Research Article Open Access
Monitoring of Pollution Using Density, Biomass and Diversity Indices of Macrobenthos from Mangrove Ecosystem of Uran, Navi Mumbai, WestCoast of India
| Prabhakar R. Pawar* | |
| Veer Wajekar Arts, Science and Commerce College, Mahalan Vibhag, Phunde, MH, India | |
| Corresponding Author : | Prabhakar Pawar R Veer Wajekar Arts Science and Commerce College, Mahalan vibhag Phunde Tal. – Uran Dist. Raigad, Navi Mumbai-400 702 Maharashtra, India Tel: +91-9869616135 Fax: 912227221035 E-mail: prpawar1962@rediffmail.com prabhakar_pawar1962@yahoo.co.in |
| Received June 09, 2015; Accepted June 18, 2015; Published June 20, 2015 | |
| Citation: Pawar PR (2015) Monitoring of Pollution Using Density, Biomass and Diversity Indices of Macrobenthos from Mangrove Ecosystem of Uran, Navi Mumbai, West Coast of India. J Bioremed Biodeg 6:299. doi:10.4172/2155-6199.1000299 | |
| Copyright: © 2015 Pawar PR. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
In this study, density, biomass and diversity indices of selected macrobenthos were assessed from substations along Sheva creek and Dharamtar creek mangrove ecosystems of Uran [Raigad], Navi Mumbai, west coast of India from April 2009 to March 2011. A total of 86 species of macrobenthos representing 61 genera and 45 families were identified comprising of gastropods, pelecypods, cephalopods, polychaetes, sponges, crabs, prawns and shrimps. Higher values of density, biomass and diversity indices were recorded during pre-monsoon and post-monsoon than the monsoon. Diversity values in the study area ranged from 0.203 to 0.332 indicating heavy pollution and the macro benthic fauna is under stress due to discharge of domestic wastes and sewage, effluents from industries, oil tanking depots and also from maritime activities of Jawaharlal Nehru Port Trust [JNPT], hectic activities of Container Freight Stations [CFS], and other port wastes. This study reveals that macro benthic fauna from mangrove ecosystems of Uran is facing the threat due to anthropogenic stress.
| Keywords |
| Biomass; Community structure; Diversity indices; Jawaharlal Nehru Port; Pollution; Population density; Species composition; Uran |
| Introduction |
| Plants ecosystems are a habitat for a wide variety of species, some occurring in high densities and provide food and shelter for a large number of commercially valuable finfish and shellfishes [1,2]. Mangroves are one of the biologically diverse ecosystems in the world, rich in organic matter and nutrients and support very large biomass of flora and fauna [3]. |
| In India, 0.14% of the country’s total geographic area is under mangroves and it account for about 5% of world’s mangrove vegetation [4]. The Indian mangroves cover about 4827 Km2, with about 57% of them along the east coast, 23% along the west coast, and 20% in Andaman and Nicobar Islands [5]. Anthropogenic activities involving development projects have resulted in depletion of coastal resources, destruction of mangrove habitats, disruption of ecosystem processes, and loss of biodiversity [6]. |
