Research Article Open Access
Mycotoxicological Concerns with Sorghum, Millet and Sesame in Northern Nigeria
Apeh Daniel Ojochenemi1,2*, Ochai Daniel Ochai1, Adejumo Aderemi1, Muhammad Hadiza Lami1, Saidu Abubakar Ndaman1, Atehnkeng Joseph3, Adeyemi Rinde Henry1, Mailafiya Simeon Chidawa1 and Makun Hussaini Anthony1
1Department of Biochemistry, Federal University of Technology, Minna, Nigeria
2Department of Biosciences, Salem University, Lokoja, Nigeria
3International Institute of Tropical Agriculture (IITA), Ibadan, Nigeria
- *Corresponding Author:
- Apeh Daniel Ojochenemi
Department of Biochemistry
Federal University of Technology, Minna, Nigeria
Tel: +2349058233573
E-mail: danapeh@salemuniversity.edu.ng (or) danapeh@gmail.com
Received date: September 01, 2016; Accepted date: September 15, 2016; Published date: September 20, 2016
Citation: Apen DO, Ochai DO, Adejumo A, Muhammad HL, Saidu AN, et al. (2016) Mycotoxicological Concerns with Sorghum, Millet and Sesame in Northern Nigeria. J Anal Bioanal Tech 7:336. doi:10.4172/2155-9872.1000336
Copyright: © 2016 Apen DO, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Incidence of fungi and aflatoxin in sorghum, millet, sesame and their products in Northern Nigeria was investigated in 146 food samples including; sorghum and traditional beer (50), millet and millet dough (50), and sesame seed (50). Members of the Aspergillus, Fusarium, Pennicilium, Macrophomena, Cercospora, Phoma, Rhizopus, Alternaria and Curvularia species in order of predominance were identified. Aflatoxin analysis showed 28.6% sorghum (0.96-21.74 μg/Kg), 80% burukutu (1.27-8.82 μg/Kg), 20% pito (0.69-2.00 μg/Kg), 29% millet grain (1.05-14.96 μg/Kg), 26.3% millet dough (0.81-3.78 μg/Kg) and 21.7% sesame (0.79-60.05 μg/Kg) samples were unsafe for consumption. Fungi and aflatoxin levels were higher in sesame than millet and sorghum. Fungal load in sesame seeds increased with latitude, aflatoxin levels in millet and sorghum varied with temperature and relative humidity. Beer processing reduced the levels of aflatoxin from sorghum grain to beer, establishing a 47% and 25% carryover respectively. Higher tannin levels in the samples correlated with lower fungal loads however, Aspergillus niger, Fusarium and Pennicilium showed resistance to tannin. Legislative, regulatory and stakeholder involvement is key in the continuous effort to reduce the mycotoxin menace.
Keywords
Aflatoxin; Sesame; Sorghum; Millet
Introduction
The fungi family have continuously raised global food safety concerns. The concern is due to their ability to colonize food items and either cause physical damage or release secondary metabolites which may be toxic; the fungi genera can produce over three hundred toxic secondary metabolites known as “mycotoxins” which have detrimental biological and economic impacts. The most regulated fungi food toxin worldwide is aflatoxin. Aflatoxin is the most toxic of all known fungi toxins and is a Group 1 Carcinogen [1] it is majorly produced by the Aspergillus flavus and Aspergillus parasiticus under certain climatic conditions. Maximum permissible limit (MPLs) are been used globally in regulating levels of aflatoxin that will be allowed in food, feed and food products, a level above which the item is unacceptable. For example, the European Union (EU) MPLs for aflatoxin in ready to eat cereal and oil seed was set at 2 μg/Kg for aflatoxinb 1 and 4 μg/ Kg for total aflatoxins, while the United States set 20 μg/Kg for total aflatoxin in food. Nigeria adopted the EU MPL yet there has been no serious legislation along the food value chain, from farm to table. With increasing availability of incidence reports mostly by independent researchers, regulatory and legislative concerns should increase in Nigeria.
Documentation of mycotoxin related human health problems in Nigeria include; the death of some children who consumed mouldy groundnut cake [2], presence of aflatoxins in the urine of liver disease patients in Zaria, in blood in Southern Nigeria, in organs of children who died of kwashiorkor in Western Nigeria, and in human semen, breast milk and in the blood of umbilical cord of babies [3-5]. Acute aflatoxin toxicity results in death, while chronically it results in immune suppression, mutagenicity, teratogenicity and carcinogenesis. Hsieh [6], and strengthens hepatitis B infection (JECFA) [7]. Economically, indirect cost elements include mortality, morbidity, and the intangible cost of pain, suffering, anxiety, and reduction in the quality of life [8] while direct cost result from reject of exported food items. Africa loses an estimate of sixty-seven ($67) million dollars annually from export rejects due to high levels of mycotoxins in food and agricultural produce. Member states of the African Groundnut Council (Gambia, Mali, Niger, Nigeria, Senegal, and Sudan) have estimated the yearly cost of implementing a program to reduce mycotoxin contamination at US$7.5 million. Impact on livestock production includes mortality as well as reductions in productivity, weight gain, feed utilization, fertility, ability to resist diseases and decrease in the quantity and quality of meat, milk and egg production.
Statistics reveal that of the 6.7 million MT of sorghum produced in Nigeria 79.4% is used locally as food [9] in form of paste (tuwo), pap (akamu) and beverages (kunu, burukutu and pito). FAO statistics also reveal that of the 5 million MT of millet produced in Nigeria 85% is source of food supply; no export is recorded [9], millet is usually consumed as paste, pap, beverage, and dough (fura). Sesame on the other hand has local use of upto 86.2% of the 994, 800T produced [9]. Sesame in consumed as soup, across Nigeria and as cake (ridi) in the Northern part. Since bulk of these food items are consumed locally, a call is placed on public health concerns. This research is an enquiry into the incidence of fungi and levels of aflatoxin in sorghum, millet and sesame in Northern Nigeria and carryover to their products.
