Nanocatalytic Conversion of Waste Palm Oil Grade III and Poplar Plant’s Wood Sawdust into Fuel
Received: 22-Aug-2017 / Accepted Date: 23-Aug-2017 / Published Date: 31-Aug-2017 DOI: 10.4172/2576-1463.1000170
Abstract
The aim of this research was to study the pyrolysis oil production from Palm Oil Grade III (POG-III) and popular wood sawdust crude oil. Today, worldwide studies have been undertaken on the biomass usage and co-conversion of biomass and coal to seek out alternative fuels for supplying energy in an environmental friendly way. Substitute fuels have become more and more vital due to descending petroleum reserves, increasing economic circumstances and awareness of the increased environmental penalties of emissions from petroleumfuelled engines. In this study, biodiesel fuel was manufactured by the nanocatalytic to elucidate their thermal behaviour under pyrolysis conditions and to assess major decomposition products in terms of their yields. Transesterification of Palm Oil Grade III (POG-III) at 80°C temperature by using nano Co as nanocatalyst. The prepared biodiesel was characterized by FT-IR and GC-MS. Results revealed the presence of esters, alkanes, alkenes, saturated and unsaturated hydrocarbons with carbon chain in the range C9-C27. Prepared biodiesel is cost effective and highly efficient. Besides Palm oil popular wood sawdust crude oil was also used. All products were obtained at low temperature and at low atmospheric pressure.
Keywords: Catalytic pyrolysis; Biodiesel; Cobalt
23148Introduction
In recent years, diminishing fossil fuel sources, growing demand, cost of petroleum-based fuels, and environmental hazards as a result of their combustion have encouraged researchers to investigate possibility of using alternative fuels as new energy resource. It is necessary to reduce consumption of the petro fuels due to the negative effects on human life. As known fossil energy sources have been exhausted rapidly nowadays, it is predicted that fossil fuel sources will be depleted in the near future [1]. Waste energy sources were focused in different research works presented in the literature as renewable energy source [2]. Recycling is the applied process for advancement of petroleumbased wastes by converting them into recyclable products such as diesel fuel, gasoline and heavy oil.
Production of diesel-like fuel from waste oils such as industrial and engine waste oils, wood pyrolytic oils, fresh and waste fats and vegetable oils is an excellent way for producing alternative fuel sources. Industrial and engine waste oils, wood pyrolysis oils, fresh and waste fats and vegetable oils have been proposed as pyrolytic raw material to produce gasoline and diesel-like fuels. There is plenty of the waste engine oil in the world. Average amounts of used engine lubricating oils are produced worldwide every year [3]. Municipal and industrial wastes like Waste Cooking Oil (WCO), Waste Plastics Oil (WPO) and Waste Lubricating Oil (WLO) which have a high calorific value are considered as efficient source of energy production [4]. Among the important substitute fuel sources waste lubricant oils and biofuel are the best replacements for existing petro fuels, since waste generated oils represent more than 60% of used lubricant oils.
It should be collected and re-used in order to decrease detrimental effects on environment, and underground and surface water, since it pollutes the atmospheric air as a result of burning, and has negative effect on living organisms, underground and surface water when it is discharged into soil or water. Conversion of the waste engine oils into diesel like fuels by using pyrolytic distillation, and by utilization of the product as a diesel fuel has positive effects on environment and atmospheric air, and also has economic value [5].
A very large amount of used oils are disposed through dumping on the ground or in water and land fillings [6]. Fuels or lubricating oil base stock can be produced by refining and treating the used oils. Used oils on the other hand are a severe threat to the environment as they contain metal content and other contaminants [7]. Due to the hazardous properties waste oils that are found in rivers, lakes are damaging to the aquatic life. A single liter of that waste oil can be damaging to a million liters of clean water. Soil pollution is one of the outcomes of waste oil spill [8]. Several classes of heterogeneous catalysts, such as supported enzymes oxides and Oxo salts impregnated oxides inorganic complexes, layered double hydroxides metal carboxylates zeolites and ion-exchange resins [9-16] have been reported in the literature as being active for the transesterification (alcoholysis) of vegetable oils.
Currently, most of the biodiesel production processes are based on the Transesterification of vegetable oils using heterogeneous nano catalysts, which are known for their high activity and low cost, making them suitable for large-scale industrial operation [17]. In fact, this class of catalysts offers economical, ecological, and technical advantages, which are critical parameters for improvement of the efficiency and sustainability of biodiesel, manufacture [18]. Transition metal oxides and oxosalts have also been investigated as transesterification catalysts due to their elevated Lewis acidity, depending on the metal oxidation state and ion radius size [19]. In this sense, molybdenum- and tungsten-containing solids have been extensively studied in a number of reactions in which the acidity profile plays an important role, such as isomerization. Cyclic alkene oxidation alcohol dehydration and vegetable oils transesterification [20].