| ¨Mumbai, a major metropolis and generates 0.85 million m3/d of liquid effluent and 14,600 t/d of solid waste, which without any treatment are discharged in the coastal region in and around Mumbai [7]. Estimates of area of mangroves in Mumbai varied from 248.7 Km2 [8] to 200 Km2 [9] to 92.94 Km2 [10] to 26.97 Km2 [11,12] reported that Mumbai has lost 40% of all its mangroves in the past decade because of overexploitation and unsustainable demand for housing, slums, sewage treatment, and garbage dumps. |
| Mangroves are inhabited by a variety of macrobenthic invertebrates, which have a profound effect on sediment structure and their biochemical processes by enhancing the porosity and water flow through the sediments [13]. Macrobenthic fauna have been studied more widely than others because of their high commercial value [14]. They play an important role in the cycling of matter and energy in mangrove ecosystems [15,16]. Benthic communities are highly affected by all the environmental parameters governing the distribution and diversity variation of the macrofaunal community in Pondicherry mangroves [17]. |
| Investigation of the population density and biomass of the organisms in creeks and estuaries is useful for the environmentalists to get enough information about the life span of important resource fauna [18]. It gives a lot of information about the inflow of the young ones, the fry, total biomass, maturity, spawning, breeding and fecundity of the organisms of that region [19]. Understanding the structure of the benthic faunal communities in relation to the impacts of pollution is an important part of monitoring changes in mangrove ecosystems in India [20]. Pearson and Rosenberg [21] have demonstrated that diversity indices provide important insights into faunal communities at different stages in succession. In biodiversity investigations, assessment of diversity indices is essential to determine the community structure of a particular ecosystem [22,23]. |
| The coastal environment of Uran [Navi Mumbai] has been under considerable stress since the onset of industries like Oil and Natural Gas Commission [ONGC], Liquid Petroleum Gas Distillation Plant, Grindwell Norton Ltd., Gas Turbine Power Station, Bharat Petroleum Corporation Limited Gas Bottling Plant, Jawaharlal Nehru Port [JNP, an international port], Nhava-Seva International Container Terminal [NSICT], Container Freight Stations [CFS], etc. These activities affect the quality of mangrove ecosystems [24]. Although many studies have been undertaken to evaluate the macrobenthos of mangrove ecosystems in India, no scientific studies have been carried out on community structure of macrobenthos from mangroves of Uran, Navi Mumbai; hence, the present study is undertaken. Objective of the present study is to evaluate the community structure of macrobenthos in relation to the impacts of pollution from mangrove ecosystems of Uran, Navi Mumbai with respect to population density, biomass and diversity indices. |
| Materials and Methods |
| Study area |
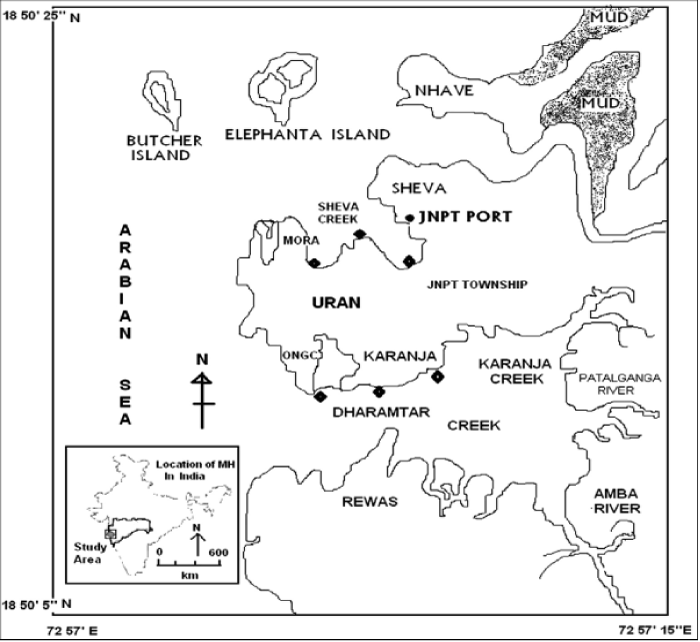
| Geographically, Uran [Lat. 180 50’ 5” to 180 50’ 20” N and Long. 720 57’ 5” to 720 57’ 15” E] with the population of 23,254 is located along the eastern shore of Mumbai harbor opposite to Coloba. Uran is bounded by Mumbai harbor to the northwest, Thane creek to the north, Dharamtar creek and Karanja creek to the south, and the Arabian Sea to the west. Uran is included in the planned metropolis of Navi Mumbai and its port, the Jawaharlal Nehru Port [JNPT] (Figure 1). |