Methodology
Sampling location, sample collection and preparation
Sampling was according to Commission Regulation (EU) No 178/2010 of 2 March 2010; Ten incremental samples making up an aggregate from the same lot of foods were collected from every (n=4) 4th trader in the market. After mixing, 200 g sub samples were taken and divided into two groups. The first was used for fungi assay and the second for aflatoxins determination.
Thirty five (35) sorghum, 15 sorghum based traditional beer (200 ml each), 30 millet, 20 millet dough (fura) and 46 sesame samples were collected from several towns in Northern Nigeria. Sorghum and its product were collected within Minna and Bida, Millet was collected within 3 microclimatic zones in 12 Local Government Area of Niger state while Sesame was collected from three agro-ecological zones (3 states) namely; Jigawa (Sudan Savannah), Nassarawa (Derived savannah) and Niger (Sahel savannah). To observe carryover of aflatoxins, Sorghum intended for beer production was collected and 200 ml of its beer (burukutu and pito; they are both fermented products however, pito undergoes further fermentation) was collected post production, millet and millet dough were sampled independently. Each grain (100 g) was blended for 30 seconds using a high speed blender (Waring Commercial, Springfield, MO, USA). Samples were packaged into transparent nylon bags, labeled accordingly and stored for subsequent use.
Fungi assay
Media preparation and fungi isolation: The method described by Cotty was adopted. A Modified Rose Bengal Media also known as Clean up media (CU) was used. One gram (1 g) of the blended sample was weighed into 10 mls sterile-distilled water (for the liquid samples 1 ml was measured into 9 ml sterile-distilled water) and mixed with a vortex mixer. Samples were plated and spread in three dilutions (50 μl, 100 μl and 200 μl) on CU media to allow collection of isolates from plates with fewer than 15 colonies. Plates were incubated for 72 hours at 31°C. After 72 hours, the various fungi colonies were counted with the aid of a magnifying lens and a colony counter. Aspergillus colonies that counted between 1-15 were transferred into a 5/2 media containing 50 mL/L 5% V-8 juice, 2% Bacto (technical) agar set at pH 5.0-5.2, all other fungi were transferred into a full strength PDA Media containing lactic acid.
Fungi identification: Isolates were classified on the basis of conidial morphology and colony characteristics. Isolates with abundant small sclerotia (average diameter <400 mm) were classified as strain S. Isolates with smooth conidia and large sclerotia (average diameter over 400 mm) were classified as the L strain of A. flavus. A. tamarii was identified by colony and spore morphology. Microscopic examination coupled with atlas guide was used to determine other fungi genera and species. Quantities of fungi were calculated as colony-forming units (CFU) per gram.
Tannin analysis: The level of tannin was determined by UV spectrophotometer according to Krishnaiah.
Aflatoxin determination: As earlier described by Atehnkeng et al. [10], 20 g of powdered sample (20 ml for beer sample) was extracted with 10 ml 70% methanol (ratio of 1:5) using a high speed blender (Waring Commercial, Springfield, MO, USA) for 3 min, and a Lab-line orbit shaker set at 400 rpm for 30 minutes. Filtration followed for 20 min through a Whatman No. 1 (185 mm) filter paper. The filtrate was then poured into a separating funnel and 20 mls of deionized water plus 25 mls of dichloromethane (DCM) was also added. This was allowed to stand for separation. On separation, DCM (lower) layer was dispensed into a polypropylene cup set with a filter paper on which 40 g of anhydrous sodium sulphate (Na2SO4) has been added. This whole process was repeated twice with the addition of 10 mls DCM. The DCM portion was kept in a fume hood for 24 hours to evaporate leaving its solute in the polypropylene cup. After which 1 ml DCM was used to dissolve the extract into a 1 ml eppendorf tube for analysis. Aflatoxins were quantified using scanning densitometer, CAMAG TLC Scanner 3 with winCATS 1.4.2 software (Camag AG, Muttenz, Switzerland). The minimum detection limit of the scanner was 1.0 ng/g. Based on spiked recovery controls using 5, 10, 15, 20, and 25 ng/g levels, over 85% of the aflatoxin present was recovered by this method.
Discussion
Fungal contamination
Fungal species belonging to the Aspergillus, Pennicilium and Fusarium genera have been shown to be the most common mycotoxigenic fungi involved with the human food chain [11], These fungi are prevalent in grains, nuts, seeds, fruits, tubers and grainbased products [12,13]. The occurrence of these genera of fungi is common in agricultural products in Nigeria [14-16]. Fusarium spp grow optimally at temperatures 25-30°C and humidity below 90%. Pennicilium spp grows optimally when a relative humidity is above 60%, and at temperature of 25°C [17]. A. flavus has a minimum growth temperature of 12°C, a maximum growth temperature of 48°C and an optimum growth temperature of 37°C, with rapid growth between 30- 55°C [18]. On the basis of moisture content, A. flavus growth occurs at 13-13.2% for starchy substrate [18]. DS lies within latitudes 6°8′ and 9°30′ N and longitudes 2°40′ and 12°15′ E and has a bimodal rainfall distribution averaging between from 1300 mm to 1500 mm annually, and maximum temperatures varying from 25 to 35°C. The SGS zone lies within latitudes 8°4′ and 11°3′ N and longitudes 2°41′ and 13°33′ E, with a bimodal rainfall averaging between from 1000 mm to 1300 mm per year, and maximum temperatures ranging from 26 to 38°C. SuS is in the far North of the Country between latitudes 12°82' and 13°88'N and longitudes 38°9' and 13°89'E. The annual rainfall is between 650 and 1000 mm and the relative humidity is below 40% except in the few wet months when it averages 60%, it also has a higher average yearly temperature compared to the DS and SGS with dry season lasting from 6 ± 8 months. It is clear therefore that contamination was heightened with increase in average temperature.
