Morphological and Physiological Adaptations of Coronopus didymus against Herbicides
Received: 26-Nov-2018 / Accepted Date: 06-Dec-2018 / Published Date: 13-Dec-2018
Abstract
Weeds are the notorious plants which cause considerable damage with devastating effects on the agricultural sector of agro based economy of Pakistan. The study under consideration is well direct at studying effects of weedicides on weed and they produce adaptation against them. One of the most detrimental weed in Pakistan is Coronopus didymus which is our topic of concern in this study. Different herbicides were sprayed post emergent to study the morphological and physiological adaptive components of this species. Based on the result from experimentations it was concluded that this plant showed less resistance against weedicides. Coropus didymus showed significant increase in physiological parameter i.e. photosynthetic pigmentation and mineral uptake against Sulphosulfuran and Novex, while Trump and Bromoxynil caused reduction in morphological parameter. Instead of separate application of specific herbicide a mixture of two or more two herbicides produces and fruitful result.
Keywords: Coronopus didymus; Herbicides; Weed; Morphology; Sulphosulfuran
Introduction
Coronopus didymus L. is a main element of the weed flora of family Brassicaceae which badly affects crop production in Pakistan.
Weeds are regarded as crucial pest complex affecting human being and generally unwanted, unappealing, or worrying plants, particularly those which grow where not needed. Weeds are locally profuse, economically less significant and occupy agitate habitats and are not original members of plant community. Weeds show three characteristics namely,
• Growth on the unwanted areas (anthropomorphic definition)
• They occur as colonizers in succession (ecological definition) and
• Invasive plants (bio-geographical definition) [1].
Weeds compete with cereal crops for obtaining water, light, nutrients, anchorage crop pest and diseases by adopting different methods to make themselves well able to cope with often changing and uncertain environmental conditions [2]. Weeds have the ability to adjust with, produce resistance and tolerance to or completely disappear in response to any environmental conditions that may try to reshape their life and normal growth [3].
Roughly 45 weed species of different kinds are documented in the fields of wheat crops in Pakistan [4]. Phalaris minor Retz., Rumex dentatus L., Coronopus didymus (L.)., Chenopodium album L., and Poaannua L. Sm., Medicago denticulata Willd., have been documented as the weeds happening with high frequency and in dense numbers in Pakistan [5].
Earlier studies have shown that competition of weeds and cereals for various environmental resources are largely owing to morphological and physiological features of plant species [6]. Significant features mainly effecting photo synthetically dynamic emission interception include size of seeds, inclination of leaves , height of plants, early growth vigor, rudimentary root and shoot growth rates along with tillering capacity [7].
Morphological changes which lead to differing survival strategies were studied long ago because they help the plants in adaption to a given environment in variable living habitats [8]. Phenotypic variability is a manifestation of the genetic rudiments of the individuals including their affiliation with the related environment. Therefore, morphological demonstrations usually have links to habitat conditions [9,10]. Hence, the plants populations occupy and are provided sustainability in often changing habitats through the demonstration of morphological parameters [11]. The variability of morphological characters is also connected with habitat conditions. Phenotypes having different plant modules demonstrate variations in environmental conditions. The population growth under comparable environment conditions manifests similar morphological parameters. In addition, the changes in different morphological traits in the field are produced by the environmental circumstances and give revolutionary outcomes which are helpful for the plants to acclimate to the environment [12,13].
The phenotypic change ability is resulted not only because of physiological and morphological characters but also by the interrelationship of specific development plans, the genotypes of a certain environment [14]. Ability of the weds to avoid herbicidal toxicity is gained by changes in growth habits, physical and/or physiological variations namely setting emergence time, morphological traits or by having some phonological adaptive sequences (such as germination patterns changes).These are caused by the inherited traits by means of which plants intend to thrive against various herbicidal applications [15].
Coronopus didymus was revealed to be the second highest detrimental weed, causing 75% reduction in wheat crop output and resulted in extreme losses in the growth of plant. Additionally, members of the same family also exhibit allelopathic interventions which resulted in the lack productivity and growth of species under consideration by giving out volatile allelochemicals which cause harm to the plants [16,17].