| The mangrove ecosystem of Uran is a tide-dominated and the tides are semidiuranal. The average tide amplitude is 2.28 m. The flood period lasts for about 6-7 h and the ebb period lasts for about 5 hrs. The average annual precipitation is about 3884 mm of which about 80% is received during July to September. The temperature range is 12–36°C, whereas the relative humidity remains between 61% and 86% and is highest in the month of August. Four species of true mangroves representing three genera and three families were recorded during present study. The dominant species are Avicennia marina, Avicennia officinalis, Acanthus ilicifolius, and Ceriops tagal. The average tree height is 2.4 m and the canopy coverage is greater than 90%. |
| Sampling procedures |
| The present study was carried out for a period of two years, i.e., from April 2009 to March 2011. Two study sites, namely Sheva creek, site I [Lat. 18050’20” N and Long. 72057’50’’ E] and Dharamtar creek, site II [Lat.18050’50” N and Long. 72057’10” E] separated approximately by 10 km, were selected along the coast. At each site, three sampling stations separated approximately by 1 km were established for assessment of density, biomass and diversity indices of selected macrobenthos. |
| The selected sites were visited fortnightly at spring low tide from April 2009 to March 2011. The intertidal area was divided into 3 zones i.e. High water zone [HWZ], mid water zone [MWZ] and Low water zone [LWZ] following Bhatt [25] and Parulekar [26]. From selected sites, macrobenthos were collected and processed as per the recommendations of Holme and McIntyre [27]. Identification of macro benthos was done following the work of Hornell [28], Menon [29], Subrahmanyam [30-32], Chhapgar [33,34], Apte [35] and Khan and Murugesan [23]. |
| Population study of selected macro fauna |
| The abundantly recorded macrobenthic fauna from mangrove ecosystem is considered for population studies and their density, biomass and diversity Indices were assessed following standard methods [27,36,37]. |
| Population density: Number of selected macrobenthos present in one m2 area was considered for assessment of population density. The macro benthos collected from fixed transects of one m2 area, each from upper, middle and lower littoral zones were counted and average number of each species was recorded. Density of each species was expressed as average No/m2 [36]. |
| Biomass: Macrobenthos of different species collected from one m2 area were shelled and average wet weight was measured [38]. Biomass of each species was obtained by multiplication of average wet weight with average density and was expressed as g/m2. |
| Diversity indices: Following indices were calculated for the quantification of biodiversity and comparison of species diversity |
| Index of Frequency [F] OR Importance Probability [Pi] Smith and Smith [39] |
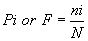 |
| Where, |
| ni=Number of individuals of each species in the community. |