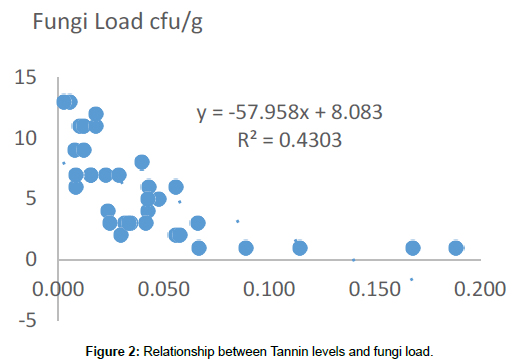
Tannin in this work has been demonstrated to have fungicidal activity (Figure 2). There is a significant (p<0.05) correlation between high levels of tannin in our samples and reduced fungi load. This validates the claims of [19] that plants with higher concentrations of tannin are seen to be less susceptible to mycoflora attack. Aspergillus niger, Fusarium and Pennicilium spp however demonstrated some degree of resistance to the presence of tannin in this work. Tannin resistance by some microbes is probably due to the presence of tannase, a key enzyme in the degradation of hydrolysable tannins, produced by a reduced group of microorganisms, this enzyme is increasingly used in a number of processes [20]. Knudson et al. [21] first reported that tannic acid could be degraded by a strain of Aspergillus niger. In our work A. niger was found to be present in samples with high tannin concentration; implying resistance. Lewis and Starkey [22] reported that pure cultures of some soil fungi including Fusarium, Penicillium and Aspergillus grew on media containing tannins and are also capable of degrading tannery waste constituents [23]. This can explain the repeated resistance to high tannin concentration by members of the Fusarium and Penicillium genera.
Aflatoxin contamination
This study looked at occurrence of aflatoxins with a view to estimate the impact on food safety. Natural occurrence of aflatoxin has been reported to be 1.0: 0.1 when limited only to AFB1 and AFB2 and 1.0: 0.1: 0.3: 0.03 when all four aflatoxins occur (AFB1, AFB2, AFG1 and AFG2) [24-26]. Our work showed occurrence of 1:0.08:0.08 for sorghum, 1:0.07 for sorghum product, 1:0.43 (millet), 1:0.21 (millet dough) and 1:0.04:0.4 (sesame) for AFB1, AFB2 and AFG1 respectively.
Contamination of sorghum grain (54.4%) by aflatoxin is similar to Makun et al. who reported 31.25% and 57.85% in field and stored sorghum samples. Uriah and Ogbadu (1980) found 100% contamination of sorghum samples in Northern Nigeria while Opadokun [27] found 6.9% contamination. Aflatoxin contamination of sorghum (0.96-21.74 μg/Kg) is similar to Odoemelam and Osu [28], 27.22-36.13 μg/kg, and Makun et al. [15] 0-54 μg/kg for field samples but not as high as [29] 30.32-211.20 μg/kg for AFB1; 2.40-208.00 μg/kg for AFG1, and Makun, et al. [15] 0-1164 μg/kg in stored samples. We can infer a decrease in levels of marketed sorghum product over the years which could be a result of improved good practices.
Findings on aflatoxin B1 in burukutu and pito samples are similar to earlier reports [30,31]. Alozie et al. [30] reported the presence of aflatoxins within the range of 0.2- 2.0 mg/kg in sorghum based local beers. Okoye and Ekpenyong [31] analyzed 20 pito and 20 burukutu samples in Jos metropolis for aflatoxin B1 contamination and found that 75% burukutu samples was contaminated with 1.7-140 μg/kg while 85% pito samples was contaminated with 16-140 μg/kg aflatoxin.
In South African beer, Odhay and Naicker [32] detected AFB1 (200- 400 μg/kg) at unsafe levels, Sibanda et al. [33] reported upto 50 μg/ kg AF in sorghum based local beer from Lesotho, Matumba, et al. [34] also found aflatoxin at levels above the CODEX permissible limit in sorghum based traditional opaque beer from Malawi. However, in Botswana Nkwe, et al. [35] detected no aflatoxin in 46 samples of traditional sorghum malt, wort, and beer. From most studies carried out around the world, especially in Africa and Nigeria on aflatoxin contamination in traditional beer reported above, our study presents a relatively lower contamination of beer product in Minna/Bida similar to the sorghum case. This is likely due to lower aflatoxin level in the grain as discussed above and slight variations in production processes.
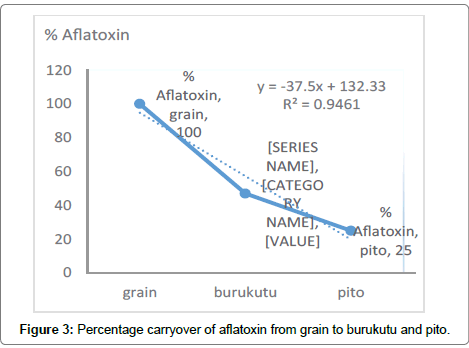
Although fermentation process reduces mycotoxins in contaminated products, evidence had shown a significant carryover of mycotoxins into sorghum based traditional African beer [36]. This study indicates a significant (p<0.05) decrease in the amount of toxins that was carried over from the grain to product. Pito is fermented twice and fermentation is not stopped before consumption, burukutu is fermented once. Our study show that there was 47% and 25% carryover of aflatoxin from grain to burukutu and pito respectively (Figure 3). Oluwafemi and Ikeowa (2005) had demonstrated 50% carryover of aflatoxin B1 after 72 hours of maize fermentation (50 μg/kg -1 to 25 μg/ Kg). Yuan et al. [37] also established that spontaneous fermentation was safe based on the results of aflatoxin B1 test. Oluwafemi, showed upto 89% loss of AF at laagering stage. pH reduction coupled with non-specific interaction or absorption of AF by solid particles which is later removed by filtration process are responsible for reduction in aflatoxin levels [38,39]. One of such solid particle that can interact with aflatoxins is mannan-oligosaccharides present on the fermentums cell wall [40].
