on the amount of trapped S2- after acidification of H2S [25,61]. Reversephase (RP)-HPLC for the determination of H2S-derived methylene blue was used in measuring the sulfide content in brain, liver, and kidney from sulfide-treated mice. After exposure of mice to 60 μg/g Na2S, tissue contents of H2S were all significantly increased [73]. Shen et al. [102] reported in 2011 a novel and sensitive method to detect physiological levels of free H2S in cell lysates, tissue homogenates, and body fluids.
This method is built on the rapid reaction of monobromobimane with H2S under basic conditions at room temperature to produce sulfide-dibimane (SDB). SDB is stable, in which it favors over the unstable H2S for biological assays. SDB is also more hydrophobic than most physiological thiols. RP-HPLC can separate SDB with a gradient elution and then analyze it by fluorescent detection. The sensitivity of this SDB-based RPHPLC analysis reaches the H2S level as low as 5 nM, which is in sharp contrast to the methylene blue-based spectrophotometry method which has a low limit of 2 μM [102]. When the SDB-based method was applied to wild-type mice, heterozygous CSE knock-out (CSE HT) mice, and homozygous CSE KO mice, clear differentiation in plasma level of H2S was achieved. CSE HT mice have lower plasma level of H2S than that of wild-type mice, but higher level than that of CSE KO mice [102]. Sensitive and selective detection of H2S has been one of the hot spots as well as one of the bottlenecks in H2S study. New methodologies are being continuously devised and reported and the existing methods improved and adapted to new applications [27]. The quick oxidation and scavenging of H2S in biological samples are the biggest challenges for accurate and rapid measurement of H2S levels. At this moment, the spectrophotometry- based method is still of the choice to determine tissue or cell production of H2S, whereas sulfur ion-specific electrodes and polarographic H2S sensors hold potential for real time measurement of H2S net levels in blood or other body fluids. For analyzing H2S in air samples, such as exhaled air from lungs, chromatography analysis of H2S would be more suitable. Furthermore, the fluorescence-based quantitative or semi-quantitative methods would be useful for detecting H2S production in specific cellular organelles.
Biological Roles of H2S and Its Effect on Ion Channels
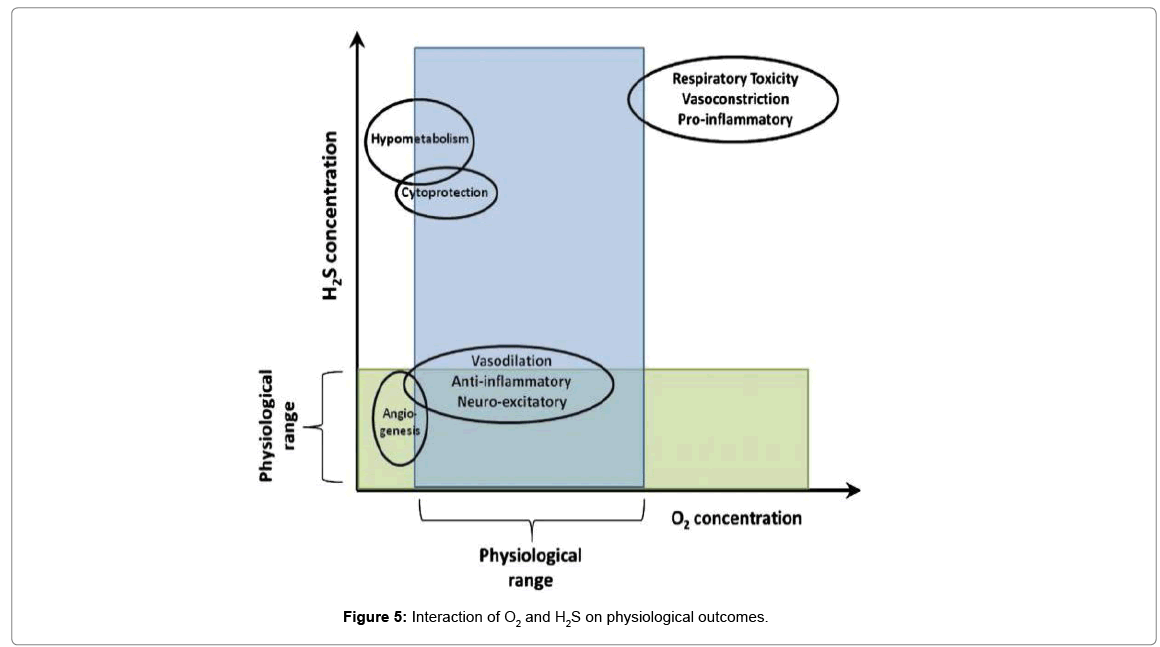
Ion channels are pore-forming membrane proteins that help establish and control the small voltage gradient across plasma membrane of cell or intracellular organelle membranes. These channels, individually or collectively, participate in the regulation of cell differentiation, muscle contractility, neurotransmitter release, or hormone secretion. Like NO and CO, H2S can easily diffuse without the need for transporters and has diverse biological actions by interacting with various channels (Figure 5).
Important factors that determine the biological actions of H2S include, but are not limited to, differences in the solubility of H2S in aqueous vs. lipid phases, proximity of the target to H2S detoxifying enzymes, heme redox state, and inter- and intra-cellular differences in O2 tension [17]. H2S plays a role in many physiological processes. However, high O2 can reverse many of the beneficial roles of H2S seen at lower O2 concentrations, resulting in, for example, vasoconstriction rather than vasodilation. Additionally, under hypoxic and normoxic conditions, H2S promotes angiogenesis. However, at higher concentrations of both O2 and H2S, an inhibition of cellular proliferation is seen. H2S has a narrow therapeutic window within which it is cytoprotective. At high concentrations it can be pro-apoptotic and pro-inflammatory. Finally, the larger doses of H2S necessary to induce a hypometabolic effect, can, if pushed further, result in cardiac and respiratory toxicity.
H2S and ATP-sensitive k+ (KATP) channels
ATP-sensitive K+ (KATP) channels are composed of pore forming subunits (Kir6.x) and sulfonylurea receptor (SUR) subunits that couple cellular electrical activity to metabolism in a variety of tissues. Hydrogen sulfide is an endogenous opener of KATP channels in many different types of cells. However, the molecular mechanism for an interaction between H2S and KATP channel proteins remains unclear. The whole-cell patch-clamp technique and mutagenesis approach were used to examine the effects of H2S on different KATP channel subunits, rvKir6.1 and rvSUR1, heterologous expressed in HEK-293 cells.
H2S stimulated co-expressed rvKir6.1/rvSUR1 KATP channels, but had no effect on KATP currents generated by rvKir6.1 expression alone. Intracellularly applied sulfhydryl alkylating agent (N-ethylmaleimide, NEM), oxidizing agent (chloramine T, CLT) and a disulfide bondoxidizing enzyme (protein disulfide isomerase) did not alter H2S effects on this recombinant channels. CLT, but not NEM, inhibited basal rvKir6.1/rvSUR1 currents, and both abolished the stimulatory effects of H2S on KATP currents, when applied extracellularly. After selective cysteine residues (C6S and C26S but not C1051S and C1057S) in the extracellular loop of rvSUR1 subunits were point-mutated, H2S lost its stimulatory effects on rvKir6.1/rvSUR1 currents [103]. By targeting KATP channels, H2S regulates the processes of inflammation, nociception, pain, and cell death and exerts its beneficial protective effects against ischemia damage, hypertension, inflammation, nociceptiveness and apoptosis, etc. [27]. Extensive experiments on vascular tissues strongly suggest that H2S-induced vasorelaxation is mainly caused by opening ATP-sensitive potassium channels (KATP) on the vascular smooth muscle cells [38,104,105]. In isolated piglet cerebral arteriole SMCs, a recent study showed that H2S activated KATP channels at physiological steady state voltage (-50 mV), which was antagonized by glibenclamide [106]. Electrophysiological study provides direct evidence that exogenous H2S increases macroscopic or unitary KATP currents, which is blocked by glibenclamide in isolated rat aortic and mesenteric SMCs [38,107]. Zhao et al. confirmed an important role of KATP channels in high-dose H2S-induced vasorelaxation in isolated rat aortas [38]. Consistent with the role of KATP channels in mediating the effects of H2S, reduced endogenous synthesis of H2S decreased KATP channel activity [1]. Moreover, exogenous H2S administration activated KATP channels and hyperpolarized the membrane of vascular smooth muscle cells isolated from rat mesenteric arteries [107]. H2S-induced hyperpolarization of SMC membrane is also abolished by glibenclamide. The opening of KATP channels in myocardium has been seen to play a pivotal role in cardioprotection during I/R injury, which is specifically seen in cardiac ischemic preconditioning [108]. It was observed that in the perfused rat heart preparation, NaHS concentration-dependently limited the size of infarction induced by left coronary artery ligation, and this protective effect was abolished by KATP channel blockers glibenclamide and 5-hydroxydecanoate [106]. Reperfusion of the isolated Langendorffperfused heart with NaHS after ischemia attenuated arrhythmias and improved cardiac function during I/R.
These effects of NaHS were blocked by glibenclamide, which suggests that H2S produces a cardioprotective effect against I/R injury during reperfusion, at least in part by opening KATP channels [109]. The patch-clamp data provide additional electrophysiological evidence that convincingly shows the effect of H2S on KATP channels. Exposure of single cardiac myocytes to NaHS increased single-channel activity of KATP channels by increasing the open probability of these channels without altering single-channel conductance [110]. This increase in the open probability can be blocked by glibenclamide. In the heart, H2S and its donors cause the negative inotropic and chronotropic action through activating sarcKATP and mitoKATP channels. The cardioprotective effect of H2S involves not only by the opening of KATP channels, but also though activation of cardiac ERK and/or Akt pathways in addition to preserving mitochondrial structure and function [111,112]. H2Sinduced neuroprotection and suppression of glutamate toxicity was also partially mediated by the activation of KATP channels. Glibenclamide and glipizide dose-dependently suppress H2S-induced protection of HT22 cells from oxidative stress. Neuroprotection was increased by the simultaneous application of H2S and pinacidil or the combined application of cysteine and pinacidil. While all these results support the involvement of plasma membrane KATP channels in the effects of H2S, opening (with diazoxide) or blocking (with 5-hydroxydecanate, 5-HT) of mitochondrial KATP (mitoKATP) channels did not modulate protection by H2S [113,114]. Distrutti et al. [115] have demonstrated that the systemic administration of different H2S donors inhibits visceral nociception by opening KATP channels. The activation of KATP channels in the peripheral nociceptive system has been seen to be involved in the modulation of nociception [116]. For instance, peripheral antinociceptive drugs that directly block ongoing hypernociception induced by PGE2, such as morphine and dipyrone, exert their effects by opening KATP channels stimulated by the NO-cGMP anti-nociceptive pathway [116]. Cunha et al. [117] tested the hypothesis that the anti-nociceptive effect of H2S on direct hypernociception induced by PGE2 is dependent on KATP channels in the periphery. Supporting this hypothesis, glibenclamide prevented the anti-nociceptive effect of exogenous H2S in rodents. A possible direct hypernociceptive effect of glibenclamide was excluded, as glibenclamide administration alone in the rat paw did not produce mechanical hypernociception [118]. Local administration of a KATP channel opener also directly blocks hypernociception induced by PGE2, which further supports the findings.
Electrophysiologically, it has been shown that KATP channel activation reduces the enhanced excitability of rat nociceptive sensory neurons induced by PGE2 [117]. A key event in inflammation is the recruitment of circulating leukocytes into the damaged tissue. Andruski et al. [119] used intravital fluorescence microscopy to look at leukocyte behavior in an intact rodent knee joint and later surmised that local treatment of acutely inflamed knee joints with an H2S donor limited leukocyte recruitment and trafficking and decreased synovial blood flow. These anti-inflammatory effects of H2S were mediated via the KATP channel because responses could be blocked by glibenclamide treatment. Intraarticular administration of NaHS had no effect on joint pain sensation or secondary allodynia in the rat, although this observation needs to be corroborated in other animal species. Thus it is conceivable that H2S may function as an endogenous regulator of joint function and that its action is distinctly anti-inflammatory [119]. However, exogenously administered H2S acts on sensitive neurons and promotes the opening of KATP channels and subsequent antinociception [117]. The effects of H2S on KATP channels also exert influence on pain cognizance. Research has clarified that parenteral administration of either NaHS or an H2S-releasing derivative of mesalamine inhibited dose-dependently visceral nociception in a colorectal distension (CRD) model in the rat. Administration of L-cysteine also reduced rectal sensitivity to CRD. The inhibitory effect of NaHS on CRD-induced pain or antinociception was completely reversed by pretreating rats with glibenclamide [115]. Also, glibenclamide inhibited colonic smooth muscle relaxation induced by the highest dose of NaHS. The antinociceptive and muscle relaxant effects of NaHS were mimicked by pinacidil. These results show that H2S functions as a negative regulator of visceral nociception by activating KATP channels and attenuating pain. NaHS-induced antinociceptive effects are not dependent on the activity of capsaicinsensitive pathways that can induce smooth muscle contraction [120], although CRD-induced pain is closely related to increased contractility of colorectal smooth muscles. NaHS induced antinociception only at relatively low doses, but caused intestinal smooth muscle relaxation at high doses. Due to the crucial role of KATP channels in the regulation of pancreatic insulin secretion, multiple studies have examined the effect of H2S on-cells. KATP currents were limited after lowering endogenous H2S level in INS-1E cells, derived from rat insulinoma cell line, by CSEtargeted short interfering mRNA transfection, which was blocked by gliclazide and stimulated by diazoxide [121].