The current research will lead to the study of morpho-physiological adaptive components against herbicides and will be helpful in the discovery of some new deterrent measures against the modifications of weeds. This will lead to the improved yield of different crops and will support the economy of the Pakistan.
Materials and Methods
Pesticides are agrochemicals formulated to combat the attack of pests on agricultural crops. In modern agricultural practices, pesticides are widely used on crops for pre and post-harvest applications. In the present work post emergent spray of herbicides was done. Sulfosulfuran, Novex, Trump, Bromoxynil are the weedicides which were applied in different treatments namely T1, T2, T3 and T4 respectively and T0 was kept as control (i.e) without any herbicidal application. The post emergent spray of herbicide was done in five equal part of a selected field of wheat. After 15 days of spray data was collected from each field. Fresh samples were collected for morphological study. The plants were carefully uprooted and placed in paper bags. They were brought back to the laboratory for morphometric analysis including shoot length (cm), root length (cm), leaf area (cm2), number of total leaves, number of fresh leaves, number of dry leaves, fresh weight (g) and dry weight (g).
The photosynthetic pigments of Coronopus didymus i.e. Chlorophyll a, b, and carotenoids were measured with the Arnon’s method (1949). Fresh leaves weighing 0.2 g were selected and extracted overnight by using 80% acetone at the temperature 0°C-4°C. Centrifuging of the extract was done for 5 minutes at 10,0000 x g readings were taken at 645, 663 and 480 nm absorbance by using a spectrophotometer (Hitachi-220, Japan). The contents of chlorophyll a, b and carotenoids were calculated by using the following formulae:
Chl a (mg/g f.wt.)= (12.7(OD 663)-2.69(OD 645) × V/1000 × W)
Chl b (mg/g f.wt.)= (22.9(OD 645)-4.68(OD 663) × V/1000 × W)
Carotenoids (mg/g f.wt.)=Acar/ Em × 100
Where
V = volume of the sample
W= weight of fresh tissue
Acar=(OD 480)+ 0.114(OD 663) - 0.638(OD 645)
Em = 2500
Mineral analysis of Coronopus didymus was done by the use of nitric acid and hydrogen peroxide. The oven dried ground plant material (0.1 g) was digested with nitric acid and hydrogen peroxide for different mineral analysis (Ca2+, Mg2+, Na+, and K+, Mg2+, Fe3+, Zn2+, Pb2+, Cu+, Cr3+ by using the Wolf method (1982). The extraction volume was made up to 20 mL. The extract was filtration of extract was carried out and was used for the determination of Na, K, Ca, Mg, Fe, Zn, Pb, Ni, Cu and Cr ionic content. Different concentrations of minerals i.e. K+, Mg2+, Ca2+, Na+ were determined with flame photometer (Genwiey PFP7).
Emission intensity of sodium and potassium was recorded by using their respective filters in flame photometer. Amount of each of the mineral elements was noted from the respective standard curve. Mineral content was calculated as mg kg-1 of the sample. Micro minerals i.e. Cu, Mg, Fe, Zn, Pb and Cr were determined by Atomic Absorption Spectrophotometer (AAS) by using the standard solutions of the above minerals and their respective cathode lamp. Uptake of different minerals was calculated by using following formula
Micro mineral (mg/L)=ppm from graph x dilution factor/Weight of Sample.
Statistical analysis
The morphological and physiological parameters are analyzed and interpreted by different statistical techniques i.e. one way and two way anova by SPSS software and Minitab along with Microsoft excel toolbox.
Results and Discussion
Plants showed a variable behavior in response to different herbicides. Coronopus didymus showed significant adaptive components in different morphological and physiological parameters as compared to that recorded at control level.