Similar to our findings is the report of Ezekiel [41] who found higher concentration aflatoxin B1 relative to aflatoxin B2 in fonio millet samples. In our earlier work Makun, et al. [42] millet sampled from kontagora a less humid and high temperature region showed undetectable levels of aflatoxin, in this work, low concentration of Aflatoxin B1 1.38 ± 0.33 and AFB2 1.00 ± 1.00 was detected, this is an indication of season variability influencing fungi proliferation and mycotoxin production. Bandyopadhyay. Kumar and Leslie [43] reported mean content of total aflatoxins of 4.6 μg/kg in pearl millet sampled around West Africa, while about 50% of millet grain sampled in India was found to contain aflatoxin in concentrations between 12 and 44 μg/kg [44].
Sesame seed in this work had 50%, 4.35% and 6.52% contamination by AFB1, AFB2 and AFG1 respectively. Contamination ranged from 0.79-60.05 μg/kg with a mean ± SD value of 13.67 ± 13.59. This was higher than the other samples, it was also higher than the report of Ezekiel et al. [41] who found 0.08-1.4 μg/kg of AFB1 in Jos, Nigeria. Idris et al. [45] reported aflatoxin incidence of 7/16 (43.75%) in unrefined sesame oil from Sudan.
The influence of environmental variables on fungi proliferation and mycotoxin production has been studied and established. AflD gene (Nor-1) a key gene involved in the aflatoxin biosynthetic pathways requires certain conditions of temperature, humidity and water activity to be optimally expressed. According to Wu et al. [46] the natural occurrence of aflatoxins varies according to localities and years. The authors found out that the optimal temperature in favor of aflatoxin contamination in peanuts in China province rose along with decrease of the latitude. In this work (Table 4), aflatoxin levels in marketed sesame seeds increased with increasing latitude from derived savannah to southern guinea savannah and then to sudan savannah. In millet, toxins were found to be more predominant in the relatively hotter and more humid region compared to the region having higher temperatures and lower humidity and lower temperature and higher humidity region. During the sampling period Minna had a relative humidity which ranged between 76-88%, temperature of 28.3°C while Bida had a relatively lower humidity between 73-85% and temperature of 28.9°C this is directly related to higher toxin concentration in sorghum samples from Bida than Minna.
Food safety, health and economic impact
The current EU maximum permissible limit of 2.0 μg/Kg for AFB1 and 4.0 μg/Kg for total AF (www.R-Biopharm.com) have been adopted in Nigeria. Based on this 28.6% sorghum, 80% burukutu, 20% pito, 29% millet grain, 26.3% millet dough and 21.7% sesame samples were unsafe for human consumption from our study. These have grievous effects on food safety, health, economy as well as international trade. Data from previous studies on the burden of aflatoxin contamination of maize and groundnut in Nigeria show that at prevalence rates of 20 μg/kg the monetized burden was estimated to be between $112 and $942 million (in 2010) [47]. It is noteworthy that in 2010, Nigeria GDP was $197 billion so the estimate of 20 μg/kg constitutes roughly 0.5% of Nigeria GDP. Aflatoxin contamination in maize and groundnuts reported in Nigeria has been estimated to cause as many as 7,761 liver cancer cases per year out of the estimated 10, 130 total liver cancer cases [47]. Studies have found evidence that chronic exposure to aflatoxin is associated with several human health effects, including liver cancer [48]. being a group 1 human carcinogen [1], liver and kidney related diseases [49], immunologic suppression and growth impairment [50]. High levels of exposure (i.e., acute exposure) may result in acute aflatoxicosis. Aflatoxin has also been shown to cause immune suppression, particularly suppression of cell-mediated immune responses, in human [51], thus presenting additional burden to the HIV epidemic. Considering the findings in this study, steps towards elimination of fungi and aflatoxin in food is necessary [52].
It will be very promising if grains are stored in controlled environment as opposed to the traditional methods, also the findings presented earlier suggests that tannin rich varieties of sorghum and millet can reduce the presence of fungi on food. Such species can be promoted among local farmers for cultivation, if this is done, the antinutritional concerns associated with tannin in food can be further be handled during processing.