Endogenously produced H2S by overexpression of the CSE gene significantly aggrandized whole cell KATP currents in INS-1E cells. Exogenous H2S markedly increased the open probability of single KATP channels by twofold in inside-out patches, but single-channel conductance and ATP sensitivity of KATP channels were not changed by H2S [121].
H2S and Ca2+-sensitive K (Kca) channels
Other than KATP, small, intermediate, and large conductance calcium-dependent potassium channels (SKCa, IKCa and BKCa) have also been demonstrated as possible mediators of H2S vasodilator effects in resistance vessels [122,123]. It has been observed that H2Sinduced vasorelaxation of rat aortic ring was not affected by iberiotoxin or charybdotoxin. This observation suggests that big-conductance Ca2+-sensitive K (BKCa) channels might not be responsible for the H2S-induced vasorelaxation in conduit vessels [124]. Both H2S and NaHS evoked concentration-dependent relaxation of in vitro perfused rat mesenteric artery beds (MAB) [125]. The vascular effects of H2S on MAB were related to the stimulation of charybdotoxin/apamin sensitive K+ channels in the vascular endothelium, in addition to the activation of KATP channels in vascular SMCs. Similarly, a combination of charybdotoxin and apamin abrogates the vasorelaxant effect of H2S in the endothelium intact rat aorta. These data suggest that small to medium conductance KCa channel (SKCa and IKCa) in MAB and aorta is activated by H2S. Therefore, H2S might fulfill the role of EDHF [115]. The stimulation of SKCa and IKCa channels by H2S was also indirectly demonstrated in isolated rat mesenteric arteries as well as in isolated vascular endothelial cells, based on the changes in membrane potential [122]. One recent patch-clamp study showed that NaHS arrested heterologously expressed BKCa channels in HEK-293 cells transfected stably with human BKCa channel –subunits [126,127]. NaHS decreased the open probability and shifted the BKCa-channel activation curve rightward without altering its conductance, suggesting that the inhibitory action of H2S on BKCa-channel. The same conclusion of H2Sinduced inhibition of BKCa channels was drawn in type I glomus cells of mouse carotid body [128]. In sharp contrast, a recent report showed that NaHS augments whole cell BKCa currents and enhances singlechannel BKCa activity in rat pituitary tumor cells (GH3) by increasing channel open probability [129]. The above three patch-clamp studies used NaHS at the same concentration range (~300μM), but the conclusions are opposite.
No explanation has been given, but it might be related to specific BKCa channel subtypes in different types of cells [130]. Another study by Jackson-Weaver et al. [131] examined the myogenic tone of rat mesenteric arteries and cerebral arteries as well as the membrane potential of vascular SMCs. Although the authors did not directly record changes in KCa channel currents, their results nevertheless showed that exogenous H2S dilated and hyperpolarized rat arteries and that these effects of H2S were blocked by iberiotoxin and paxillin. Thus the stimulation of iberiotoxin sensitive BKCa channels by H2S is suggested [131].
H2S and chloride (cl-) channels
The ATP-binding cassette superfamily includes cystic fibrosis transmembrane conductance regulator (CFTR) Cl- channels and sulfonylurea receptors, which are components of KATP channels. Both subunits also share key sequence homologies [27]. The Cl- channel blockers 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB) and indyanyl oxyacetic acid (IAA-9) suppress protection by H2S, while levamisole, which is an opener of Cl- channels, competently stops glutamate toxicity [113]. This research purports that CFTR Cl- channels may also be involved in protection by H2S against oxidative stress. The recent findings that a decrease in transmembrane Cl- gradients causes cell death in hippocampal pyramidal neurons and that the expression of CFTR gene is reduced in the hypothalamus of patients with AD [132] suggest that homeostasis of transmembrane Cl- gradients is required for normal cell survival. Subsequently, the effect of H2S on Clchannels in the CNS has been studied. In the research, H2S was seen to activate CFTR Cl- channels in HT22 neuronal cell lines which led to neuroprotection during oxytosis. This was demonstrated through dose-dependent suppression of neuroprotection due to H2S using specific CFTR blockers, NPPB and IAA-94, and confirmed using CFTR activator levamisole [113]. Together with the recent observation of H2S activating Cl-/HCO3 ‑ transporters in smooth muscle cells [133], the results suggest possible regulation of Cl- fluxes by H2S in the CNS with neuroprotective consequences. The regulation of inhibitory Cl- currents coincides with the regulation of inhibitory K+ channels and therefore strongly purports a key role for H2S in modulating excitability [130].
H2S and calcium (Ca2+) channels
It is well recognized that voltage-activated Ca2+ channels (VDCC) regulate intracellular Ca2+ concentration ([Ca2+]i) and consequently impact Ca2+ signaling in excitable cells. Ca2+ channels are classified, based on their electrophysiological features, as high voltage-activated (HVA) and low voltage activated (LVA) types. The former include L-, N-, P-/Q- and R-type channels, and the latter are actually T-type channels [27]. In addition to Ca2+ channels in the membrane, [Ca2+]i is controlled by intracellular Ca2+ stores. [Ca2+]i changes due to extracellular Ca2+ entry may be facilitated by VDCC, transmitter-gated Ca2+-permeant ion channels, transient receptor potential (TRP) ion channels, and Ca2+ pumps located in the plasma membrane [134]. Channels that affect intracellular Ca2+ stores include ryanodine receptor (RyR) channels, inositol trisphosphate receptor (IP3R) channels and sarcoendoplasmic reticular Ca2+ ATPases (SERCA) [134].
H2S and l-type voltage-activated Ca2+ channels (L-type VDCC)
Voltage-activated Ca2+ channels (Cav) are expressed at high density in excitable cells, mostly in neurons, cardiac conduction system and smooth muscles. H2S modulates cardiovascular homeostasis and exerts cardioprotective effects in different models of in vitro, ex vivo and in vivo ischemia/reperfusion [135-140]. Indeed, whole patch clamp experiments in rat cardiomyocytes revealed that NaHS negatively modulates L-type Ca2+ channels composed by the CaV1.2 subunits [141,142]. More specifically, NaHS (up to 1 mM) causes a dose dependent reduction in the Ca2+ current peak. This effect is only partial: the current density diminishes by 50% at 1 mM NaHS [143]. The mechanism could involve a direct modification of Cav free sulfhydryl groups (143). The H2S donor also affects the recovery from depolarization induced inactivation, without altering the steady state activation and inactivation curves. Accordingly, the shortening of single cardiomyocytes and contraction of isolated rat papillary muscles are depressed. Electric field-induced Cai transients in single cardiomyocytes are also reduced by 100 M NaHS [141,142]. Consistently, H2S exerts a negative inotropic effect in isolated perfused rat and papillary muscles when NaHS is administrated at concentrations ranging from 1 μM up to 1 mM [36,144]. More recently, it has been reported its negative chronotropic action in human atrial fibers by blocking L-type Ca2+ channels and an enhancement in the repolarization phase by opening KATP channels (50–200 M μNaHS) [145].
Interestingly, according to a recent study, H2S can reverse the negative inotropic effect induced by NO by causing an increase in the peak amplitude of the electrically stimulated Cai transients [140]. These apparently discrepant data may be reconciled when considering that, under such conditions; the modulation of the Cai toolkit responsible for the positive inotropic effect is not accomplished by H2S, but by a new thiol-sensitive endogenous modulator deriving from the interaction between the two gasotransmitters [140]. Interestingly, in this report, H2S was provided by NaHS at low micromolar doses (10 μM). The negative effect of H2S on Ca2+ influx is not limited to the cardiovascular system. Similarly to rat cardiomyocytes, 100 μM NaHS suppresses voltage-gated Ca2+ currents in INS-1E cells (rat insulinoma cell line) and native pancreatic beta-cells: these currents are sensitive to both nifedipine and Bay K-8664, a pharmacological profile consistent with L-type Ca2+ channels [130]. On the other hand the effects of NaHS on neurons, that can express both CaV1.2 and CaV1.3 subtypes, seem to be opposite [146]. In cultured rat cerebellar granule neurons (CGN), NaHS (50-300 M) induces cell death as well as Cai signals sensitive to nifedipine and nimodipine, L-type Ca2+ channel blockers [130].
However, no electrophysiological recordings were conducted and a direct activation of L-type Ca2+ channels by NaHS remains to be demonstrated yet. Moreover, there is no evidence about the molecular nature (i.e. CaV1.2 or CaV1.3) of L-type channels in these cells. Taken together, these evidences suggest that L-type Ca2+ channels are inhibited by H2S in the myocardium, whereas they are enhanced by the same H2S doses in the CNS. Future investigations will unveil whether this feature depends on the different molecular make-up of L-type channels, i.e., CaV1.2 in ventricular cardiomyocytes vs. CaV1.3 in the cerebellum, or on their associated subunits. Alternatively, an intermediate sensor coupled to the channel complex, whose nature varies between the heart and the CNS, might mediate the regulation of L-type Ca2+ channels by H2S. NaHS increases Cai also in astrocytes, hippocampal slices and microglia, through currents sensitive to Ca2+ channel inhibitors (La3+ and Gd3+) and in a concentration range (100–500 M) similar to that affecting VOCs [130,147]. It appears that H2S-triggered Cai waves are due to influx through Ca2+ channels on plasma membrane and, to a lesser extent, to the release from intracellular Ca2+ stores [130,147].
In contrast, a recent report showed that NaHS-induced Cai increase in isolated rat colonic crypts was not dependent on extracellular Ca2+, but was affected by blockade of either ryanodine receptors (RyRs) or sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) [148]. T-type Ca2+ channels are encoded by the three members of the CaV3 subfamily and display different biophysical and pharmacological features as compared to L-type Ca2+ channels: activation at lower membrane potentials, faster inactivation, slower deactivation, smaller permeability to Ba2+, insensitivity to dihydropyridines and block by ZnCl2 [149]. T-type Ca2+ currents are involved in a great number of physiological processes, such as neuronal firing, hormone secretion, smooth muscle contraction, myoblast fusion, and fertilization [149]. Moreover, they play critical roles in mediating either somatic or visceral nociceptive information. Similarly to capsaicin, NaHS, injected intracolonically at 0.5-5 nM per mouse, triggers visceral nociceptive responses in vivo, which are completely abolished by mibefradil, an unspecific T-type channel blocker, and insensitive to verapamil and to the KATP channel blocker glibenclamide [130]. Therefore, H2S may function as a novel nociceptive messenger through the activation of peripheral T-type Ca2+ channels, particularly during inflammatory processes. However, since mibefradil is not selective for T-type channels, this conclusion should be confirmed by future investigations [150]. Furthermore, both intraplantar (1 nM/paw) and intratechal (0.01-0.1 nM/animal) administration of NaHS caused a prompt hyperalgesia in rats, an effect that was abolished by mibefradil, ZnCl2 or antisense oligodeoxynucleotides (ODNs) selectively targeting rat CaV3.2 [151- 153]. The finding that DL propargylglycine (PPG) and -cyanoalanine, two CSE inhibitors, abolish the l-cysteine-induced hyperalgesia and attenuate the lipopolysaccharide-induced hyperalgesia, an effect reversed by NaHS, supports these observations [151,152]. Moreover, mibefradil suppressed the phosphorylation of ERK induced by the infusion of NaHS, a pronociceptive stimulus in the pancreatic duct, albeit at higher concentrations than those reported above (500 nM/rat) [154]. Finally, the neuropathic allodynia/hyperalgesia induced in rats by damaging the right L5 spinal nerve [155] or by systemic injection of paclitaxel [156], an anticancer drug, was strongly attenuated by either mibefradil or CSE inhibitors, or by antisense ODNs against rat CaV3.2. In addition, CaV3.2 was significantly up-regulated in the ipsilateral L4, L5 and L6 dorsal root ganglia of rats subjected to spinal nerve injury, but not treated with paclitaxel [155].
A redox modulation of CaV3.2 has been proposed, since NaHS increases the amplitude of T-type Ca2+ currents in a neuroblastoma cell line without affecting their kinetics. This effect was reversed by the oxidizing agent, 5,5-dithio-bis(2-nitrobenzoic acid) (DTNB), and mimicked by the reducing compound, dithiothreitol (DTT) [152]. It should be pointed out that the elevation in the density of T-type Ca2+ currents was observed at 0.5-1.5 mM NaHS. The enhancement of T-type Ca2+ current by the exogenous application of H2S, in turn, induces neuronal differentiation, as revealed by neurite outgrowth and functional expression of high voltage-activated Ca2+ currents, including L-, P/Q- and N type channels [157]. Once again, these effects arose when NaHS was administrated at 1.5-13.5 mM. Interestingly, earlier reports demonstrated that L-cysteine selectively potentiates recombinant CaV3.2-dependent, but not CaV3.1- and CaV3.3-, currents [158]. A mechanistic link between H2S and the onset of the Cai waves might be provided by the protein-kinase A (PKA)/cAMP pathway. Accordingly, H-89, a rather selective PKA blocker, hinders NaHSevoked Cai signals in both neurons and microglial cells [159,160]. Moreover, PKA-dependent phosphorylation may increase the Ca2+ permeability of T-type channels, NMDA receptors and RyRs [159].