| N=Total number of all individuals of all species in the community. |
| Index of dominance [c] Simpson, [40] |
 |
| Where, |
| ni=Number of individuals of each species in the community |
| N=Total number of all individuals of all species in the community |
| Rarity Index [R] Ludwig and Reynolds, [41] |
 |
| Where, F=Index of frequency |
| Shannon’s Index of General Diversity [Η'] Shannon and Weaver, [42] |
| Η'=- Σ Pi. Log e Pi |
| Where, Pi=Importance Probability |
| log e=ln [ log natural] OR |
| =log10 X 2.303 |
| Results |
| Species composition of macrobenthos |
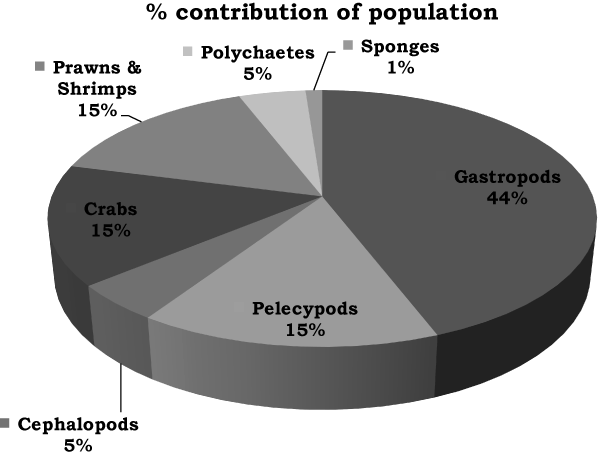
| A total of 86 species of macrobenthos representing 61 genera and 45 families were recorded from the mangroves of Uran coast. Varied diversity of macrobenthos belonging to gastropods, pelecypods, cephalopods, polychaetes, sponges, crabs, prawns and shrimps is recorded from both sites. Of the recorded species, 44.19% belonged to gastropods, 15.12% each to pelecypods, crabs and prawns and shrimps, 4.65% each to cephalopods and polychaetes and 1.16% to sponges (Figure 2). |
| Population studies of selected macrobenthos |
| Among the recorded macrobenthos, abundantly recorded species like Thais cariniferas, Perinereis cultrifera and Uca annulipes were selected for assessment of density, biomass and diversity Indices following standard methods [27,36,37]. |
| Population density |
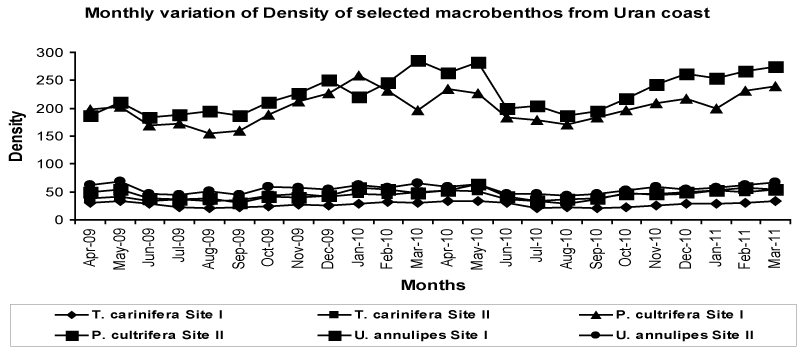
| Population density of macrobenthos in mangroves of Uran is high during pre-monsoon and post-monsoon than the monsoon (Figure 3 and Table 1). Maximum density of P. cultrifera in the range of 164 ± 8 to 222 ± 25 no/m2 was recorded at site I and 188 ± 5 to 270 ± 17 no/m2 at site II. Minimum density was noted for T. carinifera in the range of 24 ± 4 to 33 ± 2 at site I and 34 ± 4 to 50 ± 4 at site II. U. annulipes has moderate density in the range of 37 ± 4 to 55 ± 6 at site I and 46 ± 2 to 66 ± 3 at site II. |
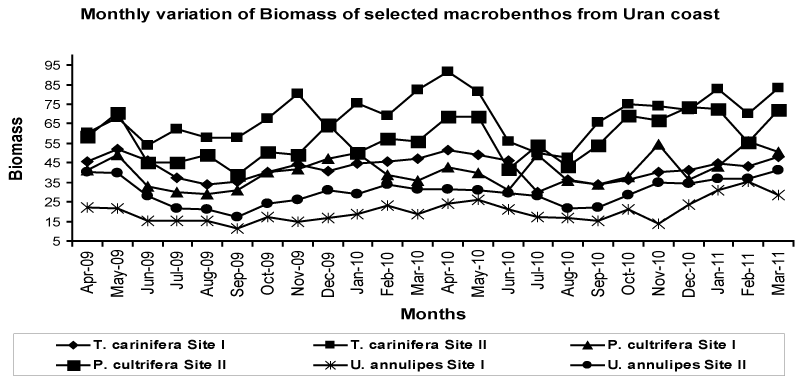
| Biomass |
| The biomass follows the trend of population density with lowest value in monsoon and highest values in pre-monsoon and postmonsoon (Figure 4 and Table 2). Biomass of T. carinifera was highest in the range of 36.60 ± 5.81 to 48.47 ± 2.24 g/m2 at site I and 54.90 ± 6.97 to 81.25 ± 8.08 g/m2 at site II. P. cultrifera shows moderate biomass in the range of 30.80 ± 1.48 to 49.20 ± 5.19 g/m2 at site I and 44.58 ± 3.74 to 70.39 ± 2.75 g/m2 at site II. Lowest biomass in the range of 14.33 ± 1.58 to 26.96 ± 5.63 g/m2 at site I and 22.13 ± 3.82 to 39.48 ± 1.66 g/ m2 at site II was recorded for U. annulipes. The degree of biomass can be put as T. carinifera > P. cultrifera > U. annulipes. |
| Diversity indices |
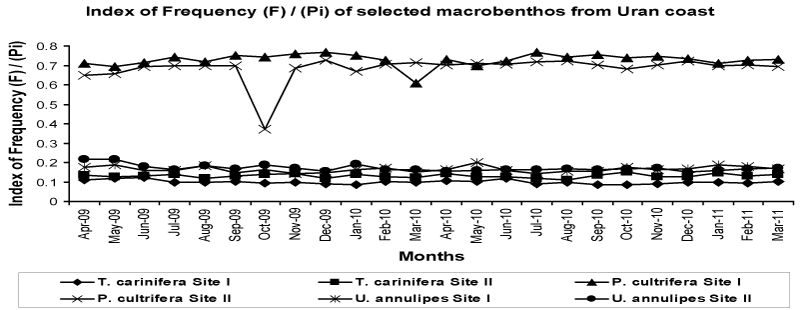
| The index of frequency [F] or Importance Probability [Pi] shows seasonal variation and the values were high in pre-monsoon and postmonsoon than the monsoon. Highest F was recorded for P. cultrifera during pre-monsoon [0.767 at Site I and 0.725 at Site II, Dec 2009] followed by U. annulipes during pre-monsoon [0.198 at Site I, May 2010 and 0.218 at Site II, Apr 2009]. The lowest values were recorded for T. carinifera [0.084 at Site I, Jan 2010 and 0.112 at Site II, Aug 2010]. The index of frequency can be placed in order of P. cultrifera > U. annulipes > T. carinifera (Figure 5). |