Results
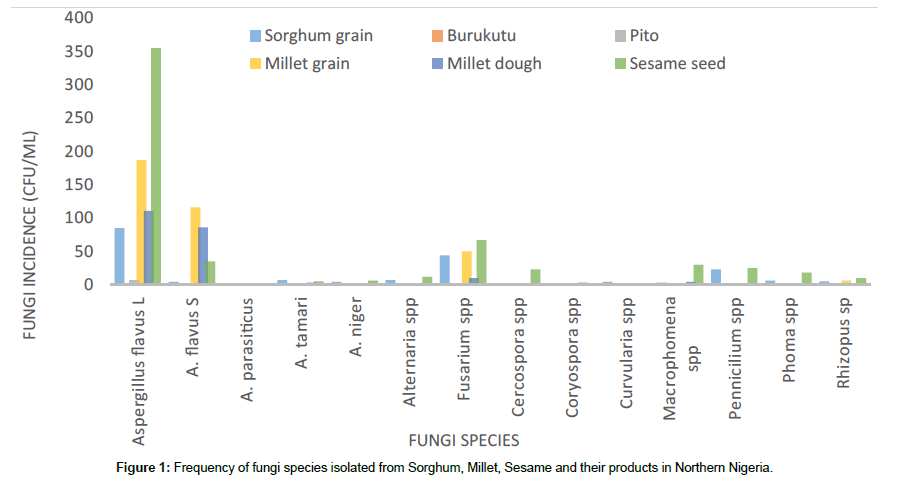
Ten fungi genera were isolated from the samples, these include Aspergillus spp, Alternaria spp, Fusarium spp, Cercospora spp, Coryospora spp, Curvularia spp, Macrophomena spp, Pennicilium spp, Phoma spp and Rhizopus spp, among which Coryospora spp was only found in millet and millet dough. The Aspergillus genera was further divided into: A. flavus (L-strain), A. flavus (S-strain), A. niger, A. tamarii and A. parasiticus. Members of Aspergillus (A. flavus (L-strain) and A. flavus (S-strain)), Pennicilium and Fusarium where most predominant (Figure 1).
Sesame had the higher fungi presence (150 – 550000 cfu/ml) followed by millet (33.3-1800 cfu/ml), sorghum (16.7-2200 cfu/ml), millet dough (0.00-1500 cfu/ml), burukutu (0-50 cfu/ml) and pito (0- 33.3 cfu/ml). Sorghum samples from Minna had heavier fungi presence than Bida. In millet samples, the fungi mean values for the different local government areas decreased in the order; Chanchaga (1050 ± 50.0 cfu/ml), Agaie (1000 ± 300 cfu/ml), Lapai (733 ± 667 cfu/ml), (Bosso (543.7 ± 118.2 cfu/ml), Kuta (493 ± 261.1 cfu/ml), Suleja (383 ± 117 cfu/ml), Bida (300 ± 100 cfu/ml), Kontogora (283 ± 117 cfu/ml), and Katcha (49.75 ± 31.81 cfu/ml), these show that the higher temperature regions have higher fungi load, higher fungi load occurred in stored (619.3 ± 164.9 cfu/ml) than marketed (475.8 ± 121.9 cfu/ml) and millet dough (352.6 ± 80.3 cfu/ml), which is an indication of decreasing fungi contamination upon processing. Sesame showed a higher fungi load in Jigawa state (SuS) (150-550000) followed by Niger state (SGS) (200- 400000) and then Nassarawa state (DS) (350-300000) (Table 1).
| S/N | Fungi species | Sorghum grain | Burukutu | Pito | Millet grain | Millet dough | Sesame seed | ||||||
| n=35 | n=10 | n=5 | n=31 | n=19 | n=46 | ||||||||
| Incidence | Frequency (%) | Incidence | Frequency (%) | Incidence | Frequency (%) | Incidence | Frequency (%) | Incidence | Frequency (%) | Incidence | Frequency (%) | ||
| 1 | A. flavus L | 85 | 44 | 6 | 100 | 187 | 50.7 | 111 | 50 | 355 | 60.4 | ||
| 2 | A. flavus S | 4 | 2.1 | 116 | 31.4 | 86 | 38.7 | 35 | 6 | ||||
| 3 | A. parasiticus | 0 | 0 | 1 | 0.3 | 1 | 0.5 | 2 | 0.3 | ||||
| 4 | A. tamari | 7 | 3.6 | 0 | 3 | 1.4 | 5 | 0.9 | |||||
| 5 | A. niger | 4 | 2.1 | 0 | 0 | 6 | 1 | ||||||
| 6 | Alternaria spp | 7 | 3.6 | 0 | 0 | 12 | 2 | ||||||
| 7 | Fusarium spp | 44 | 22.8 | 2 | 50 | 50 | 13.6 | 10 | 4.5 | 67 | 11.4 | ||
| 8 | Cercospora spp | 1 | 0.5 | 2 | 0.5 | 0 | 23 | 3.9 | |||||
| 9 | Coryospora spp | 0 | 0 | 1 | 0.3 | 3 | 1.4 | 0 | |||||
| 10 | Curvularia spp | 4 | 2.1 | 2 | 0.5 | 2 | 0.9 | 0 | |||||
| 11 | Macrophomena spp | 3 | 1.6 | 2 | 0.5 | 4 | 1.8 | 30 | 5.1 | ||||
| 12 | Pennicilium spp | 23 | 11.9 | 2 | 50 | 2 | 0.5 | 1 | 0.5 | 25 | 4.3 | ||
| 13 | Phoma spp | 6 | 3.1 | 0 | 0 | 18 | 3.1 | ||||||
| 14 | Rhizopus spp | 5 | 2.6 | 6 | 1.6 | 1 | 0.5 | 10 | 1.7 | ||||
| Cfu/ml | 16.7- 2200 | 0- 50 | 0- 33.3 | 33.3-1800 | 0.00-1500 | 150 - 550000 | |||||||
** Nigeria adopts the EU regulatory limit of 2 μg/kg for AfB1 and 4 μg/kg for total aflatoxin.
ND – Not detected.
Table 1: Incidence and frequency of fungi species isolated from Sorghum, Millet, Sesame and their products in Northern Nigeria.