H2S and t-type voltage-activated Ca2+ channels (T-type VDCC)
In addition to KATP channels, T-type VDCC also has critical roles to play in the processing of either somatic [149] or visceral [161] nociceptive information and in control of pain [152]. However, unlike KATP, T-type VDCC’s antinociceptive effects are dependent on the activity of capsaicin-sensitive pathways [120]. Similar to capsaicin, NaHS, administered intracolonically, triggered visceral nociceptive behavior that was accompanied by referred abdominal hyperalgesia/ allodynia [162]. These responses are completely abolished by preadministered intraperitoneally mibefradil [162]. In contrast, mibefradil at the same dose failed to attenuate the intracolonic capsaicin-induced visceral nociception. Neither L-type VDCC blocker verapamil nor KATP channel blocker glibenclamide modified the intracolonic NaHS-evoked visceral nociception. Furthermore, researchers found that intraperitoneal NaHS facilitated intracolonic capsaicin-evoked visceral nociception, which was also abolished by intraperitoneal pretreatment with mibefradil. Similarly, intraplantar administration of NaHS induced prompt mechanical hyperalgesia in rat hindpaw, which is blocked by mibefradil but not by glibenclamide [152]. Therefore, H2S likely functions as a novel nociceptive messenger through the activation of T-type VDCC during inflammation. Furthermore, PPG or BCA (CSE inhibitors) abolished the L-cysteine-induced hyperalgesia and attenuated the lipopolysaccharide-induced hyperalgesia, an effect being reversed by NaHS [152]. Like the reducing agent dithiothreitol, NaHS increased T-type VDCC currents without alteration of their kinetics in undifferentiated NG108 –15 cells, an effect being abolished by an oxidizing agent 5,5’-dithio-bis(2-nitrobenzoic acid) (DTNB). Suppression of T-type VDCC by DTNB at a high concentration was reversed by NaHS and dithiothreitol at subeffective concentrations. T-type VDCC is also involved in pancreatic nociception in rodents [163]. Either NaHS or capsaicin induced the expression of Fos protein in the superficial layers of the T8 and T9 spinal dorsal horn of rats or mice [27]. The induction of Fos by NaHS but not capsaicin was abolished by mibefradil. In conscious mice, repeated doses of cerulein produced pancreatitis, accompanied by abdominal allodynia/hyperalgesia. Pretreatment with PPG prevented the allodynia/hyperalgesia, but not the pancreatitis. A single dose of mibefradil reversed the established pancreatitis-related allodynia/hyperalgesia. Taken together, H2S appears to function as a novel nociceptive messenger through sensitization of T-type VDCC in the peripheral tissues, particularly during inflammation [164]. In patch-clamp studies using undifferentiated NG108 –15 cells, NaHS enhanced T-type VDCC currents, which may prove that H2S activates these channels [152]. These authors also reported that intraplantar [151,152] and intrathecal [151] injections of NaHS promptly induced hyperalgesia in rats through T-type VDCC activation. Further investigation suggested that the Cav3.2 isoform of T channels was activated by H2S, demonstrated by the abolishment of H2S induced-hyperalgesia using a general T-type channel blocker mibefradil, and similar results were produced using ZnCl2 (Cav3.2 specific inhibitor) and also with intrathecal administration of Cav3.2- specific antisense nucleotides to the rat [151]. Using high (4.5-13.5 mM) concentrations of NaHS on undifferentiated NG108 –15 cells, the same group was able to demonstrate that H2S induced neurite outgrowth, which was found to be related to the activation of Cav3.2 isoform T-type channels demonstrated with the abolishment of neurite outgrowth using general T-type channel inhibitor mibefradil, intracellular Ca2+ chelator BAPTA-AM, and Cav3.2 isoform specific blocker ZnCl2 [157]. Interestingly, they also discovered that H2S induced high-voltage-activated Ca2+ currents that were composites of L-type, N-type, and P/Q-type channel activation [157]. Therefore, by compiling the evidence by various authors, T-type channel activation, in particular the Cav3.2 isoform, by H2S appears to regulate rhythmic neuronal activity, pain sensation, and differentiation of neurons and boosting of synaptic communication, similar to putative processes regulated by H2S-related L-type channel activation.
H2s and transient receptor potential (trp) ion channels
The mammalian TRP superfamily consists of 28 different proteins that may be subdivided into six main subfamilies. They are TRPC (canonical), TRPV (vanilloid), TRPM (melastatin), TRPP (polycystin), TRPML (mucolipin), and TRPA (ankyrin) [165]. Several members that make up this protein superfamily have been found to be nonselective cation channels, of which many are located on primary sensory neurons and involved in somatosensory procedures, such as the transduction of chemical, thermal, and mechanical stimuli. TRPV1 (also called capsaicin receptor) is a nonselective cation channel with high permeability of Ca2+ and activated by capsaicin and other vanilloid compounds [165]. However, TRPA1 is activated by a variety of plantderived and environmental irritants all of which interact with cysteine residues in the ion channel proteins and is present on capsaicin-sensitive primary sensory neurons, which upon activation elicit pain, protective reflexes, and local release of neurotransmitters in the periphery [166].
H2S and capsaicin receptor (TRPV1)
H2S and its donors activate TRPV1 ion channels in GI tract, airway, pancreas, and urinary bladder, which cause colonic mucosal Clsecretion, gut motility, airway constriction, acute pancreatitis, detrusor muscle contraction, and bladder contractility through a neurogenic inflammation mechanism [167-170]. Serosal application of NaHS and L-cysteine stimulates luminal Cl- secretion by guinea pig and human colonic tissues [171]. This effect is blocked by TTX, desensitization of afferent nerves with capsaicin, or by the TRPV1 antagonist capsazepine. As such, the stimulatory effects of H2S on TTX-sensitive Na+ channels as well as TRPV1 channels are theorized [130]. Interestingly, the secretory effect of NaHS is not observed in a human colonic epithelial cell line (T84 cells) [171].
It appears that H2S-stimulated mucosal secretion cannot be realized in the absence of either TTX-sensitive Na+ channels and/or TRPV1 channels from sensory nerve endings. In addition, NaHS-induced Clsecretion in rat distal colon is inhibited by serosally applied glibenclamide and tetrapentylammonium, which also block K+ channels (KATP and KCa) [148]. As glibenclamide may inhibit CFTR, this result could also be interpreted as the direct activation of CFTR by H2S to increase Clsecretion. Similar to capsaicin, H2S donors induce CGRP and substance P release from the sensory nerves in the guinea pig airways and cause in vivo bronchoconstriction and microvascular leakage in a capsazepinesensitive manner. This adds to the irritant action of H2S in the respiratory system [170]. It has been found that NaHS induces a dose-dependent contraction of isolated bronchial and tracheal rings in vitro and this effect is denigrated by the desensitization of sensory nerves with high concentration of capsaicin, by TRPV1 antagonists (capsazepine), as well as by a mixture of neurokinin NK1 (a substance P receptor) and NK2 receptor (CGRP receptor) antagonists. Interestingly, intraperitoneal injection of NaHS to healthy mice induced substantial inflammatory reaction in the lung, as evidenced by increased concentration of substance P, pro-inflammatory cytokines, TNF-αand IL-1β and lung MPO activity [172]. These effects were abolished by a specific NK1 receptor antagonist, but not by NK2 receptor antagonists. In addition, the inflammatory effect of H2S was abolished by capsazepine and was not observed in mice lacking substance P and neurokinin-A due to the knockout of their common precursor gene, preprotachykinin-A [172]. These data indicated that H2S per se may induce neurogenic inflammation, even in the absence of other, often harmful, elements. Further research is still required to solve whether H2S acts as an endogenous ligand of TRPV1 or not [130]. Activation of TRPV1 has been reported to mediate neurogenic inflammation in cerulein-evoked pancreatitis [173]. Intravenous injection of the TRPV1 agonist capsaicin activated a dose-dependent increase in Evan blue aggregation in the rat pancreas. This effect was halted by the pretreatment with the TRPV1 antagonist capsazepine or the neurokinin-1 receptor antagonist CP96, 345. Capsazepine also limited cerulein-induced Evans blue, MPO and histological severity of inflammation in the pancreas, but no effect was seen on serum amylase [173]. Consequently, enhanced plasma H2S levels have recently been demonstrated in cerulein-induced pancreatitis [167], and administration of PPG reduces the morphological changes in acute pancreatitis, which consists mainly of edema, inflammation and acinar cell injury/necrosis. In contrast to its vasorelaxant effect, NaHS actually created concentration-dependent contractile responses in the detrusor muscle of the rat urinary bladder [174]. This response generated rapid and persistent tachyphylaxis similar to the responses of capsaicin. However, this cannot be seen as a direct effect of H2S on the muscle because it was destroyed by the combination of NK1 and NK2 receptor-selective antagonists as well as by high-capsaicin pretreatment, which could desensitize capsaicin-sensitive primary afferent neurons. The response to NaHS is mostly resistant to TTX, as is the effect of capsaicin in this organ. The results may be able to provide pharmacological proof that H2S stimulates capsaicin-sensitive primary afferent nerve terminals with the consequent release of tachykinins, which subsequently produces contractile responses of the detrusor muscle. Furthermore, ruthenium red, a nonspecific blocker of TRPV1 channels, blocked the H2S-induced contractile response [168], but TRPV1-selective antagonist capsazepine and SB366791 failed to do so. It has also been theorized that H2S may stimulate the TRPV1 receptor by a different way from those known activators.
H2S and ankyrin (TRPA1)
TRPA1 is activated by a variety of plant-derived and environmental irritants, such as allyl isothiocyanate (AI), cinnamaldehyde (CA), allicin and acrolein, all of which interact with cysteine residues in the ion channel proteins [175]. Interestingly, acrolein and similar aldehydes are formed endogenously during inflammation. TRPA1 was initially characterized as a noxious cold receptor [176] and lately its role in mechanosensation has been suggested [177,178]. In the rat bladder, TRPA1 is expressed in unmyelinated sensory nerve fibers with similar pattern to that of TRPV1. Interestingly, TRPA1 is also present in the urothelium, detected at both transcriptional and protein levels. The stimulation of TRPA1 channels induced detrusor overactivity. TRPA1 appears to be consistently colocalized with TRPV1 in the bladder afferents, which suggests a role of TRPA1 in bladder chemosensation and mechanotransduction [169]. Following pretreatment with protamine sulfate, NaHS increased maximal bladder pressure and reduced voided and infused volumes. NaHS evoked a time- and concentrationdependent increase in [Ca2+]i in Chinese hamster ovary cells expressing mouse or human TRPA1, but not in untransfected cells. This indirect evidence for the activation of TRPA1 by H2S needs to be validated with more direct electrophysiologial recording.
Should this role be confirmed, H2S may function as a TRPA1 activator potentially involved in inflammatory bladder disease and in lower urinary tract infection. Furthermore, bacterial metabolite H2S induced by potential pathogens such as Escherichia coli [179] might activate TRPA1 in lower urinary tract infections. In generally, H2S is the first identified gaseous opener of KATP channels in vascular SMCs and regulates vascular tone by relaxing smooth muscle cells. In the heart, H2S and its donors cause the negative inotropic and chronotropic action through activating sarcKATP and mitoKATP channels and inhibiting L-type Ca2+ channel activity and exert cardioprotection during I/R injury. H2S-induced reduction of blood pressure can be related to the activation of peripheral KATP channels in resistant vessel SMCs. The regulation of insulin secretion from pancreatic β-cells by H2S is via enhancing KATP channel and suppressing L-type Ca2+ channel activities. By elevating [Ca2+]i, H2S may mediate glutamate-induced neurotoxity and neuronal cell death, but conflicting reports describe the protective effect of H2S on neuron cells from oxidative glutamate toxicity by activating KATP and Cl- channels. H2S-induced hyperalgesia in the colon seems to depend on the sensitization of T-type Ca2+ channels. On the other hand, H2S has a pronociceptive role through evoking the excitation of capsaicin-sensitive TRPV1-containing sensory neurons. H2S and its donors also activate TRPV1 and TRPA1 channels in nonvascular smooth muscle such as urinary bladder, airways and GI tract, regulating smooth muscle contractility. The opening of KATP channels by H2S has been confirmed in cardiovascular, endocrine, and nervous systems, which constitute a major molecular mechanism for many cellular effects of H2S. However, the molecular interaction of this gasotransmitter with KATP channel complex has not been clear and the relative contribution of cysteine sulfhydration in KATP channel proteins by H2S merits further investigation [27]. The effects of H2S on voltagedependent L-type Ca2+ channels or BKCa channels are inconclusive.
Biological Roles of H2S Vascular System
H2s and myogenic tone
Initial studies suggested that in the vascular wall H2S is produced only by smooth muscle cells (SMCs) [38]. However, now it is clear that H2S are produced by endothelial cells, perirenal, epidydimal and perivascular white adipose tissue, as well as in brown adipose tissue [8,180-184].