Photosynthetic pigments adaptations of Coronopus didymus against herbicides
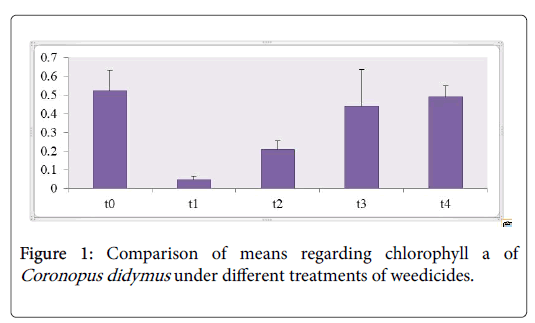
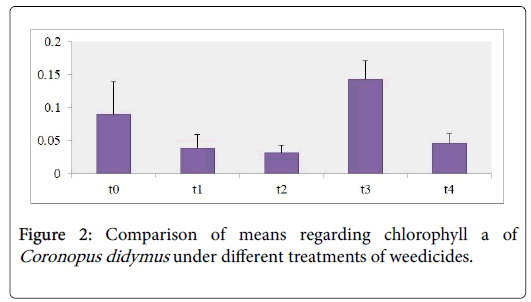
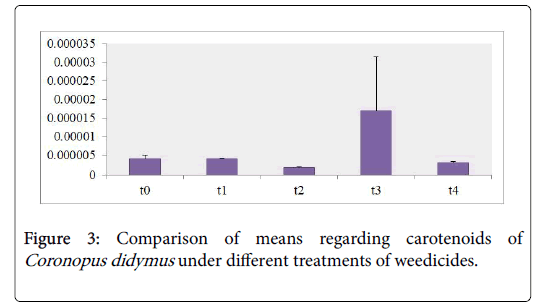
Photosynthetic pigments analysis revealed that nearly the content of Chl a,b and carotenoids of Coronopus didymus from all herbicidal treatments were decreased significantly as shown in the Table 1. The maximum modifications were observed in Chl b 43% and carotenoids 200% increase against the treatment of Trump in T3 while sulfosulfuranin T1 caused a decrease in the content of Chl a,b and carotenoids 91%, 62% and 20% as compared to T0 respectively as shown in the Figures 1-3.
| Photosynthetic pigments | Chlorophyll a | Chlorophyll b | Carotenoids |
|---|---|---|---|
| F value | 3.850* | 2.803* | 0.958 NS |
| NS=Non-significant (P>0.05); * = Significant (P<0.05); ** = highly significant (P<0.01) | |||
Table 1: Analysis of variance of the data regarding photosynthetic pigments content of Coronopus didymus.