In this study, aflatoxin B1 was the most occurring in sorghum, millet, sesame and their products, AFB2 was next then AFG1 (Tables 2 and 3).
| S/N | Product | No. Of Samples | AFB1 | AFB2 | AFG1 | Natural Occurrence AFB1:AFB2:AFG1 | |||||||
| No. of +ve Samples | % Conamination | Range | No. of +ve Samples | % Conamination | Range | No. Of +ve Samples | % Conamination | Range | |||||
| 1 | Sorghum grain | 35 | 19 | 54.29 | 0.96- 17.33 | 4 | 11.43 | 1.26- 2.24 | 1 | 2.86 | 7.11 | 1:0.08:0.08 | |
| 2 | Burukutu | 10 | 9 | 90 | 1.27- 7.50 | 2 | 20 | 1.32- 1.61 | ND | 0 | - | 01:00.1 | |
| 3 | Pito | 5 | 3 | 60 | 0.69-2.00 | ND | 0 | - | ND | 0 | - | ||
| 4 | Millet grain | 31 | 19 | 61.29 | 1.05- 10.06 | 12 | 38.71 | 1.86- 4.90 | ND | 0 | - | 01:00.4 | |
| 5 | Millet dough | 19 | 10 | 52.63 | 0.81-3.95 | 3 | 15.79 | 1.13-2.07 | ND | 0 | - | 01:00.2 | |
| 6 | Sesame seed | 46 | 23 | 50 | 0.79- 37.25 | 2 | 4.35 | 2.46-3.92 | 3 | 6.52 | 5.87-22.80 | 1:0.04:0.4 | |
| TOTAL | 146 | 83 | 56.85 | 23 | 15.75 | 4 | 2.74 | ||||||
Table 2: Aflatoxin Levels in Sorghum, Millet, Sesame and their Products.
| S/N | PRODUCT | No. Of Samples | No. Of +ve Samples | % of Contaminated Samples | Samples above 2µg/Kg | Samples above 4µg/Kg | Range (Total AF) | Mean ± SD |
|---|---|---|---|---|---|---|---|---|
| µg/Kg | µg/Kg | |||||||
| 1 | Sorghum grain | 35 | 19 | 54.3 | 10 | 6 | 0.96- 21.74 | 5.31 ± 6.28 |
| 2 | Burukutu | 10 | 9 | 90 | 8 | 3 | 1.27- 8.82 | 4.47 ± 2.87 |
| 3 | Pito | 5 | 3 | 60 | 1 | - | 0.69-2.00 | 1.38 ± 0.66 |
| 4 | Millet grain | 31 | 19 | 61.2 | 9 | 10 | 1.05-14.96 | 5.99 ± 4.70 |
| 5 | Millet dough | 19 | 10 | 52.6 | 5 | - | 0.81- 3.78 | 2.66 ± 1.09 |
| 6 | Sesame seed | 46 | 23 | 50 | 10 | 7 | 0.79- 60.05 | 13.67 ± 13.50 |
| 146 | 56.8 |
Table 3: Total Aflatoxin (B1+B2+G1+G2) Levels in Sorghum, Millet, Sesame and their Products.
| Product | Location | Agro-ecological zones | AFB1 | AFB2 | AFG1 | Total Aflatoxin |
|---|---|---|---|---|---|---|
| Sesame | Agro-ecological Zonation | Derived savannah (Nassarawa) | 5.3 ± 9.66 | 2.46 ± 0.00 | 17.89 ± 6.94 | 6.59 ± 15.15 |
| Southern guinea savannah (Niger) | 7.82 ± 15.57 | 0.00 ± 0.00 | 5.87 ± 0.00 | 5.66 ± 13.69 | ||
| Sudan savannah (Jigawa) | 3.68 ± 5.68 | 0.00 ± 0.00 | 0.00 ± 0.00 | 3.68 ± 5.68 | ||
| `Millet | High Temperatures and High Humidity LGA | Kuta | 1.56 ± 0.75 | 0.67 ± 0.67 | ND | 2.24 ± 1.41 |
| Bosso | 2.22 ± 0.68 | 0.82 ± 0.31 | ND | 2.81 ± 0.90 | ||
| Chanchaga | ND | ND | ND | ND | ||
| Paikoro | ND | ND | ND | ND | ||
| Agaie | 4.03 ± 4.03 | 1.77 ± 1.77 | ND | 5.8 ± 5.8 | ||
| High Temperatures and Low Humidity LGA | Kontogora | 1.38 ± 0.33 | 1.00 ± 1.00 | ND | 2.38 ± 1.33 | |
| Kagara | ND | ND | ND | ND | ||
| Wushishi | ND | ND | ND | ND | ||
| Low Temperature and High Humidity LGA | Suleja | 4.15 ± 4.15 | 1.45 ± 1.45 | ND | 5.60 ± 5.60 | |
| Katcha | 0..26 ± 0.26 | ND | ND | 0.27 ± 0.27 | ||
| Lapai | 2.25 ± 1.74 | ND | ND | 2.25 ± 1.738 | ||
| Bida | 0.41 ± 0.41 | 0.69 ± 0.69 | ND | 1.11 ± 1.11 | ||
| Sorghum | Minna | Tungangoro | 7.47 ± 8.60 | 1.90 ± 0.00 | ND | 8.10 ± 9.68 |
| Gwadabe | 4.40 ± 4.65 | 1.6 ± 0.5 | ND | 5.20 ± 5.46 | ||
| Bosso | 1.33 ± 0.13 | ND | ND | 1.33 ± 0.13 | ||
| New Market | 2.44 ± 2.48 | ND | ND | 2.44 ± 2.48 | ||
| Bida | Bida Main Mkt | 3.90 ± 4.00 | ND | ND | 3.90 ± 4.00 | |
| Bida Mammy Mkt | 8.00 ± 5.05 | 2.20 ± 0.00 | 7.1 ± 0.00 | 11.12 ± 9.78 |
*ND – not detected
Table 4: Environmental Influence on Aflatoxin Contamination.