One of the first physiological roles that prompted investigators to regard H2S as the ‘‘third gaseous signaling molecule’’ was vasodilation. Intravenously administered H2S or its donors decrease blood pressure in experimental animals and deficiency of endogenous H2S has been implicated as a pathogenic factor in arterial hypertension [185]. In 2001, Zhao et al. showed that H2S decreased blood pressure in rats in vivo and caused vascular smooth muscle cell (VSMC) relaxation in vitro [38]. H2S-mediated vasodilation has also been shown in the smooth muscle of the ileum and the vas deferens [186]. H2S produced in vascular smooth muscle and endothelial cells dilates blood vessels in part by activating ATP-sensitive potassium channels (KATP) in smooth muscle cells and inducing cell hyperpolarization [185] and in part by stimulating endothelium-derived NO production [104]. Studies suggest that H2S liberates NO. from S-nitrosothiols [187]. Others show that endothelial denudation and nitric oxide synthase (NOS) inhibitors shift the concentration-response curve for H2S [104]. However, H2S increases eNOS phosphorylation and subsequent NO. production in an Akt-dependent manner [188]. Teague et al. [186] reported a summation effect between H2S and NO on the sublimation of the twitch responses of the ileum to electrical activation. The enhancing effect of H2S on NO-induced vasorelaxation is still controversial. Zhao et al. [38] observed that pretreatment of aortic ring preparations with H2S inhibited the vasorelaxant effect of the NO-producing agent SNP [38]. However, Ali et al. [189] have shown that H2S induced vasoconstriction and increased the mean arterial pressure in rats likely by scavenging endothelial NO. It is likely that the interaction of NO and H2S may alter the vasorelaxant properties of these two gasotransmitters. Also, the common molecular target for NO and H2S may become desensitized after firstly encountering one of them. The production of H2S in the presence of NO is a different story. H2S production by CSE in vascular tissues is increased by SNP, while the expression of CSE is up-regulated by another NO-producing agent, SNAP [38]. CSE contains 12 cysteine residues that are potential targets for S-nitrosylation. S-nitrosylation of CSE has the potential to increase the enzymatic activities [72]. Perfusion of the mesenteric system with 1 mmol/L cysteine (precursor of H2S) resulted in an increase of endogenous H2S production and a dilation of the mesenteric circulation [125]. Cheang et al. showed that KATP channels were not involved in mediating effects of H2S in rat coronary arteries [190]. These authors suggested voltage-dependent potassium (KV) channels as possible mediators of NaHS-evoked vasorelaxation.
Schleifenbaum et al proposed H2S as a vasorelaxing factor released from perivascular adipose tissue and acting via the stimulation of special Kv type channels – KNCQ channels [191]. Additionally, small, intermediate, and large conductance calcium-dependent potassium channels (SKCa, IKCa and BKCa) have also been demonstrated as possible mediators of H2S vasodilator effects in resistance vessels [122,123]. An H2S-evoked increase in cyclic guanosine monophosphate (cGMP) levels could also be involved in H2S-induced vasorelaxation of smooth muscle cells. Bucci et al. confirmed that H2S results in vasorelaxation by non-selectively inhibiting endogenous phosphodiesterase (PDE) [192]. This effect would increase tissue levels of cyclic nucleotides, such as cGMP. Recently, conflicting reports have emerged showing that the contribution of the KATP channels to H2S-induced vasodilation is minimal and that vasodilation is due to metabolic inhibition (i.e., decrease in ATP), intracellular pH changes, and modulation of Cl-/ HCO3 - channels [193]. A change in the intracellular acid-base balance is one of the factors that influence the vasoactivity of vascular smooth muscle cells. In another statement, acidification has a vasorelaxant effect, whereas the alkalinization of the intracellular environment causes vasoconstriction in most of the vascular bed. According to data published by Lee et al, H2S could modify the pH equilibrium in cells by activating the Cl-/HCO3- exchanger and thereby induce acidification [133]. However, the vasoactive response of vessels to H2S differs in dependence on several factors, for example, the type of vessel (conduit arteries, resistance arteries) endothelium, the substance used for precontraction and the concentration of H2S applied [1,104]. H2S relaxes small mesenteric arteries much more potent than aortic tissues [125]. Although rat aortic and mesenteric artery tissues produce similar levels of H2S, H2S is nearly six fold more potently in relaxing rat mesenteric artery beds than relaxing rat aortic tissues. The higher sensitivity of mesenteric arteries to H2S speaks for the importance of H2S in regulating peripheral resistance. The mechanisms for differential vasorelaxant effects of H2S are not clear yet, but several possibilities exist. One explanation is the tissue-type specific distribution of the molecular targets of H2S. For example, the expression of KATP channels possibly differs in various vascular tissues with different isoforms. The second explanation is that sensitivities of contractile proteins to H2S and to intracellular calcium level may vary between conduit and resistant arteries [125].
Also, different types of blood vessels face different sheer stress levels, possess different cellular components (smooth muscle cells, endothelial cells and connective tissues, etc.) and have different stiffness. Finally, oxygen-dependent sensitivity of blood vessels to H2S should also be considered. It has been reported that H2S induced vasorelaxation at physiological O2 levels and this vasorelaxation occurred much faster at below physiological O2 levels. With higher than physiological O2 levels (200 mM), H2S has the tendency to induce vasoconstriction [72]. This could result from the product of H2S oxidation, which may mediate vasoconstriction. Blood in small peripheral vessels has lower oxygen partial pressure, and these small vessels consume oxygen at higher rate due to the high content of smooth muscle cells and low collagen. The situation is just opposite in large conduit arteries [72]. The difference in tissue oxygen level may explain different vascular effects of H2S. Another note worth taking is that the release of NO from S-nitrosoglutathione by H2S is oxygen dependent [72]. H2S functions as a vasodilator in cerebral circulation. Topical application of H2S to the newborn pigs induces dilation of pial arterioles [194]. Leffler et al. [194] further showed that L-cysteine per se dilated pial arterioles. Additionally, others have shown that transgenic mice deficient in CBS are chronically hypertensive [37]. Three lines of evidence were given to demonstrate the effect of L-cysteine was the outcome of CSE-generated H2S. First, PPG at 10 mM blocked the vasorelaxant effect of L-cysteine, but AOA at 1 mM failed to do the same. Second, CSE proteins were detected in cerebral microvessels. While CBS proteins were detected in brain parenchyma, it was not detectable in cerebral microvessels. Third, H2S concentration in cerebrospinal fluid was increased about fourfold after L-cysteine treatment, measured by GC-MS, which was again blocked by PPG. Whether this vasodilatory effect of H2S is unique to newborn animal or ubiquitous to cerebral circulation at other stages of development is not known. The stimulus used to precontract vascular tissues also significantly affects the effect of H2S. While H2S relaxed phenylephrine- or norepinephrine-precontracted aortic tissues, high concentration of KCl (60 mM)-induced vascular contraction was essentially not affected by H2S. In effect, though H2S inhibits KCl (20 mM)-induced contractions of aortic tissues, it does not change the contraction of ileum induced by the same concentration of KCl [186]. Therefore, different vascular tissues manifest different sensitivities to H2S. Sodium hydrosulphide (NaHS) at concentrations over 100 μmol/L evoked the relaxation of precontracted isolated rat arteries [7,38,189].
Higher concentrations of H2S (sodium disulphide (NaHS): 2.8 and 14 μmol/kg; 0.1-1 mmol/L) evoked decrease of blood pressure or vasorelaxation in some types of isolated vessels [38,104]. At the same concentration level, H2S-gassed solution has much stronger vasorelaxant effects than NaHS solution does. The involvement of various signal transduction pathways in the vasodilator effects of H2S has been examined. NO and CO relax smooth muscle by activating guanylyl cyclase to increase the production of cGMP. H2S does not affect the production of cGMP, which leads to the inference that there is a different mechanism for the effect of H2S. Earlier studies also demonstrated that the vasorelaxant effects of H2S on rat vascular tissues are unlikely mediated by prostaglandin, protein kinase C or cAMP pathways [38,104,125]. Superoxide dismutase and catalase in the bath solution also did not alter the vasorelaxant effect of H2S, indicating that superoxide anion and hydrogen peroxide did not contribute to H2S-induced acute vasorelaxation. Although ODQ blocked the vasorelaxation induced by SNP, it had no effect on the vasorelaxant effect of H2S on rat aortic tissues. Therefore, under this experimental condition, the vasorelaxant effect of H2S was not mediated by the cGMP pathway [38]. KATP channel is the major molecular target of H2S for its vasorelaxant effect and smooth muscle hyperpolarization [38,195]. In the ileum, glibenclamide did not interfere with the relaxation induced by H2S [186]. This finding may be seen as there are participation of several additional signaling pathways and mechanisms [48]. Furthermore, the specific molecular targets of H2S were shown to be cysteine 6 and 26 of the extracellular portion of the rvSUR2B subunit of the KATP channel complex [103]. These vicinal thiols form a disulfide bond, which H2S reduces, increasing channel conductance and induce hyperpolarization in a tissue-dependent manner. On the other hand, some observations revealed an opposite effect of H2S on smooth muscle cells of the arterial wall. Lower concentrations of H2S (Na2S: 3 μmol/kg; 10-100 μmol/L) resulted in blood pressure increase and vasoconstriction of the same vessels [105,196-198]. Published data indicate numerous possible mechanisms of H2S-induced vasoconstriction. One possible mechanisms of H2S-induced vasoconstriction is decreased levels of cyclic adenosine monophosphate (cAMP) in smooth muscle cells. Li et al showed on the rat cerebral artery that H2S evoked a decrease of cAMP levels, an effect that was associated with the promotion of an interaction between actin and myosin [199].
The H2S-mediated decrease in cAMP concentrations stimulated the activation of myosin light chain kinase, an enzyme that mediates the interaction between actin and myosin [197]. Li et al also proved that H2S did not directly influence cAMP levels but significantly reduced forskolin-stimulated adenylyl cyclase activity in human brain vascular smooth muscle cells [199]. This result demonstrated that H2S-induced vasoconstriction was due to the inhibition of the cAMP/ adenylyl cyclase pathway. It was also shown that the administration of low concentrations of H2S (5-100 μmol/l) inhibited forskolin-induced cAMP accumulation in aortic smooth muscle. Moreover, NaHS was observed to inhibit vasorelaxing effects via β-adrenergic vasodilators and to induce vasoconstricting effects via adenylate cyclase and cAMP inhibition [200]. Ping et al. found that prostanoids could be involved in NaHS-induced vasoconstriction because the vasoconstriction evoked by H2S was markedly attenuated in the presence of a cyclooxygenase inhibitor (indomethacin, 10 μmol/l) [201]. It was concluded by the same authors that the contractile effect of H2S was mediated by an influx of extracellular Ca2+ because the effect was totally inhibited in a Ca2+- free solution and following incubation with the Ca2+ influx blocker nifedipine [1]. H2S, in contrast to NO, which has a clear vasorelaxant action, has both vasorelaxing and vasoconstricting effects on the arterial system [8]. In another terms, H2S has marked effects on the circulation, by acting as a hypoxic vasoconstrictor or vasodilator in the pulmonary and systemic circulation, respectively [202,203]. H2S is a potent vasodilator in the systemic circulation and it produce reduces cardiac output and prolongs body energy stores by redistributing blood flow to the most demanding organs [38]. Conversely, in the lung circulation, H2S may contribute to hypoxic vasoconstriction [204,205]; thereby helping to maintain a high arterial O2 saturation at the low ventilation rates [202,206].
H2S and vascular endothelial cell proliferation
The same physiological stimuli do not necessarily elicit the same functional responses from different types of cells. While H2S inhibits vascular SMC proliferation, the gasotransmitter stimulates the proliferation and migration of vascular endothelial cells either in culture or in the whole blood vessel walls. To this end, the stimulatory effect of H2S on ECs has been reported with cultured human umbilical vein endothelial cells (HUVECs) [207,208] and bEnd3 microvascular endothelial cells [209].
It should be noticed that the pro-proliferative effect of H2S donors on ECs could not be detected if the concentrations of H2S donors were higher than physiologically relevant levels. The signaling pathways underlying the stimulatory effect of H2S on EC proliferation are complex and inconclusive. The stimulation of PI-3K/Akt pathway, KATP channels, and MAPK and the inhibition of sGC/cGMP pathway by H2S have all been suggested in ECs [166]. Increased intracellular calcium concentration ([Ca2+]i) in cultured human saphenous vein endothelial cells by NaHS treatment has also been reported [210]. This increase in [Ca2+]i was mostly due to calcium release from ryanodine receptor-coupled endoplasmic reticulum and due to capacitative Ca2+ entry to a smaller extent. Also note that endothelial cells in intact blood vessels (not in culture) do not express functional ryanodine receptors, so this effect is irrelevant. To date, there is no report to link the effect of H2S on [Ca2+]i levels in ECs to H2S-stimulated EC proliferation [210]. H2S also protects ECs from the damages of different stressors. Hyperglycemia decreased the viability of ECs by increasing oxidative stress and nuclear DNA injury. This hyperglycemia stress results in impaired endothelium-dependent vasorelaxation. In cultured microvascular ECs, the hyperglycemia-induced EC damage was suppressed by supplementation of exogenous H2S to the culture media. CSE overexpression increased EC viability by 6% compared with the native ECs, facing the same hyperglycemic culture conditions. On the other hand, knocking down the expression of endogenous CSE with siRNA reduced hyperglycemia-enhanced oxidative stress in ECs [209]. Extending their observations from cultured endothelial cells, Suzuki et al. [209] overexpressed CSE gene in thoracic aortic rings isolated from Sprague-Dawley rats. This in vitro transfection preserved the endothelium-dependent vasorelaxant properties of the vascular rings in the presence of hyperglycemia [209]. The important role of CSE/H2S in protecting ECs from hyperglycemic damage was further demonstrated in CSE KO mice. The isolated thoracic aorta rings from CSE KO mice were manifested with much more severely damaged endotheliumdependent relaxations than that from WT mice when incubated with the same hyperglycemic conditions in vitro [209].