Chl a content as shown in Figure 1 depicted the decrease in all treatments including T1 showed decrease in 91% T2 21%, T3 60%, T4 16%. Chl b content as shown in Figure 2 against the herbicidal treatment revealed a decrease in all treatments including T1 62%, T2 69% , T3 55% except in T4 showed an increase in 43%. Carotenoids content of Coronopus didymus as shown in Figure 3 decreased significantly in all treatments including T1 10%, T2 30% , T4 25% except in T3 showed an increase in 200%.
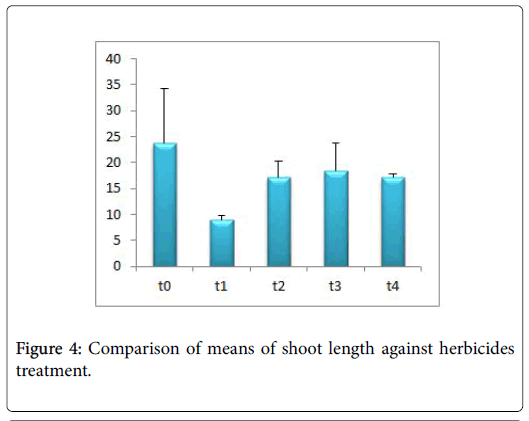
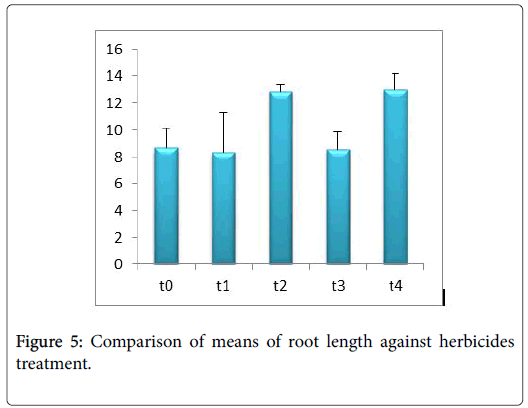
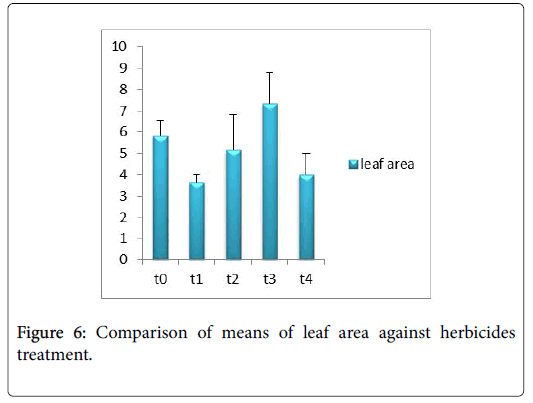
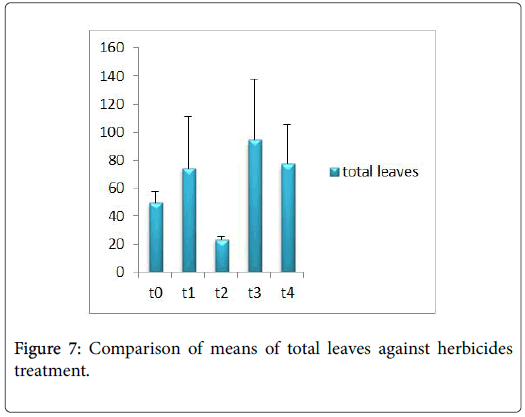
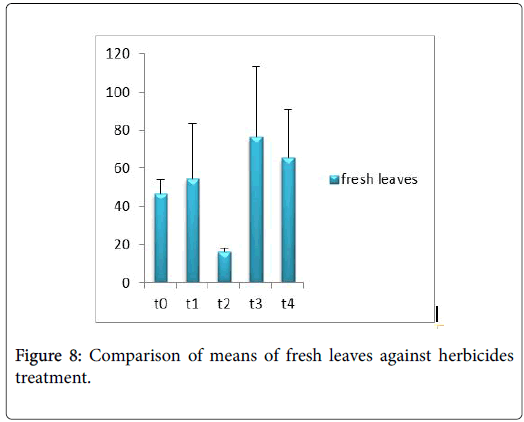
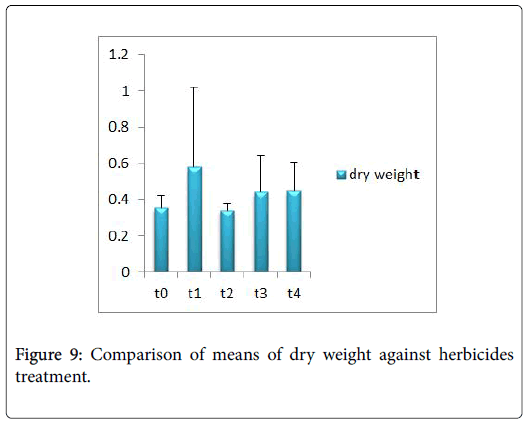
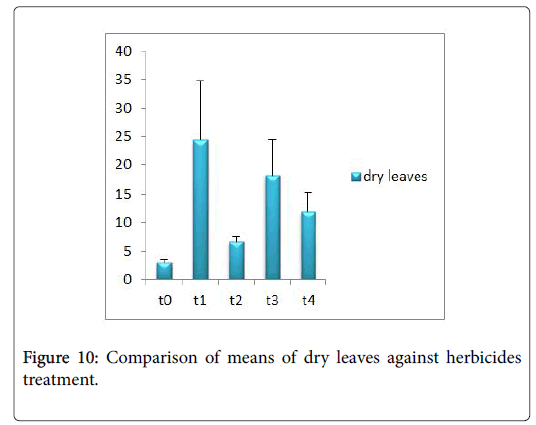
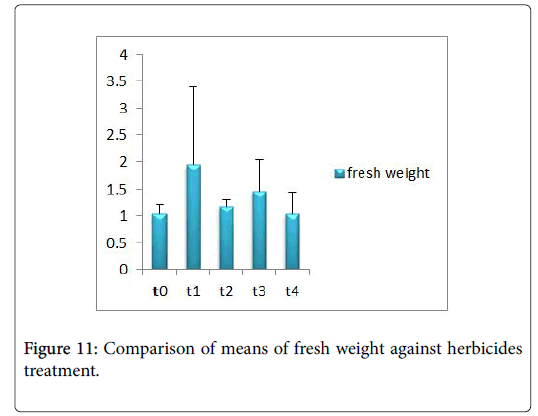
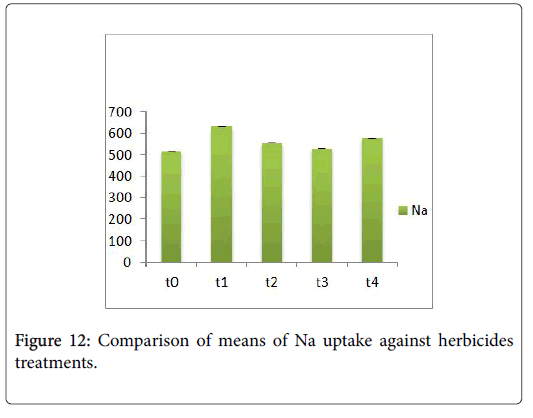
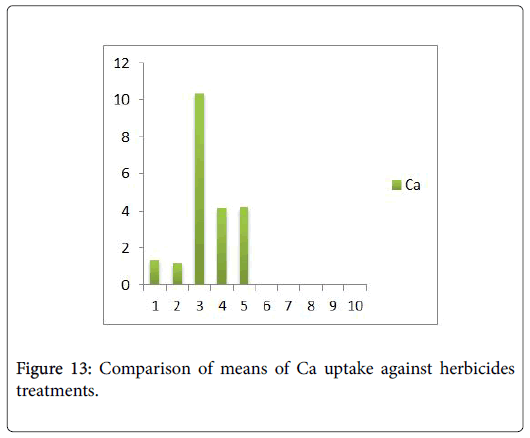
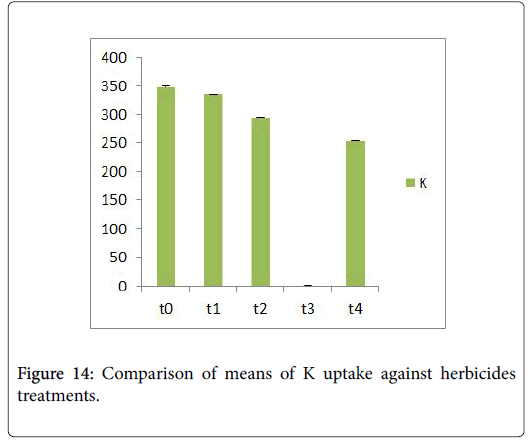
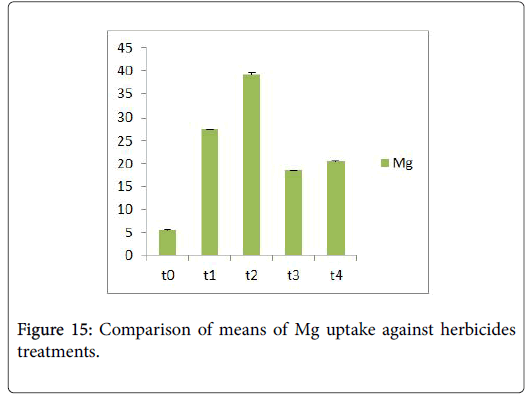
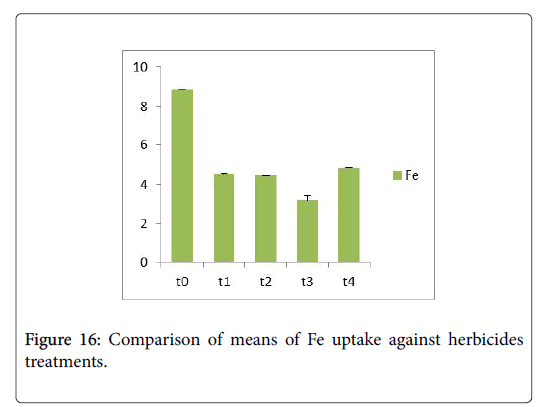
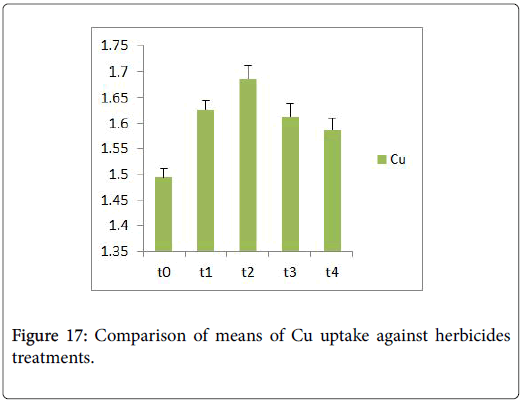
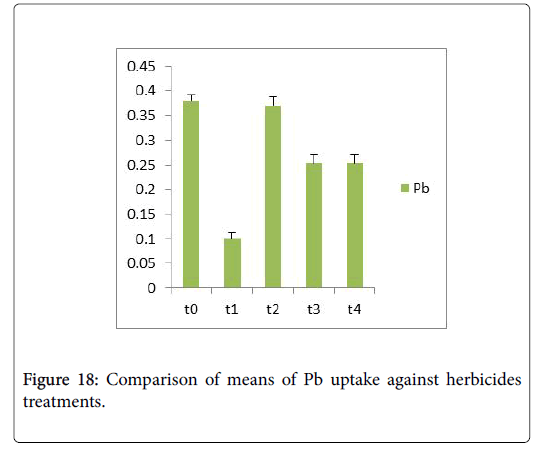
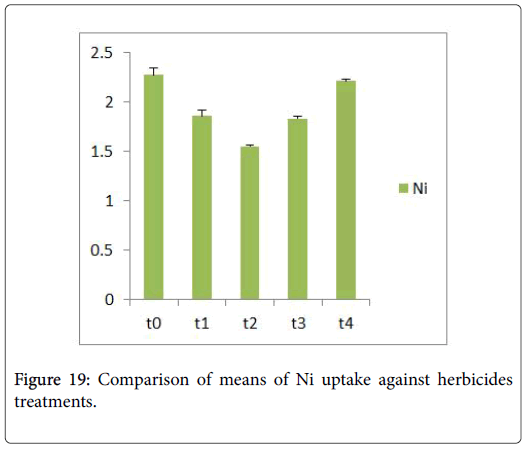
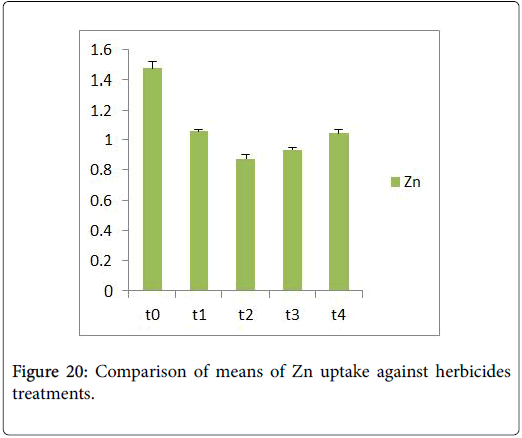
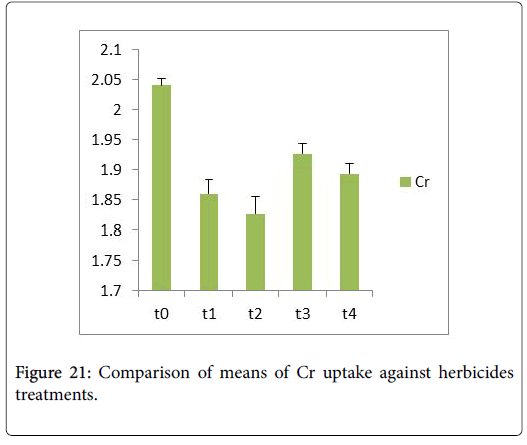