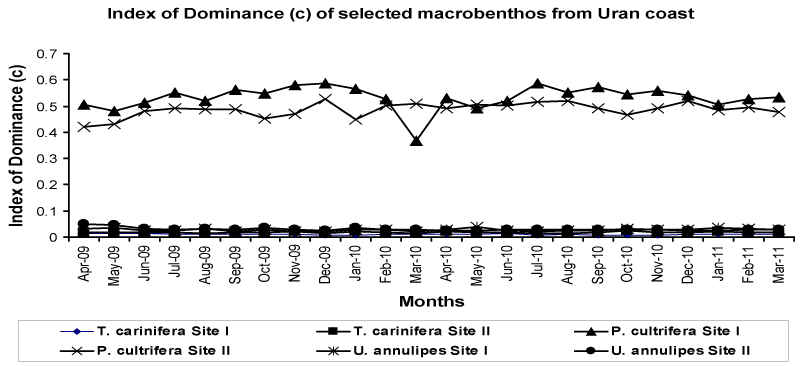
| The index of dominance was uniform at both sites where the dominance of the species can be placed in order as P. cultrifera > U.annulipes > T. carinifera. The data on index of dominance shows that the gastropod species T. carinifera is dominated over by other species (Figure 6). |
| The rarity index was according to the index of dominance. In this case, T. carinifera shows highest rarity index and found to be rare with respect to population density and biomass. Rarity index of P.cultrifera, found to be very common at both sites. Lower Rarity index of polychaetes marks the higher density of it. Among other species, U. annulipes found to be dominant over T. carinifera species assessed. The pattern of Rarity index was not significantly varied throughout the period of investigation, although, slight seasonal variation of Rarity index was recorded for all macro benthos (Figure 7). |
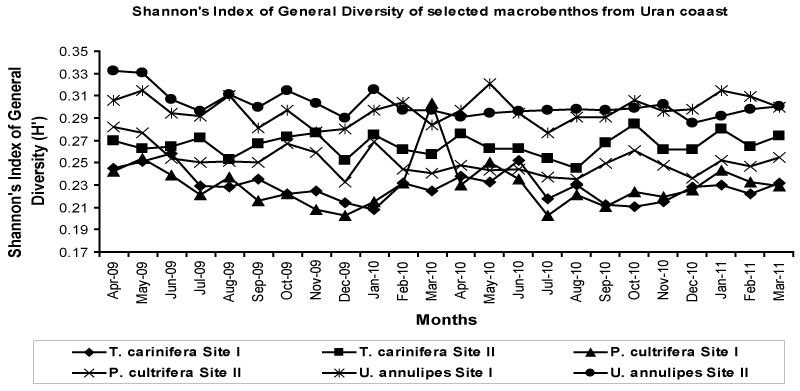
| The Shannon’s Index of General Diversity [Η'], was found to be uniform during the period of investigation. Maximum Η' was observed for U. annulipes, which was followed by T. carinifera and P. cultrifera. The Η' of all the species studied was uniform throughout the period of investigation. The data on Η' shows that species richness of P. cultrifera, U. annulipes and T. carinifera was more or less same. These results of Η' are in agreement with index of frequency, index of dominance and rarity index and did not varied significantly (Figure 8). |
| Discussion |
| Benthic macro fauna of the gastropods is dominant in Uran mangroves and is followed by pelecypods, crabs, prawns and shrimps,cephalopods, polychaetes and sponges. Similar results on species composition of macrobenthos in mangrove ecosystems were reported by Ngo-Massou e [42], Thilagavathi [1], Pravinkumar [13] and Kumar and Khan [17]. |
| Higher density of macrobenthos recorded during pre-monsoon and post-monsoon is attributed to higher total organic carbon coupled with a stable environment [1]. The results of the study are in agreement with Kurian [43], Saravanakumar [44] and Praveen kumar [13]. Results of dominance of gastropods, pelecypods, crustaceans and polychaetes in mangrove environment were also reported by Zhou [45] and Huang [46]. Low species diversity recorded during monsoon is attributed to the influx of freshwater, low temperature and lowered salinity. This is in conformity with Parulekar [26], Chandran [47], Devi [48] and Pravinkumar [13]. |
| Density and biomass of P. cultrifera and T. carinifera shows an inverse relationship. In comparison of P. cultrifera, low density of T. carinifera was observed throughout the investigation period. However, the biomass of T. carinifera was higher than that of P. cultrifera. A size variation of these organisms can be correlated to their inverse relationship in between density and biomass [18]. As compared to P. cultrifera, well-developed body organs and thick coverings of U. annulipes can be correlated to their differential density and biomass [49,50]. |