H2S and vascular smooth muscle cell proliferation
The proliferation of vascular SMCs plays a critical role in the maintenance of vascular structure and functions, and its alteration leads to vascular remodeling and various proliferative vascular diseases. However, cellular and molecular mechanisms that regulate SMC proliferation and differentiation are not fully understood. H2S is an important endogenous modulator of cell proliferation and apoptosis [211]. Serum deprivation up-regulated CSE expression and H2S production in cultured human aorta SMCs in concert with the induced SMC differentiation marker gene expressions, such as SMMHC, calponin and SM-actin [212]. Overexpression of CSE in human aortic SMCs inhibited cell growth and induced cell apoptosis [213]. Absence of endogenous H2S in vascular SMCs, such as those isolated from CSE gene deficient mice (KO mice), led to a significant surge in cell growth rate [214]. The percentage of BrdU-positive cells in cultured SMCs and in the media of the aorta was also significantly greater in CSE KO mice than in age matched and wide-type mice [214]. Clearly, endogenous CSE/H2S limits the proliferation and growth of SMCs. Furthermore, increased SMC proliferation in CSE KO mice was not secondary to the development of hypertension. The normalization of blood pressure in CSE KO mice by captopril did not reduce aortic SMC proliferation when compared with untreated age-matched CSE KO mice [214]. The endogenous level of H2S affects the effect of exogenous H2S on cell apoptosis. Yang et al. [213] found that NaHS induced apoptosis of human aortaic SMCs at concentrations 200 M. After inhibition of endogenous H2S production by PPG pretreatment or by knocking down endogenous CSE gene with short-interfering RNA approach, the proapoptotic effect of NaHS becomes significant at 50-100 M. Another study reported that exogenously applied NaHS at 100 M inhibited proliferation and induced apoptosis of vascular SMCs from CSE KO mice, but not of SMCs from wide-type (WT) mice [214]. CSE/H2S pathway is also involved in the development of balloon injuryinduced neointima formation of rat carotid arteries. The transcriptional expression levels of CSE, CSE activity and endogenous H2S production were all decreased in balloon-injured carotid arteries [215]. Treatment of the rats with NaHS significantly weakened balloon injury-induced neointimal hyperplasia and reduced vascular smooth muscle cell proliferation in the lesions in vivo. Similar observations were made in the mouse where carotid artery ligation resulted in enhanced neointima formation and down-regulation of CSE expression [212].
The mechanisms underlying the antiproliferative and/or proapoptotic effect of H2S are multifaceted. One of the focal points of these studies is the involvement of the mitogen-activated protein kinase (MAPK) superfamily, including three parallel cascades which are the stress-activated protein kinase/c-Jun NH2-terminal kinase (SAPK/ JNK) cascade, the p38-MAPK cascade, and the classical extracellular signal-regulated kinase (ERK)/MAPK cascade. In human aortic SMCs, for example, exogenous H2S induced apoptosis through activation of MAPK pathway. The phosphorylation of ERK transduces the apoptotic signal to its downstream enzyme cascades and eventually activates caspase-3. After the activities of ERK and caspase-3 were inhibited, the apoptosis of human aortic SMCs induced by H2S was significantly attenuated. Therefore, the activation of ERK and its downstream factor caspase-3 likely mediates H2S-induced cell apoptosis [211]. It is worth pointing out that in many other cell types or tissues, ERK activation serves as a proliferative/antiapoptotic signal. It has been reported that the proliferation of cultured rat aortic vascular SMCs was inhibited by NaHS. At the same concentration range (50-500 M), NaHS also inhibited ERK activity [216]. Whether activation of ERK could reverse NaHS induced proliferation inhibition was not conducted by the same researchers [216]. Therefore, it is not sure whether the decreased ERK activity can account for the reported effect of NaHS on rat vascular SMCs. In CSE overexpressed HEK-293 cells, ERK and p38 MAPK activities were significantly increased, but not in Ad-lacZ infected cells or control cells, and the cell growth was inhibited [217]. The activations of ERK and p38 MAPK were also involved in H2S-treated intestinal epithelial cells (IEC-18) [218]. The pro-apoptotic effect of H2S may also be related to the cell cycle due to the stimulation of cyclin-dependent kinases. S-diclofenac {2-[(2,6-dichlorophenyl)amino]benzene acetic acid 4-(3H-1,2,dithiol-3-thione-5-yl)phenyl ester} is a novel molecule comprising an H2S-releasing dithiolthione moiety attached by an ester linkage to diclofenac [219]. S-diclofenac induces a dose-dependent decrease in the survival of primary and immortalized rat aortic vascular SMCs. The cells in G1 phase were not affected by S-diclofenac but asynchronized SMCs manifested with an increase in apoptotic cell death. S-diclofenac stabilized p53 and induced p21, p53AlP1 and Bax. But the anti-apoptotic factor BCl-2 was not affected [219]. In CSE-overexpressed cell or exogenous H2S-treated cells, there are also an increased expression of p21Cip/WAK-1 and a down-regulation of cyclin D1 [217].
The anti-proliferative and/or pro-apoptotic effect of H2S may be of importance for the prevention of cell proliferation in disorders such as atherosclerosis, vascular graft occlusion and neointimal hyperplasia leading to restenosis after angioplasty [220].
H2S and angiogenesis
H2S can cause cell proliferation and migration [166,208]; however, there appears to be a narrow concentration range of the proliferative effect, below which no effect is seen and above which there is antiproliferation and H2S cytotoxicity [207]. In cell culture experiments, low micromolar concentrations of H2S increase endothelial cell number, proto-vessel formation, and cell migration [166]. Chicken chorioallantoic membranes, an in vivo model of angiogenesis, display increased branching and lengthening of blood vessels in response to 48 h incubation with H2S [208]. Additionally, aortic tissue isolated from transgenic mice lacking CSE, the primary H2S-producing enzyme in the endothelium, exhibit marked decreases in angiogenesis [208]. The mechanism of H2S-induced angiogenesis operates through several pathways, including activation of ATP-sensitive potassium (KATP) channels [38]. Papapetropoulos et al. showed that treatment of endothelial cells with the KATP channel inhibitor glibenclamide reduced cell migration, which was accompanied by decreased H2S-induced p38 and heat shock protein 27 (Hsp27) phosphorylation [208]. Additionally, H2S can stimulate angiogenesis through phosphatidylinositol 3-kinase (PI3K) and Akt activation [166]. H2S can also activate hypoxia inducible factor-1α (HIF-1α) and thus increase expression of vascular endothelial growth factor (VEGF) [221]. Conversely, VEGF-stimulated angiogenesis is suppressed in CSE knockout mice [166]. Endogenous H2S production is known to be upregulated during wound healing [222]. Topically applied H2S accelerates wound closure and healing [208]. Angiogenesis is very important in both acute and chronic ischemia as poorly vascularized tissue will lose function and possibly become necrotic. In models of chronic hind limb ischemia, sodium hydrosulfide (NaHS) increased capillary formation and blood flow [223]. Similar results were found in chronically ischemic hearts with improvements in cardiac function following H2S treatment [224]. These studies indicate that endogenous H2S is crucial in physiological angiogenesis and that those capabilities can be employed in disease treatment.
Conclusion
H2S can directly regulate vascular tone by acting up on specific targets receptors and induces hyperpolarization of adjacent vascular smooth muscle cells in large conduit and small resistance arteries by activating KATP and KCNQ channels, respectively. The mechanism of vascular effect of H2S is controversial with opposite data sometimes provided by different studies. These discrepancies result most likely from using different animal species, different H2S donors and their concentrations, as well as experimental conditions, such as buffer composition or oxygen levels. Generally H2S has marked effects on the circulation, by acting as a hypoxic vasoconstrictor or vasodilator in the pulmonary and systemic circulation, respectively. Although H2S is a potent vasodilator of isolated systemic vessels, its effect in living hibernating animals would probably be overwhelmed by a strong adrenergic tone that may constrict peripheral systemic blood vessels.
References
- Cacanyiova S, Berenyiova A, Kristek F (2016) The role of hydrogen sulphide in blood pressure regulation. Physiol Res 65: S273-273S289.
- Furchgott RF, Zawadzki JV (1980) The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288: 373-376.
- Palmer RM, Ferrige AG, Moncada S (1987) Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327: 524-526.
- Wang R (2002) Two’s company, three’s a crowd: Can H2S be the third endogenous gaseous transmitter? FASEB J 16: 1792-1798.
- Marks GS, Brien JF, Nakatsu K, McLaughlin BE (1991) Does carbon monoxide has a physiological function? Trends Pharmacol Sci 12: 185-188.
- Abe K, Kimura H (1996) The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci 16: 1066-1071.
- Hosoki R, Matsuki N, Kimura H (1997) The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun 237: 527-531.
- Beltowski J, Jamroz-Wisniewska A (2014) Hydrogen sulfide and endothelium-dependent vasorelaxation. Molecules.19: 21183-21199
- Wang R1 (2014) Gasotransmitters: Growing pains and joys. Trends Biochem Sci 39: 227-232.
- Mulrow C, Lawrence V, Ackermann R, Gilbert Ramirez G, Morbidoni L, Aguilar C, et al. (2000) Garlic: Effects on cardiovascular risks and disease, protective effects against cancer and clinical adverse effects. Evid Rep Techn Asses 20: 1-4.
- Barnes L, Anderson L, Phillipson J (2007) Herbal medicines. (3rd edn). London, Grayslake, IL: Pharmaceutical Press.
- Benavides GA, Squadrito GL, Mills RW, Patel HD, Isbell TS, et al. (2007) Hydrogen sulfide mediates the vasoactivity of garlic. Proc Natl Acad Sci U S A 104: 17977-17982.
- Banerjee SK, Maulik SK (2002) Effect of garlic on cardiovascular disorders: A review. Nutr J 1: 4.
- Moss GA (2010) Water and health: A forgotten connection? Perspect Public Health 130: 227-232.
- Reigstad L, Jorgensen S, Lauritzen S, Schleper C, Urich T (2011) Sulfuroxidizing chemolithotrophic proteobacteria dominate the microbiota in high arctic thermal springs on Svalbard. Astrobiology 11: 665-678.
- Matz H, Orion E, Wolf R (2003) Balneotherapy in dermatology. Dermatol Ther 16: 132-140.
- Stein S, Bailey S (2013) Redox biology of hydrogen sulfide: Implications for physiology, pathophysiology and pharmacology. Redox Biol 1: 32-39
- Kimura H (2014) Hydrogen sulfide and polysulfides as biological mediators. Molecules 19: 16146-16157.
- Zhang X, Bian JS (2014) Hydrogen sulfide: A neuromodulator and neuroprotectant in the central nervous system. ACS Chem Neurosci 5: 876-883.
- Blackstone E, Morrison M, Roth MB (2005) H2S induces a suspended animation-like state in mice. Science 308: 518.
- Beltowski J, Atanassova P, Chaldakov G (2010) Hydrogen sulfide: Synthesis and function in the adipose tissue. Adipobiology 2: 41-50.
- Cooper CE, Brown GC (2008) The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: Chemical mechanism and physiological significance. J Bioenerg Biomembr 40: 533-539.
- Brown GC (2001) Regulation of mitochondrial respiration by nitric oxide inhibition of cytochrome c oxidase. Biochim Biophys Acta 1504: 46-57.
- Doeller JE1, Isbell TS, Benavides G, Koenitzer J, Patel H, et al. (2005) Polarographic measurement of hydrogen sulfide production and consumption by mammalian tissues. Anal Biochem 341: 40-51.
- Furne J1, Saeed A, Levitt MD (2008) Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am J Physiol Regul Integr Comp Physiol 295: R1479-1485.
- Beltowski J (2007) Hydrogen sulfide (H2S) – the new member of gasotransmitter family. Biomed Rev 18: 75-83.
- Wang R (2012) Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol Rev 92: 791-896.
- Kimura H (2011) Hydrogen sulfide: its production, release and functions. Amino Acids 41: 113-121.
- Beltowski J, Tokarzewska D (2010) Adipose tissue and homocysteine metabolism. Biomed Rev 20: 7-15.
- Chiku T, Padovani D, Zhu W, Singh S, Vitvitsky V, et al. (2009) H2S biogenesis by human cystathionine gamma-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J Biol Chem 284: 11601-11612.
- Singh S, Padovani D, Leslie R, Chiku T, Banerjee R (2009) Relative contributions of cystathionine ß-synthase and cystathionase to H2S biogenesis via alternative trans-sulfuration reactions. J Biol Chem 284: 22457-22466.