Conclusion
The present report is a major investigation into the incidence of fungi and the presence of aflatoxins in major food items in Northern Nigeria. Members of the Aspergillus, Fusarium, Pennicilium family were most predominant, the L-strain of Aspergillus flavus was the most occurring. Fungi load generally increased with increase in average temperature and was inversely proportional to tannin concentration. Macrophomena spp is reported in Nigerian sorghum for the first time. Aflatoxin levels was higher in sesame, followed by millet and then sorghum. Carryover of aflatoxin from sorghum grain to burukutu and pito was 47% and 25% respectively. However, in all sample types, the safe samples were more than the unsafe samples based on the EU MPLs adopted in Nigeria. The work necessitates implementation of management and intervention strategies by concerned stakeholders and regulatory bodies. The local food regulatory agency and standards organization should heighten effort in the control and regulation of mycotoxins.
Appreciation
The research grant obtained from the University Board of Research (now Directorate of Research, Innovation and Development) of the Federal University of Technology, Minna, Nigeria and the technical assistant by staff of plant pathology and mycotoxin department of the International Institute of Tropical Agriculture (IITA) Ibadan which made this work possible are greatly treasured.
References
- IARC (International Agency for Research on Cancer) (1987) Overall evaluations of carcinogenicity: an updating of IARC Monographs volumes 1 to 42. Report of an IARC Expert Committee. Lyon, (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Supplement 7).
- Ikeorah J, Okoye Z (2005) Four decades of research on aflatoxins in Nigeria: A review of NSPRI experience. A paper presented at the Regional Workshop on Mycotoxins organized by National Agency for Food and Drug Administration and Control (NAFDAC) in collaboration with International Atomic Energy Agency (IAEA), Held at Meidan Hotels. Victoria Garden City, Lagos, Nigeria.
- Adegoke GO1, Allamu AE, Akingbala JO, Akanni AO (1996) Influence of sundrying on the chemical composition, aflatoxin content and fungal counts of two pepper varieties--Capsicum annum and Capsicum frutescens. Plant Foods Hum Nutr 49: 113-117.
- Oluwafemi F, Ikeowa MC (2005) Fate of Aflatoxin B1 during Fermentation of Maize into \"ogi\". Nigerian Food Journal 23: 51-56.
- Adejumo O, Atanda O, Raiola A, Somorin Y, Bandyopadhyay R, et al. (2013) Correlation between aflatoxin M1 content of breast milk, dietary exposure to aflatoxin B1 and socioeconomic status of lactating mothers in Ogun State, Nigeria. Food Chem Toxicol 56: 171-177.
- Hsieh D (1988) Potential human health hazards of mycotoxins. Third Joint Food and Agriculture Organization/ W.H.O./United Nations Environment Program International Conference of Mycotoxins. Elsevier, Amsterdam, The Netherlands, p: 69-80.
- JECFA (2001) Joint FAO/WHO Expert Committee on Food Additives, Safety evaluation of certain mycotoxins in food. WHO Food Additives Series 47 and FAO Food and Nutrition Paper 74: 701.
- Lubulwa AG, Davis JS (1994) An economic evaluation of postharvest tropical fruit research: some preliminary results. Australian Centre for International Agricultural Research.
- FAO (2013) Sorghum and millets in human nutrition.
- Atehnkeng J, Ojiambo PS, Donner M, Ikotun T, Sikora RA, et al. (2008) Distribution and toxigenicity of Aspergillus species isolated from maize kernels from three agro-ecological zones in Nigeria. Int J Food Microbiol 122: 74-84.
- Sweeney MJ, Dobson AD (1998) Mycotoxin production by Aspergillus, Fusarium and Penicillium species. Int J Food Microbiol 43: 141-158.
- Kumar V, Basu MS, Rajendran TP (2008) Mycotoxin research and mycoflora in some commercially important agricultural commodities. Crop Protection 27: 891-905.
- Sánchez-Hervás M, Gil JV, Bisbal F, Ramón D, Martínez-Culebras PV (2008) Mycobiota and mycotoxin producing fungi from cocoa beans. Int J Food Microbiol 125: 336-340.
- Jonathan SG, Esho EO (2010) Fungi and aflatoxin detection in two stored oyster mushrooms (Pleurotus ostreatus and Pleurotus pulmonarius) from Nigeria. Electronic Journal of Environmental, Agricultural and Food Chemistry 9: 1722-1730.
- Makun HA, Gbodi TA, Akanya HO, Salako EA, Ogbadu GH (2009) Fungi and some mycotoxins found in mouldy Sorghum in Niger State, Nigeria. World Journal of Agricultural Sciences 5: 5-7.
- Makun HA, Anjorin ST, Moronfoye B, Adejo FO, Afolabi OA (2010) Fungal and aflatoxin contamination of some human food commodities in Nigeria. African Journal of Food Science 4: 127-135.
- Pitt JI, Hocking AD (1997) Fungi and Food Spoilage. Blackie Academic and Professional, p: 596.
- Agrios GN (2005) Plant Pathology. Elsevier Academic Press, p: 922.
- Awika JM, Rooney LW (2004) Sorghum phytochemicals and their potential impact on human health. Phytochemistry 65: 1199-1221.
- Aguilar CN, Gutierrez-Sanchez G (2001) Sources, properties, and potential uses of tannin acyl hydrolase (3.1.1.20) Food Science and Technology International 7: 373-382.
- Knudson L (1913) Tannic acid fermentation. Journal of Biology and Chemistry 14: 159-202.
- Lewis JA, Starkey RL (1969) Decomposition of plant tannins by some soil microorganisms. Soil Science 107: 235-241.
- Mahadevan A, Muthukumar G (1980) Aquatic microbiology with reference to tannin degradations. Microbiologia 72: 73-79.