Morphological parameters of Coronopus didymus altered non significantly except the number of dry leaves increased significantly as shown in Table 2. All the features including shoot length, root length, fresh leaves, dry leaves, fresh and dry weight showed an increase against herbicidal treatments as in Figures 4-10, except leaf area which is reduced 36% in T1, 10% in T2 and 31% in T4 as in Figure 11. Mineral analysis of Coronopus didymus reflects that uptake of different minerals altered significantly as shown in Table 3. Minerals including Na, Ca, K, Mg, Cu and Cr enhanced significantly against all the treatments as in Figures 12-15. While reduction occurred in the uptake of Fe, Zn, Pb and Ni as indicated in Figures 16-19. Further, in Figures 20 and 21 minerals such as Na, Ca, K, Mg, Cu and Cr are increased significantly against all the treatments.
| Morphological Parameter | F Value |
|---|---|
| Root length (cm) | 2.047NS |
| Shoot length (cm) | 0.917NS |
| Leaf area (cm) | 1.674NS |
| Total leaves No. | 0.968NS |
| Fresh leaves No. | 0.890NS |
| Dry leaves No. | 2.392* |
| Fresh weight (g) | 0.283NS |
| Dry weight (g) | 0.180NS |
| NS = Non-significant (P>0.05); * = Significant (P<0.05); ** = Highly significant (P<0.01). | |
Table 2: Analysis of variance of the data regarding morphological adaptive components of Coronopus didymus.
| Physiological parameter Mineral uptake (mg/l) |
F value |
|---|---|
| Na | 604663.828** |
| K | 10996.570** |
| Ca | 61389.515** |
| Mg | 3732.746** |
| Fe | 408.436** |
| Zn | 65.947** |
| Pb | 62.352** |
| Ni | 46.737** |
| Cu | 10.585* |
| Cr | 15.760** |
| NS = Non-significant (P>0.05); *= Significant (P<0.05); **= Highly significant (P<0.01). | |
Table 3: Analysis of variance of the data regarding minerals uptake of Coronopus didymus .
Sulfosulfuran also caused significant alteration in Coronopus didymus plant morphology i.e. it increased root length, no. of leaves, fresh leaves, dry leaves, fresh weight and dry weight of plant except leaf area which is reduced by the action of herbicide. Plot 2 was sprayed with weedicides Novex. Novex significantly increases no. of dry leaves and fresh and dry weight of Coronopus didymus while decreases shoot root length, leaf area and fresh leaves. Chlorophyll and carotenoids contents also decrease after application of weedicides. Mineral uptake of Na, K, Ca, Mg, Cu and Cr increases. Trump causes a uniform increase in Coronopus didymus according to its mode of action. There was gradual increase observed in both the root length and dry leaves of plant before and after the application of weedicides. The reason for this increase in root length is reported by Doughty [18] that the rate of growth is highly increased after the application of weedicides.
Trump causes significant adaptations in Coronopus didymus according to its mode of action. There was gradual increase observed in leaf area, dry leaves, fresh weight and dry weight of plant before and after the application of weedicide. Bromoxynil causes significant increase in plant morphology of Coronopus didymus i.e. increase in root length, shoot length, no. of fresh leaves as well as fresh and dry weight and an increase in dry leaves. Chlorophyll increases while chlorophyll b reduces carotenoid contents decreases when Bromoxynil was sprayed. The uptake of Na, K, Ca, Mg, Cu and Cr is enhanced. Iron content was lower in grass species than in broadleaf [19,20].