- Shibuya N, Tanaka M, Yoshida M, Ogasawara Y, Togawa T, et al. (2009) 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal 11: 703-714
- Shibuya N, Mikami Y, Kimura Y, Nagahara N, Kimura H (2009) Vascular endothelium expresses 3-mercaptopyruvate sulfur transferase and produces hydrogen sulfide. J Biochem 146: 623-626.
- Xia M1, Chen L, Muh RW, Li PL, Li N (2009) Production and actions of hydrogen sulfide, a novel gaseous bioactive substance, in the kidneys. J Pharmacol Exp Ther 329: 1056-1062
- Geng B, Yang J, Qi Y, Zhao J, Pang Y, et al. (2004) H2S generated by heart in rat and its effects on cardiac function. Biochem Biophys Res Commun 313: 362-368
- Yang G, Wu L, Jiang B, Yang W, Qi J, et al. (2008) H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine gamma-lyase. Science 322: 587-590.
- Zhao W, Zhang J, Lu Y, Wang R (2001) The vasorelaxant effect of H2S as a novel endogenous gaseous K(ATP) channel opener. EMBO J 20: 6008-6016.
- Whitfield NL, Kreimier EL, Verdial FC, Skovgaard N, Olson KR (2008) Reappraisal of H2S/sulfide concentration in vertebrate blood and its potential significance in ischemic preconditioning and vascular signaling. Am J Physiol Regul Integr Comp Physiol 294: R1930-1937.
- Levitt M, Abdel-Rehim M, Furne J (2011) Free and acid labile hydrogen sulfide concentrations in mouse tissues: anomalously high free hydrogen sulfide in aortic tissue. Antioxid Redox Signal 15: 373-378.
- Ishigami M, Hiraki K, Umemura K, Ogasawara Y, Ishii K, et al. (2009) A source of hydrogen sulfide and a mechanism of its release in the brain. Antioxid Redox Signal 11: 205-214.
- Furne J, Springfield J, Koenig T, DeMaster E, Levitt MD (2001) Oxidation of hydrogen sulfide and methanethiol to thiosulfate by rat tissues: A specialized function of the colonic mucosa. Biochem Pharmacol 62: 255-259
- Wilson K, Mudra M, Furne J, Levitt M (2008) Differentiation of the roles of sulfide oxidase and rhodanese in the detoxification of sulfide by the colonic mucosa. Dig Dis Sci 53: 277-283.
- Jackson M, Melideo S, Jorns M (2012) Human sulfide: Quinone oxidoreductase catalyzes the first step in hydrogen sulfide metabolism and produces a sulfane sulfur metabolite. Biochemistry 51: 6804-6815.
- Tiranti V, D’Adamo P, Briem E, Ferrari G, Leonard J,  et al.(2004) Ethylmalonic encephalopathy is caused by mutations in ETHE1, a gene encoding a mitochondrial matrix protein. Am Jour of Hum Gen 74: 239-252.
- Tiranti V, Viscomi C, Hildebrandt T, Di Meo I, Mineri R, et al. (2009) Loss of ETHE1, a mitochondrial dioxygenase, causes fatal sulfide toxicity in ethylmalonic encephalopathy. Nature Medicine 15: 200-205
- Hildebrandt TM, Grieshaber MK (2008) Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. FEBS J 275: 3352-3361.
- Liu Y, Lu M, Hu L, Wong P, Webb D, et al.(2012) Hydrogen sulphide in the mammalian cardiovascular system. Antioxid Redox Signal 17: 141-185.
- Berzofsky JA, Peisach J, Blumberg WE (1971) Sulfheme proteins. I. Optical and magnetic properties of sulfmyoglobin and its derivatives. J Biol Chem 246: 3367-3377.
- Assendelft O (1970) Spectrophotometry of haemoglobin derivatives. (3rd edn.) Springfield: C.C. Thomas.
- Carrico RJ, Blumberg WE, Peisach J (1978) The reversible binding of oxygen to sulfhemoglobin. J Biol Chem 253: 7212-7215.
- Pietri R, Roman-Morales E, Lopez-Garriga J (2011) Hydrogen sulfide and hemeproteins: knowledge and mysteries. Antioxid Redox Signaling 15: 393-404
- Schreier S, Muellner M, Steinkellner H, Hermann M, Esterbauer H, et al.(2010) Hydrogen sulfide scavenges the cytotoxic lipid oxidation product 4-HNE. Neurotox Res 17: 249-256.
- Toohey JI (1989) Sulphane sulphur in biological systems: A possible regulatory role. Biochem J 264: 625-632
- Mustafa A, Gadalla M, Sen N, Kim S, Mu W, et al. (2009) H2S signals through protein S-sulfhydration. Science Signaling2 (ra72), pp:1-8.
- Jacob C, Anwar A, Burkholz T (2008) Perspective on recent developments on sulfur-containing agents and hydrogen sulfide signaling. Planta Med 74: 1580-1592.
- Carballal S, Trujillo M, Cuevasanta E, Bartesaghi S, Moller M, et al. (2011) Reactivity of hydrogen sulfide with peroxynitrite and other oxidants of biological interest. Free Radic Biol Med 50: 196-205
- Iciek M, Wlodek L (2001) Biosynthesis and biological properties of compounds containing highly reactive, reduced sulfane sulfur. Polish J Pharm 53: 215-225
- Chen K, Morris J (1972) Kinetics of oxidation of aqueous sulfide by O2. Environ Sci Technol 6: 529-537
- Hongfang J, Bailin C, Bin Z, Chunyu Z, Xinmin L, et al. (2006) Effects of hydrogen sulfide on hypoxic pulmonary vascular structural remodeling. Life Sci 78: 1299-1309
- Hyspler R, Ticha A, Indrova M, Zakak Z, Hysplerova L, et al. (2002) A simple, optimized method for the determination of sulphide in whole blood by GC-MS as a marker of bowel fermentation processes. J Chromatog B Anal Tech Biomed Life Sci 770: 255-259.
- Richardson CJ, Magee EA, Cummings JH (2000) A new method for the determination of sulphide in gastrointestinal contents and whole blood by micro distillation and ion chromatography. Clin Chim Acta 293: 115-125.
- Chen YH, Yao WZ, Geng B, Ding YL, Lu M, et al. (2005) Endogenous hydrogen sulfide in patients with COPD. Chest 128: 3205-3211.
- Li L, Bhatia M, Zhu Y, Zhu Y, Ramnath R, Wang Z, et al.(2005) Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. FASEB J 19: 1196-1198.
- Srilatha B, Lingxu H, Adaikan G, Moore P (2009) Initial characterization of hydrogen sulfide effects in female sexual function. J Sex Med 6: 1875-1884
- Olson KR (2009) Is hydrogen sulfide a circulating "gasotransmitter" in vertebrate blood? Biochim Biophys Acta 1787: 856-863.
- Beauchamp RO Jr, Bus JS, Popp JA, Boreiko CJ, Andjelkovich DA (1984) A critical review of the literature on hydrogen sulfide toxicity. Crit Rev Toxicol 13: 25-97.
- Goodwin LR, Francom D, Dieken FP, Taylor JD, Warenycia MW, et al. (1989) Determination of sulfide in brain tissue by gas dialysis/ion chromatography: postmortem studies and two case reports. J Anal Toxicol 13: 105-109.
- Warenycia MW, Goodwin LR, Benishin CG, Reiffenstein RJ, Francom DM, et al. (1989) Acute hydrogen sulfide poisoning. Demonstration of selective uptake of sulfide by the brainstem by measurement of brain sulfide levels. Biochem Pharmacol 38: 973-981
- Yusuf M, Kwong Huat BT, Hsu A, Whiteman M, Bhatia M, et al. (2005) Streptozotocin-induced diabetes in the rat is associated with enhanced tissue hydrogen sulfide biosynthesis. Biochem Biophys Res Commun 333: 1146-1152.
- Sparatore A, Perrino E, Tazzari V, Giustarini D, Rossi R, et al. (2009) Pharmacological profile of a novel H2S-releasing aspirin. Free Radic Biol Med 46: 586-592.
- Koenitzer J, Isbell T, Patel H, Benavides G, Dickinson D, et al.(2007) Hydrogen sulfide mediates vasoactivity in an O2-dependent manner. Am J Physiol Heart Circ Physiol 292:H1953–60.
- Mitchell T, Savage J, Gould D (1993) High-performance liquid chromatography detection of sulfide in tissues from sulfide-treated mice. J Appl Toxicol 13: 389-934.
- Fischer E (1883) Bindung von Methylenblau als Reaktion auf Schwefelwasserstoff. Ber Dtsch Chem Ges 16: 2234-2236.
- Fogo J, Popowsky M (1949) Spectrophotometric determination of hydrogen sulfide. Anal Chem 21: 732-734
- Stipanuk MH, Beck PW (1982) Characterization of the enzymic capacity for cysteine desulphydration in liver and kidney of the rat. Biochem J 206: 267-277.
- Siegel LM (1965) A direct microdetermination for sulfide. Anal Biochem 11: 126-132.
- Gustafsson L (1960) Determination of ultramicro amounts of sulphate as methylene blue-I: The colour reaction. Talanta 4: 227-235.
- Geng B, Chang L, Pan C, Qi Y, Zhao J, et al. (2004) Endogenous hydrogen sulfide regulation of myocardial injury induced by isoproterenol. Biochem Biophys Res Commun 318: 756-763.
- Bamesberger W, Adams S (1969) Improvements in the collection of hydrogen sulfide in cadmium hydroxide suspension. Environ Sci Technol 3: 258-261
- Tao W, Tsai C (2002) H2S sensing properties of noble metal doped WO3 thin film sensor fabricated by micromachining. Sens Actuator 81: 237-247.
- Demming A (2013) Nanotechnological selection. Nanotechnology 24: 020201.
- Kong J, Chapline M, Dai H (2001) Functionalized carbon nanotubes for molecular hydrogen sensors. Adv Mater 13: 1384-1386
- Qi P, Vermesh O, Grecu M, Javey A, Wang Q, et al. (2003) Toward large arrays of multiplex functionalized carbon nanotube sensors for highly sensitive and selective molecular detection. Nano Lett 3: 347-351.
- Wang J, Bunimovich Y, Sui G, Savvas S, Wang J, et al. (2006) Electrochemical fabrication of conducting polymer nanowires in an integrated microfluidic system. Chem Commun 29: 3075-3077.
- Xue X, Chen Y, Liu Y, Shi S, Wang Y,et al.(2006) Synthesis and ethanol sensing properties of indium-doped tin oxide nanowires. Appl Phys Lett 88: 201907
- Sun Z, Yuan H, Liu Z, Han B, Zhang X (2005) A highly efficient chemical sensor material for H2S: Fe2O3 nanotubes fabricated using carbon nanotube templates. Adv Mater 17: 2993-2997.
- Kolmakov A, Zhang Y, Cheng G, Moskovits M (2003) Detection of CO and O2 using tin oxide nanowire sensors. Adv Mater 15: 997-1000
- Li C, Zhang D, Liu X, Han S, Tang T, et al.(2003) In2O3 nanowires as chemical sensors. Appl Phys Lett 82: 1613-1615.
- Wan O, Li Q, Chen J, Wang H, He X, et al. (2004) Fabrication and ethanol sensing characteristics of ZnO nanowire gas sensors. Appl Phys Lett 84: 3654-3656.
- Tamaki J, Yamada Y, Yamamoto Y, Matsuoka M, Ota I (2000) Sensing properties to dilute hydrogen sulfide of ZnSb2O6 thick-film prepared by dip-coating method. Sens Actuators B 66: 70-73.
- Kong J, Franklin NR, Zhou C, Chapline MG, Peng S, et al. (2000) Nanotube molecular wires as chemical sensors Science 287: 622-625.
- Klein J, Henning K (1984) Catalytic oxidation of hydrogen sulfide on activated carbons. Fuel Mag 63: 1064-1067.
- Wu X, Zhang W, Wu D, Sammynaiken R, Wang R, et al. (2006) Using carbon nanotubes to absorb low-concentration hydrogen sulfide in fluid. IEEE Trans Nanobiosci 5: 204-209.
- Wu X, Sammynaiken R, Zhang W, Wu D, Yang Q, et al. (2007) Measurement of low concentration and nano-quantity hydrogen sulfide in aqueous solution: Measurement mechanisms and limitations. Meas Sci Technol 18: 1315-1320
- Wu X, Zhang W, Sammynaiken R, Meng Q, Wu D, et al. (2009) Measurement of low concentration and nano-quantity hydrogen sulfide in sera using unfunctionalized carbon nanotubes. Meas Sci Technol 20: 105801.
- Searcy DG1, Peterson MA (2004) Hydrogen sulfide consumption measured at low steady state concentrations using a sulfidostat. Anal Biochem 324: 269-275.
- Gemerden H, Tughan C, Wit R, Herbert R (1989) Laminated microbial ecosystems on sheltered beaches in Scapa Flow, Orkney Islands. FEMS Microbiol Ecol 62: 87-101.
- Hu LF, Lu M, Tiong CX, Dawe GS, Hu G, et al. (2010) Neuroprotective effects of hydrogen sulfide on Parkinson's disease rat models. Aging Cell 9: 135-146.