- Abbas HK, Reddy KRN, Salleh B, Saad B, Abel CA, et al. (2010) An overview of mycotoxin contamination in foods and its implications for human health. Toxin Reviews 29: 3-26.
- Kensler TW, Roebuck BD, Wogan GN, Groopman JD (2011) Aflatoxin: a 50-year odyssey of mechanistic and translational toxicology. Toxicol Sci 120 Suppl 1: S28-48.
- Mehrdad T, Mohammad HS, Hassan Y, Salam AI (2011) Aflatoxin in Agricultural Commodities and Herbal Medicine. Aflatoxins - Biochemistry and Molecular Biology.
- Opadokun JS (1992) Occurrence of Aflatoxin in Nigeria food crops. Book of Proceeding, pp: 50-60.
- Odoemelam SA, Osu CI (2009) Aflatoxin B1 contamination of some edible grains marketed in Nigeria. E-Journal of Chemistry 6: 308-314.
- Uriah N, Ogbadu L (1980) Influence of woodsmoke on aflatoxin production by Aspergillus flavus. European Journal of Applied Microbiology and Biotechnology 14: 51-53.
- Alozie TC, Rotimi CN, Oyibo BB (1980) Production of aflatoxin by Aspergillus flavus (UBMI) in some Nigerian indigenous beverages and foodstuffs. Mycopathologia 70: 125-128.
- Okoye ZS, Ekpenyong KI (1984) Aflatoxin B1 in native millet beer brewed in Jos suburbs. Trans R Soc Trop Med Hyg 78: 417-418.
- Odhav B, Naicker V (2002) Mycotoxins in South African traditionally brewed beers. Food Addit Contam 19: 55-61.
- Sibanda L, Marovatsanga LT, Pestka JJ (1997) Review of mycotoxin work in sub-Saharan Africa. Food Control 8: 21-29.
- Matumba L, Monjerezi M, Khonga EB, Lakudzala DD (2011) Aflatoxins in Sorghum, Sorghum malt and traditional opaque beer in southern Malawi. Food Control 22: 266-268.
- Nkwe DO, Taylor JE, Siame BA (2005) Fungi, aflatoxins, fumonisin B1 and zearalenone contaminating sorghum-based traditional malt, wort and beer in Botswana. Mycopathologia 160: 177-186.
- Hell K, Mutegi C (2011) Aflatoxin control and prevention strategies in key crops of Sub-Saharan Africa. African Journal of Microbiology Research 5: 459-466.
- Yuan ML, Lu ZH, Cheng YQ, Li LT (2008) Effect of spontaneous fermentation on the physical properties of corn starch and rheological characteristics of corn starch noodle. Journal of Food Engineering 85: 12-17.
- Oluwafemi F (2004) Fate of aflatoxin in cereals and cereal products during processing. Journal of Food, Agriculture & Environment 2: 57-60.
- Chu FS, Chang CC, Ashoor SH, Prentice N (1975) Stability of aflatoxin B-1 and ochratoxin A in brewing. Appl Microbiol 29: 313-316.
- Devegowda G, Arvind BIR, Morton MG (1996) Saccharomyces cerevisiae and mannanoligosaccharides to counteract aflatoxicosis in broilers. Proceedings of Australian poultry science symposium Sydney pp: 103-106.
- Ezekiel CN, Sulyok M, Warth B, Krska R (2012) Multi-microbial metabolites in Fonio millet (Acha) and Sesame grains in Plateau State, Nigeria. European Food Research and Technology 3: 10-14.
- Makun HA, Apeh DO, Adeyemi HRY, Nagago T, Okeke JO, et al. (2014) Determination of Aflatoxins in Sesame, Rice, Millet and Acha from Nigeria using HPLC. Chemical Science Transaction 3: 1516-1524.
- Bandyopadhyay R, Kumar M, Leslie JF (2007) Relative severity of aflatoxin contamination of cereal crops in West Africa. Food Addit Contam 24: 1109-1114.
- Dwivedi PK, Tyagi RPS, Bansode PC (1990) Chemical Food safety of traditional grains. Food Sci Technol (India) 27: 111-112.
- Idris YM, Mariod AA, Elnour IA, Mohamed AA (2010) Determination of aflatoxin levels in Sudanese edible oils. Food Chem Toxicol 48: 2539-2541.
- Wu LX, Ding XX, Li PW, Du XH, Zhou HY, et al. (2015) Aflatoxin contamination of peanuts at harvest in China from 2010 to 2013 and its relationship with climatic conditions. Food Control.
- Countrystat (2012) Country STAT, Food and agriculture data network.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans (2002) Some traditional herbal medicines, some mycotoxins, naphthalene and styrene. IARC Monogr Eval Carcinog Risks Hum 82: 1-556.
- Wagacha JM, Muthomi JW (2008) Mycotoxin problem in Africa: Current status, implications to food safety and health, and possible management strategies. International Journal of Food Microbiology 212: 347-368.
- Khlangwiset P, Shephard GS, Wu F (2011) Aflatoxins and growth impairment: a review. Crit Rev Toxicol 41: 740-755.
- Jiang Y, Jolly PE, Ellis WO, Wang JS, Phillips TD, et al. (2005) Aflatoxin B1 albumin adduct levels and cellular immune status in Ghanaians. Int Immunol 17: 807-814.
- USAID (United States Agency for International Development) Preliminary Livelihoods Zoning: Northern Nigeria. In: Famine Early Warning Systems Network (FEWS NET) Indefinite Quantity Contract, AFP-I-00-05-00027-00, managed by Chemonics International.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14199
- [From(publication date):
October-2016 - Aug 18, 2025] - Breakdown by view type
- HTML page views : 13045
- PDF downloads : 1154