References
- Rejmanek M, Richardson DM, Higgins SI, Pitcairn MJ, Grotkopp E (2005) Ecology of invasive plants: State of the art. Invasive alien species: A new synthesis 104–161
- Rodenburg J, Riches CR, Kayeke JM (2010) Addressing current and future problems of parasitic weeds in rice. Crop Prot 29: 210-221.
- Hager AG, Refsell D (2008) Weed resistance to herbicides. In: 2008 Illinois Agricultural Pest Management Handbook. Department of Crop Science, University of Illinois.
- Qureshi R, Bhatti GR (2001) Determination of weed communities in wheat field of district Sukhur. Pak J Bot 33: 109-115.
- Siddiqui I, Bajwa R (2001)Variation in weed composition in wheat fields of Lahore and Gujranwala divisions. Pak J Biol Sci 4: 492-504.
- Didon UE (2002) Variation between barley cultivars in early response to weed competition. J Agron Crop Sci 188: 176-184.
- O'Donovan JT, Harker KN, Clayton GW, Hall LM (2000) Wild oat (Avenafatua) interference in barley (Hordeumvulgare) is influenced by barley variety and seeding rate. Weed Technol 14: 624-629.
- Gambino P, Vilela A (2011) Morphological traits and allocation patterns related to stress-tolerance and seed-yield in wild and domesticated evening primrose; Oenotherabiennis L. (Onagraceae). Ind Crops Prod 34: 1269-1276.
- Jaleel CA, Manivannan P, Lakshmanan GA, Gomathinayagam M, Panneerselvam R (2008) Alterations in morphological parameters and photo synthetic responses of Catharanthusroseus in soil which is water deficits. Colloids Surf B Biointerfaces 61: 298-303.
- Mandak B, Zakravsky P, Dostál P, Placková I (2011) Population genetic structure of the noxious weed Amaranthus retroflexusin Central Europe. Funct Ecol Plants 206: 697-703.
- Urbas P, Zobel K (2000) Adaptive and inevitable morphological plasticity of three herbaceous species in a multi-species community: Field experiment with manipulated nutrients and light. Acta Oecol 21: 139-147.
- Mahmood S, Abbas A (2003) Local population differtiation in Trifoliumalexandrium L.in response to various disturbance regimes. J Bio Sci 9: 773-781.
- Hussain A, Mahmood S (2004) Response flexibility in TrifoliumalexendrinumL. of phenomenon of adaptation to spatial and temporal disturbed habitat. J Biol Sci 4: 380-385.
- Bellavance ME, Brisson (2010) Spatial dynamics and morphological plasticity of common reed and cattails (Typha) in freshwater marshes and roadside ditches. Aquat Bot 93: 129-134.
- Chen LJ, Lee DS, Song ZP, Shu HS, Lu BR (2004) Gene flow from cultivated rice (Oryza sativa) to its weedy and wild relatives. Ann Bot 93: 67-73.
- Waddington J (1978) Growth of brome grass, barley, and alfalfa under greenhouse in soil having rape seed and wheat residues. Can J Plant Sci 58: 241-248.
- Oleszek W (1987) Allelopathic influences of volatiles of some Cruciferae species on barnyard grass, lettuce, and wheat growth. Plant Soil 102: 271-273.
- Doughty KJ, Porter AJR, Morton AM, Kiddle G, Bock CH, et al. (1991) Variation in the glucosinolates content of oilseed rape leaves. Ann Appl Biol 118: 469-477.
- Owen MK, IA Zelaya (2005) Herbicide-resistant crops and weed resistance to herbicides. Pest Manag Sci 61: 301-311.
- Siddiqui I (2010) Effect of six problematic weeds on Growth and yeild of wheat. Pak J Bot 42: 2461-2471.
Citation: Khan ZI, Aziz A, Ahmad K, Ashfaq A, Bashir H, et al. (2018) Morphological and Physiological Adaptations of Coronopus didymus against Herbicides. J Ecol Toxicol 2: 116.
Copyright: © 2018 Khan ZI, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 4676
- [From(publication date): 0-2018 - Dec 20, 2025]
- Breakdown by view type
- HTML page views: 3698
- PDF downloads: 978