- Ubuka T (2002) Assay methods and biological roles of labile sulfur in animal tissues. J Chromatogr B Analyt Technol Biomed Life Sci 781: 227-249
- Cassinelli M (1994) Hydrogen Sulfide, NIOSH Manual of Analytical Methods (NMAM). (4th edn.) Cincinnati, OH: National Institute of Occupational Safety and Health.
- Shen X, Pattillo CB, Pardue S, Bir SC, Wang R, et al. (2011) Measurement of plasma hydrogen sulfide in vivo and in vitro. Free Radic Biol Med 50: 1021-1031
- Jiang B, Tang G, Cao K, Wu L, Agarwal R (2010) Molecular mechanism for H2S induced activation of K (ATP) channels. Antioxid Redox Signal 12: 1167-1178.
- Zhao W, Wang R (2002) H(2)S-induced vasorelaxation and underlying cellular and molecular mechanisms. Am J Physiol Heart Circ Physiol 283: H474-480.
- Drobna M, Misiak A, Holland T, Kristek F, Grman M, et al. (2015) Captopril partially decreases the effect of H2S on rat blood pressure and inhibits H2S-induced nitric oxide release from S-nitrosoglutathione. Physiol Res 64: 479-486.
- Liang GH, Adebiyi A, Leo MD, McNally EM, Leffler CW, et al. (2011) Hydrogen sulfide dilates cerebral arterioles by activating smooth muscle cell plasma membrane KATP channels. Am J Physiol Heart Circ Physiol 300: H2088-2095.
- Tang G, Wu L, Liang W, Wang R (2005) Direct stimulation of KATP channels by exogenous and endogenous hydrogen sulfide in vascular smooth muscle cells. Mol Pharmacol 68: 1757-1764.
- Gross GJ, Fryer RM (1999) Sarcolemmal versus mitochondrial ATP-sensitive K+ channels and myocardial preconditioning. Circ Res 84: 973-979.
- Johansen D, Ytrehus K, Baxter G (2006) Exogenous hydrogen sulfide (H2S) protects against regional myocardial ischemia-reperfusion injury: Evidence for a role of KATP channels. Basic Res Cardiol 101: 53-60.
- Zhang Z, Huang H, Liu P, Tang C, Wang J (2007) Hydrogen sulfide contributes to cardioprotection during ischemia-reperfusion injury by opening KATP channels. Can J Physiol Pharmacol 85: 1248-1253.
- Elrod J, Calvert J, Morrison J, Doeller J, Kraus D, et al.(2007) Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. PNS of USA 104: 15560-15565.
- Hu L, Wong P, Moore P, Bian J (2007) Hydrogen sulfide attenuates lipopolysaccharide induced inflammation by inhibition of p38 mitogen-activated protein kinase in microglia. J Neurochem 100: 1121-1128.
- Kimura Y, Dargusch R, Schubert D, Kimura H (2006) Hydrogen sulfide protects HT22 neuronal cells from oxidative stress. Antioxid Redox Signal 8: 661-670
- Kimura Y, Kimura H (2004) Hydrogen sulfide protects neurons from oxidative stress. FASEB J 18: 1165-1167.
- Distrutti E, Sediari L, Mencarelli A, Renga B, Orlandi S, et al. (2006) Evidence that hydrogen sulfide exerts antinociceptive effects in the gastrointestinal tract by activating KATP channels. J Pharmacol Exp Ther 316: 325-335.
- Soares AC, Duarte ID (2001) Dibutyryl-cyclic GMP induces peripheral antinociception via activation of ATP-sensitive K(+) channels in the rat PGE2-induced hyperalgesic paw. Br J Pharmacol 134: 127-131.
- Cunha TM, Dal-Secco D, Verri WA Jr, Guerrero AT, Souza GR, et al. (2008) Dual role of hydrogen sulfide in mechanical inflammatory hypernociception. Eur J Pharmacol 590: 127-135.
- Sachs D, Cunha FQ, Ferreira SH (2004) Peripheral analgesic blockade of hypernociception: Activation of arginine/NO/cGMP/protein kinase G/ATP-sensitive K+ channel pathway. Proc Natl Acad Sci U S A 101: 3680-3685
- Andruski B, McCafferty DM, Ignacy T, Millen B, McDougall JJ (2008) Leukocyte trafficking and pain behavioral responses to a hydrogen sulfide donor in acute monoarthritis. Am J Physiol Regul Integr Comp Physiol 295: R814-820.
- Yao K (1975) Effects of several unusual sulfur-containing amino acids on rat liver cystathionine-gamma-lyase. Physiol Chem Phys7: 401-408.
- Yang W, Yang G, Jia X, Wu L, Wang R (2005) Activation of KATP channels by H2S in rat insulin-secreting cells and the underlying mechanisms. J Physiol 569: 519-531
- Mustafa AK, Sikka G, Gazi SK, Steppan J, Jung SM, et al. (2011) Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ Res 109: 1259-1268.
- Jackson-Weaver O, Osmond J, Riddle M, Naik J, Gonzalez Bosc L, Walker B, et al. (2013) Hydrogen sulfide dilates rat mesenteric arteries by activating endothelial large-conductance Ca2+-activated K+ channels and smooth muscle Ca2+ sparks. Am J Physiol Heart Circ Physiol 304: H1446-1454.
- Patel M, Shah G (2010) Possible role of hydrogen sulfide in insulin secretion and in development of insulin resistance. J Young Pharmacists 2: 148-151
- Cheng Y, Ndisang JF, Tang G, Cao K, Wang R (2004) Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am J Physiol Heart Circ Physiol 287: H2316-2323.
- Telezhkin V, Brazier SP, Cayzac S, Müller CT, Riccardi D, et al. (2009) Hydrogen sulfide inhibits human BK(Ca) channels. Adv Exp Med Biol 648: 65-72.
- Volpato G, Searles R, Yu B, Scherrer-Crosbie M, Bloch K, et al. (2008) Rapidly reversible inhibitor of cardiac and metabolic function in the mouse. Anesthesiology108: 659-68.
- Sitdikova G, Weiger T, Hermann A (2010) Hydrogen sulfide increases Ca2+-activated K+ (BK) channel activity of rat pituary tumor cells. Pflüger Arch 459: 389-397.
- Tang G, Wu L, Wang R (2010) Interaction of hydrogen sulfide with ion channels. Clin Exp Pharmacol Physiol 37: 753-763.
- Jackson-Weaver O, Paredes D, Gonzalez Bosc L, Walker B, Kanagy N (2011) Intermittent hypoxia in rats increases myogenic tone through loss of hydrogen sulfide activation of large-conductance Ca2+-activated potassium channels. Circ Res 108: 1439-1447.
- Lahousse S, Stopa E, Mulberg A, de la Monte S (2003) Reduced expression of the cystic fibrosis transmembrane conductance regulator gene in the hypothalamus of patients with Alzheimer’s disease. J Alzheimers Dis5: 455-462.
- Lee SW, Cheng Y, Moore PK, Bian JS (2007) Hydrogen sulphide regulates intracellular pH in vascular smooth muscle cells. Biochem Biophys Res Commun 358: 1142-1147.
- Bian J, Yong Q, Pan T, Feng Z, Ali M, et al.(2006) Role of hydrogen sulfide in the cardioprotection caused by ischemic preconditioning in the rat heart and cardiac myocytes. J Pharmacol Exp Therap 316: 670-678.
- Lefer DJ (2007) A new gaseous signaling molecule emerges: Cardioprotective role of hydrogen sulfide. Proc Natl Acad Sci U S A 104: 17907-17908.
- Ji Y, Pang QF, Xu G, Wang L, Wang JK, et al. (2008) Exogenous hydrogen sulfide post-conditioning protects isolated rat hearts against ischemia-reperfusion injury. Eur J Pharmacol 587: 1-7.
- Calvert J, Jha S, Gundewar S, Elrod J, Ramachandran A, et al.(2009) Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res 105: 365-374.
- Elsey DJ, Fowkes RC, Baxter GF (2010) Regulation of cardiovascular cell function by hydrogen sulfide (H2S). Cell Biochem Funct 28: 95-106.
- Yong Q, Cheong J, Hua F, Deng L, Khoo Y, et al.(2011) Regulation of heart function by endogenous gaseous mediators-crosstalk between nitric oxide and hydrogen sulfide. Antioxid Redox Signal 14: 2081-2091
- Mancardi D, Pla A, Moccia F, Tanzi F, Munaron L (2011) Old and new gasotransmitters in the cardiovascular system: Focus on the role of nitric oxide and hydrogen sulfide in endothelial cells and cardiomyocytes. Curr Pharm Biotechnol 12: 1406-1415.
- Sun Y, Cao Y, Wang W, Ma S, Yao T, et al.(2008) Hydrogen sulphide is an inhibitor of L-type calcium channels and mechanical contraction in rat cardiomyocytes. Cardiovasc Res 79: 632-641.
- Zhang R, Sun Y, Tsai H, Tang C, Jin H (2012) Hydrogen sulfide inhibits L-type calcium currents depending upon the protein sulfhydryl state in rat cardiomyocytes. PLoS One 7: e37073
- Xu M, Wu YM, Li Q, Wang FW, He RR (2007) Electrophysiological effects of hydrogen sulfide on guinea pig papillary muscles in vitro. Sheng Li Xue Bao 59: 215-220.
- Xu M, Wu YM, Li Q, Liu S, Li Q, et al. (2011) Electrophysiological effects of hydrogen sulfide on human atrial fibers. Chin Med J (Engl) 124: 3455-3459
- Lipscombe D, Helton TD, Xu W (2004) L-type calcium channels: the low down. J Neurophysiol 92: 2633-2641.
- Nagai Y, Tsugane M, Oka J, Kimura H (2004) Hydrogen sulfide induces calcium waves in astrocytes. FASEB J 18: 557-559.
- Hennig B, Diener M (2009) Actions of hydrogen sulphide on ion transport across rat distal colon. Br J Pharmacol 158: 1263-1275
- Perez-Reyes E (2003) Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev 83: 117-161.
- Major T, Dhamija S, Black N, Liachenko S, Morenko B, et al.(2008) The T- and L-type calcium channel blocker (CCB) mibefradil attenuates leg edema induced by the L-type CCB nifedipine in the spontaneously hypertensive rat: a novel differentiating assay. J Pharmacol Exp Ther 325: 723-731
- Maeda Y, Aoki Y, Sekiguchi F, Matsunami M, Takahashi T, et al.(2009) Hyperalgesia induced by spinal and peripheral hydrogen sulfide: Evidence for involvement of Cav3.2 T-type calcium channels. Pain 42: 127-132
- Kawabata A, Ishiki T, Nagasawa K, Yoshida S, Maeda Y, et al. (2007) Hydrogen sulfide as a novel nociceptive messenger. Pain 132: 74-81.
- Okubo K, Matsumura M, Kawaishi Y, Aoki Y, Matsunami M, et al.(2012) Hydrogen sulfide-induced mechanical hyperalgesia and allodynia require activation of both Ca(v) 3.2 and TRPA1 channels in mice. Br J Pharmacol 166: 1738-1743
- Fukushima O, Nishimura S, Matsunami M, Aoki Y, Nishikawa H, et al. (2010) Phosphorylation of ERK in the spinal dorsal horn following pancreatic pronociceptive stimuli with proteinase-activated receptor-2 agonists and hydrogen sulfide in rats: Evidence for involvement of distinct mechanisms. J Neurosci Res 88: 3198-3205.
- Takahashi T, Aoki Y, Okubo K, Maeda Y, Sekiguchi F, et al.(2010) Upregulation of Cav3.2 T-type calcium channels targeted by endogenous hydrogen sulfide contributes to maintenance of neuropathic pain. Pain 150: 183-191.
- Okubo K, Takahashi T, Sekiguchi F, Kanaoka D, Matsunami M, et al. (2011) Inhibition of T-type calcium channels and hydrogen sulfide-forming enzyme reverses paclitaxel-evoked neuropathic hyperalgesia in rats. Neuroscience 188: 148-156.
- Nagasawa K, Tarui T, Yoshida S, Sekiguchi F, Matsunami M, et al. (2009) Hydrogen sulfide evokes neurite outgrowth and expression of high-voltage-activated Ca2+ currents in NG108-15 cells: involvement of T-type Ca2+ channels. J Neurochem 108: 676-684.
- Joksovic P, Nelson M, Jevtovic-Todorovic V, Patel M, Perez-Reyes E, et al.(2006) CaV3.2 is the major molecular substrate for redox regulation of T-type Ca2+ channels in the rat and mouse thalamus. J Physiol 574: 415-430
- Yong QC, Choo CH, Tan BH, Low CM, Bian JS (2010) Effect of hydrogen sulfide on intracellular calcium homeostasis in neuronal cells. Neurochem Int 56: 508-515.
- Lee SW, Hu YS, Hu LF, Lu Q, Dawe GS, et al. (2006) Hydrogen sulphide regulates calcium homeostasis in microglial cells. Glia 54: 116-124.
- Kim D, Park D, Choi S, Lee S, Sun M, et al. (2003) Thalamic control of visceral nociception mediated by T-type Ca2+ channels. Science 302: 117-119.
- Matsunami M, Tarui T, Mitani K, Nagasawa K, Fukushima O, et al. (2009) Luminal hydrogen sulfide plays a pronociceptive role in mouse colon. Gut 58: 751-761.