| Low biomass and high density of macrobenthos could be due to the recruitment process. Harkantra and Rodrigaes [51] have reported that salinity is the main significant factor influencing the species diversity, population density and biomass of macrobenthos in the estuarine system of Goa, west coast of India. Results of the study are in agreement with earlier reports of Kumar [52], Edgar and Barret [53], Liang [54], Tang and Yu [55] and Mahapatro [56]. |
| The diversity indices calculated for two sites are given in Figures 5 and 8. The salinity acts as a limiting factor in the distribution of living organisms, and its variation was due to dilution and evaporation [13]. Maximum values of diversity indices recorded during pre-monsoon and post-monsoon are in agreement with the earlier reports [57,58]. |
| The low species richness recorded during monsoon might be due to the low temperature, tidal fluctuation, freshwater runoff containing sewage and low salinity which in turn affected the distribution of benthos. Pawar and Kulkarni [59] have reported that the diversity indices can be used as a good measure for studying the effect of industrial pollution because industrial wastes and sewage almost always reduce the diversity of natural systems into which they are discharged. |
| Wilhm and Dorris [60] and Kumar and Khan [17] have stated that values less than 1.0 for diversity index [H] in estuarine waters indicate heavy pollution, values between 1.0 and 3.0 indicate moderate pollution, and values exceeding 3.0 indicate non-polluted water. Diversity values in the study area ranged from 0.203 to 0.321 at site I and 0.233 to 0.332 at site II. These values suggest that the mangrove ecosystem studied is heavily polluted and the macro benthic fauna is under stress due to anthropogenic factors |
| Conclusion |
| Present study shows that the mangrove ecosystem of Uran, Navi Mumbai have heavy pollution from the sewage, industrial wastes, effluents, maritime activities of Jawaharlal Nehru Port [JNPT], Container Freight Stations, and other port wastes. This deteriorates the mangrove ecosystem affecting the community structure of macrobenthos with respect to population density, biomass and diversity indices. The macro benthic fauna from mangrove ecosystems of Uran is facing the threat due to anthropogenic stress. Present information on community structure of macrobenthos from mangrove ecosystem of Uran, Navi Mumbai would be helpful as a baseline data for further monitoring of anthropogenic inputs on mangrove ecosystem of Uran. |
| Acknowledgements |
| I thank Principal, Veer Wajekar Arts, Science and Commerce College, Mahalan Vibhag, Phunde [Uran], Navi Mumbai-400 702 for providing necessary facilities for the present study. This work was supported by University Grants Commission, Western Regional Office, Pune [File No: 47-599/08 [WRO] dated 2nd Feb 2009]. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
 |
 |
 |
| Figure 6 | Figure 7 | Figure 8 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 15779
- [From(publication date):
July-2015 - Aug 19, 2025] - Breakdown by view type
- HTML page views : 11038
- PDF downloads : 4741