- Nishimura S, Fukushima O, Ishikura H, Takahashi T, Matsunami M, et al. (2009) Hydrogen sulfide as a novel mediator for pancreatic pain in rodents. Gut 58: 762-770.
- Tang XQ, Yang CT, Chen J, Yin WL, Tian SW, et al. (2008) Effect of hydrogen sulphide on beta-amyloid-induced damage in PC12 cells. Clin Exp Pharmacol Physiol 35: 180-186.
- Nilius B, Owsianik G, Voets T, Peters JA (2007) Transient receptor potential cation channels in disease. Physiol Rev 87: 165-217.
- Szabó C, Papapetropoulos A (2011) Hydrogen sulphide and angiogenesis: Mechanisms and applications. Br J Pharmacol 164: 853-865.
- Bhatia M, Wong FL, Fu D, Lau HY, Moochhala SM, et al. (2005) Role of hydrogen sulfide in acute pancreatitis and associated lung injury. FASEB J 19: 623-625.
- Patacchini R, Santicioli P, Giuliani S, Maggi CA (2005) Pharmacological investigation of hydrogen sulfide (H2S) contractile activity in rat detrusor muscle. Eur J Pharmacol 509: 171-177.
- Streng T, Axelsson H, Hedlund P, Andersson D, Jordt S, et al. (2008) Distribution and function of the hydrogen sulfide-sensitive TRPA1 ion channel in rat urinary bladder. Eur Urol 53: 391-399
- Trevisani M, Patacchini R, Nicoletti P, Gatti R, Gazzieri D, et al. (2005) Hydrogen sulfide causes vanilloid receptor 1-mediated neurogenic inflammation in the airways. Br J Pharmacol 145: 1123-1131
- Schicho R, Krueger D, Zeller F, Von Weyhern CW, Frieling T, et al. (2006) Hydrogen sulfide is a novel prosecretory neuromodulator in the Guinea-pig and human colon. Gastroenterology 131: 1542-1552.
- Bhatia M, Zhi L, Zhang H, Ng SW, Moore PK (2006) Role of substance P in hydrogen sulfide-induced pulmonary inflammation in mice. Am J Physiol Lung Cell Mol Physiol 291: L896-904.
- Hutter M, Wick E, Day A, Maa J, Zerega E, et al.(2005) Transient receptor potential vanilloid (TRPV-1) promotes neurogenic inflammation in the pancreas via activation of the neurokinin-1 receptor (NK-1R). Pancreas 30: 260–5.
- Patacchini R, Santicioli P, Giuliani S, Maggi CA (2004) Hydrogen sulfide (H2S) stimulates capsaicin-sensitive primary afferent neurons in the rat urinary bladder. Br J Pharmacol 142: 31-34.
- Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, et al. (2007) Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 445: 541-545.
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, et al. (2003) ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112: 819-829.
- Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, et al. (2006) TRPA1 contributes to cold, mechanical and chemical nociception but is not essential for hair-cell transduction. Neuron 50: 277-289.
- Tracey WD Jr, Wilson RI, Laurent G, Benzer S (2003) Painless, a Drosophila gene essential for nociception. Cell 113: 261-273.
- Berglin EH, Carlsson J (1985) Potentiation by sulfide of hydrogen peroxide-induced killing of Escherichia coli. Infect Immun 49: 538-543.
- Polhemus D, Lefer D (2014) Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ Res 114: 730-737.
- Feng X, Chen Y, Zhao J, Tang C, Jiang Z, et al. (2009) Hydrogen sulfide from adipose tissue is a novel insulin resistance regulator. Biochem Biophys Res Commun 380: 153-159.
- Fang L, Zhao J, Chen Y, Ma T, Xu G, et al. (2009) Hydrogen sulfide derived from periadventitial adipose tissue is a vasodilator. J Hypertens 27: 2174-2185.
- Yu XH, Cui LB2, Wu K, Zheng XL, Cayabyab FS, et al. (2014) Hydrogen sulfide as a potent cardiovascular protective agent. Clin Chim Acta 437: 78-87.
- Meng G, Ma Y, Xie L, Ferro A, Ji Y (2015) Emerging role of hydrogen sulfide in hypertension and related cardiovascular diseases. Br J Pharmacol 172: 5501-5511
- Beltowski J, Jamroz-Wisniewska A, Tokarzewska D (2010) Hydrogen sulfde and its modulation in arterial hypertension and atherosclerosis. Cardiovasc Hematol Agents Med Chem 8: 173-186.
- Teague B, Asiedu S, Moore P (2002) The smooth muscle relaxant effect of hydrogen sulphide in vitro: Evidence for a physiological role to control intestinal contractility. Br J Pharm 137: 139-145
- Whiteman M, Li L, Kostetski I, Chu S, Siau J, et al. (2006) Evidence for the formation of a novel nitrosothiol from the gaseous mediators nitric oxide and hydrogen sulphide. Bioc and Biop Res Com 343: 303-310
- Predmore B, Julian D, Cardounel A (2011) Hydrogen sulfide increases nitric oxide production from endothelial cells by an Akt-dependent mechanism. Front Physiol 2: 104
- Ali MY, Ping CY, Mok YY, Ling L, Whiteman M, et al. (2006) Regulation of vascular nitric oxide in vitro and in vivo; a new role for endogenous hydrogen sulphide? Br J Pharmacol 149: 625-634.
- Cheang W, Wong W, Shen B, Lau C, Tian X, et al.(2010) 4-aminopyridine-sensitive K+ channels contribute to NaHS-induced membrane hyperpolarization and relaxation in the rat coronary artery. Vasc Pharmacol 53: 94-98.
- Schleifenbaum J, Köhn C, Voblova N, Dubrovska G, Zavarirskaya O, et al. (2010) Systemic peripheral artery relaxation by KCNQ channel openers and hydrogen sulfide. J Hypertens 28: 1875-1882.
- Bucci M, Papapetropoulos A, Vellecco V, Zhou Z, Pyriochou A,et al. (2010) Hydrogen sulfide is an endogenous inhibitor of phosphodiesterase activity. Arterioscler Thromb Vasc Biol 30: 1998-2004.
- Kiss L, Deitch EA, Szabó C (2008) Hydrogen sulfide decreases adenosine triphosphate levels in aortic rings and leads to vasorelaxation via metabolic inhibition. Life Sci 83: 589-594
- Leffler CW, Parfenova H, Basuroy S, Jaggar JH, Umstot ES, et al. (2011) Hydrogen sulfide and cerebral microvascular tone in new-born pigs. Am J Physiol Heart Circ Physiol 300: H440-447.
- Zhao W, Ndisang JF, Wang R (2003) Modulation of endogenous production of H2S in rat tissues. Can J Physiol Pharmacol 81: 848-853.
- Kubo S, Doe I, Kurokawa Y, Nishikawa H, Kawabata A (2007) Direct inhibition of endothelial nitric oxide synthase by hydrogen sulfide: Contribution to dual modulation of vascular tension. Toxicology 232: 138-146.
- Lim JJ, Liu YH, Khin ES, Bian JS (2008) Vasoconstrictive effect of hydrogen sulfide involves downregulation of cAMP in vascular smooth muscle cells. Am J Physiol Cell Physiol 295: C1261-1270.
- Liu YH, Bian JS (2010) Bicarbonate-dependent effect of hydrogen sulfide on vascular contractility in rat aortic rings. Am J Physiol Cell Physiol 299: C866-872.
- Li S, Ping NN, Cao L, Mi YN, Cao YX (2015) H2S induces vasoconstriction of rat cerebral arteries via cAMP/adenylyl cyclase pathway. Toxicol Appl Pharmacol 289: 389-396.
- Coletta C, Papapetropoulos A, Erdelyi K, Olah G, Módis K, et al.(2012) Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc Natl Acad Sci USA 109: 9161-9166.
- Ping NN, Li S, Mi YN, Cao L, Cao YX (2015) Hydrogen sulphide induces vasoconstriction of rat coronary artery via activation of Ca(2+) influx. Acta Physiol (Oxf) 214: 88-96.
- Olson KR, Dombkowski RA, Russell MJ, Doellman MM, Head SK, et al. (2006) Hydrogen sulfide as an oxygen sensor/transducer in vertebrate hypoxic vasoconstriction and hypoxic vasodilation. J Exp Biol 209: 4011-4023.
- Revsbech IG, Shen X, Chakravarti R, Jensen FB, Thiel B, et al. (2014) Hydrogen sulfide and nitric oxide metabolites in the blood of free-ranging brown bears and their potential roles in hibernation. Free Radic Biol Med 73: 349-357
- Hochachka P, Somero G (2002) Biochemical adaptation: Mechanism and process in physiological evolution. Oxford: Oxford Univ. Press.
- Ramirez JM, Folkow LP, Blix AS (2007) Hypoxia tolerance in mammals and birds: From the wilderness to the clinic. Annu Rev Physiol 69: 113-143.
- Toien O, Blake J, Edgar D, Grahn D, Heller H, et al. (2011) Hibernation in black bears: independence of metabolic suppression from body temperature. Science 331: 906-909.
- Cai WJ, Wang MJ, Moore PK, Jin HM, Yao T, et al. (2007) The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc Res 76: 29-40.
- Papapetropoulos A, Pyriochou A, Altaany Z, Yang G, Marazioti A, et al. (2009) Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Natl Acad Sci U S A 106: 21972-21977.
- Suzuki K, Olah G, Modis K, Coletta C, Kulp G, et al.(2011) Hydrogen sulfide replacement therapy protects the vascular endothelium in hyperglycemia by preserving mitochondrial function. Proc Natl Acad Sci U S A 108: 13829–34.
- Bauer C, Boyle J, Porter K, Peers C (2010) Modulation of Ca2+ signaling in human vascular endothelial cells by hydrogen sulfide. Atherosclerosis 209: 374-380.
- Yang G, Sun X, Wang R (2004) Hydrogen sulfide-induced apoptosis of human aorta smooth muscle cells via the activation of mitogen activated protein kinases and caspase-3. FASEB J 18: 1782-1784.
- Yang G, Pei Y, Teng H, Cao Q, Wang R (2011) Specificity protein-1 as a critical regulator of human cystathionine gamma-lyase in smooth muscle cells. J Biol Chem 286: 26450-26460
- Yang G, Yang W, Wu L, Wang R (2007) H2S, endoplasmic reticulum stress, and apoptosis of insulin-secreting beta cells. J Biol Chem 282: 16567-16576.
- Yang G, Wu L, Bryan S, Khaper N, Mani S, et al. (2010) Cystathionine gamma-lyase deficiency and over proliferation of smooth muscle cells. Cardiovasc Res 86: 487-495.
- Messerschmidt A, Worbs M, Steegborn C, Wahl MC, Huber R, et al. (2003) Determinants of enzymatic specificity in the Cys-Met-metabolism PLP-dependent enzymes family: Crystal structure of cystathionine gamma-lyase from yeast and intra-familiar structure comparison. Biol Chem 384: 373-386.
- Du J, Hui Y, Cheung Y, Bin G, Jiang H (2004) The possible role of hydrogen sulfide as a smooth muscle cell proliferation inhibitor in rat cultured cells. Heart Vessels19: 75-80.
- Yang G, Wu L, Wang R (2006) Pro-apoptotic effect of endogenous H2S on human aorta smooth muscle cells. FASEB J 20: 553-555.
- Deplancke B, Gaskins H (2003) Hydrogen sulfide induces serum-independent cell cycle entry in non-transformed rat intestinal epithelial cells. FASEB J 17: 1310–2.
- Baskar R, Sparatore A, Del Soldato P, Moore PK (2008) Effect of S-diclofenac, a novel hydrogen sulfide releasing derivative inhibits rat vascular smooth muscle cell proliferation. Eur J Pharmacol 594: 1-8.
- Wang R (2011) Signaling pathways for the vascular effects of hydrogen sulfide. Curr Opin Nephrol Hypertens 20: 107-112.
- Kai S, Tanaka T, Daijo H, Harada H, Kishimoto S, et al.(2012) Hydrogen sulfide inhibits hypoxia—but not anoxia-induced hypoxia-inducible factor 1 activation in a von hippel-lindauand mitochondria-dependent manner. Antioxid Redox Signal 16: 203-216.
- Wallace J, Dicay M, McKnight W, Martin G (2007) Hydrogen sulfide enhances ulcer healing in rats. FASEB J 21: 4070-4076.
- Wang M, Cai W, Li N, Ding Y, Chen Y (2010) The hydrogen sulfide donor NaHS promotes angiogenesis in a rat model of hind limb ischemia. Antioxid Redox Signal 12: 1065-1077.
- Qipshidze N, Metreveli N, Mishra P, Lominadze D, Tyagi S (2012) Hydrogen sulfide mitigates cardiac remodeling during myocardial infarction via improvement of angiogenesis. Int J Biol Sci 8: 430-441.
Citation: Melaku L, Mossie A (2017) Molecular Characterization of Hydrogen Sulfide Role in Vascular System and Method of Endogenous Production Detections with Common Ion Channels Used to Produce Its Biological Effect. Biochem Physiol 6: 222. DOI: 10.4172/2168-9652.1000222
Copyright: © 2017 Melaku L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 7566
- [From(publication date): 0-2017 - Dec 20, 2025]
- Breakdown by view type
- HTML page views: 6455
- PDF downloads: 1111