Transesterification is the chemical process between triglycerides and short-chain alcohol in the presence of basic or acidic catalyst to produce monoalkylester [21]. The long- and branched-chain triglyceride molecules are transformed to monoalkylesters and glycerol. Commonly used short-chain alcohols are methanol, ethanol, propanol, and butanol [22]. Methanol is commercially used due to its availability and cost effectiveness [23]. The subsequent useful product is Fatty Acid Methyl Ester (FAME) commonly known as biodiesel.
Palm oil grade III is the world’s cheapest waste vegetable oil, therefore crude palm oil itself presents a promising alternative as a feedstock for Fatty Acid Methyl Ester (FAME). Nevertheless, the study on converting grade III palm oil to Fatty Acid Methyl Ester (FAME) using heterogeneous reaction is still very limited and only being explored in Asian countries, most probably due to its availability [24].
Experimental (A)
Chemicals
The cobalt nitrate hexahydrate was purchased from Sigma Aldrich USA and Sodium hydroxide was purchased from Fluka. All solvents were of analytical reagent grade and were used without further purification.
Synthesis of CoO nano particles through co-precipitation method
For the synthesis of CoO nanoparticles, 0.1 molar solution of cobalt nitrate hexahydrate and 0.1 molar solution of sodium hydroxide were separately prepared in distilled water. The sodium hydroxide solution was taken in dropping funnel and very slowly dropped to the cobalt nitrate solution with constant stirring at a temperature of 40-50°C. The dark brown precipitates of cobalt Co(OH)2 appeared after almost one third (1/3) of sodium hydroxide solution was added to cobalt nitrate solution respectively.
The addition of the sodium hydroxide solution to the salt solution continued till precipitates formation was complete in the reaction mixture. The PH was plus 10. The precipitates were then filtered and washed two times with distilled water to remove the un-reacted sodium hydroxide and the salts. The precipitates were dried in oven at 250oC. The precipitates were taken in china dish and placed in furnace.
The temperature of the furnace was raised to 500°C at a heating rate of 0.5°C min-1 and the contents were kept at 500°C for 24 h and then allowed to cool to room temperature. The calcined product was ground into powder and formed CoO nano particles. The nanoparticles were studied at XRD, SEM and BET.
Sample collection
Palm oil samples were collected from Ferozsons Tank Terminal (Pvt.) Ltd., Karachi Plot # 50, and Molasses and Edible Oil Area Port Muhammad bin Qasim, Karachi. The following initial physiochemical parameters were carried out; conductivity, organic matter, Free Fatty Acids (FFA), Dirt content and oil content [25].
Determination of moisture and volatile content
A mass of 10 g of thoroughly mixed palm oil sample was weighed into a known weight of clean petri dish which had been previously dried and cooled in desiccators. It was placed in the oven for 4 h, allowed to cool to room temperature in a desiccator for 45 min and weighed till a constant weight was obtained. The moisture and volatile matter content was expressed in percentage by mass using the formula.
% Moisture and Volatile content=((Mb-Md)/(Mb-M)) × 100 (1)
Where M=Mass (g) of petri dish;
Mb=Mass (g) of petri dish+sample;
Md=Mass (g) of petri dish+test sample after dying.
Determination of oil content
Dry palm oil sample (10 g) was weighed into thimble and extraction was done for 6 h using 150 ml of hexane as an extracting solvent. The oil extract was dried at atmospheric pressure at 103°C for 2 h, cooled for 30 min and weighed. The oil content was expressed as percentage by using the formula.
Percentage=((Mass (g) of oil in the flask drying)/(mass (g)sample)) × 100 (2)
Determination of dirt content
The dirt content of the sample was analyzed as 8.50 g of the sludge was weighed into a conical flask, 100 ml of hexane was added, and then mixture was filtered through glass fiber filter paper in a crucible and dried in the oven at 103°C for an hour. Percentage dirt content was expressed as:
(Mass of crucible+filter paper+dirt)–(Mass of crucible+filter paper/Mass of sample) × 100 (3)
Determination of free fatty acids (FFAs)
Sample (2.5 g) was weighed into conical flask, 50 ml of neutralizing solvent was carefully added to the weighed sample in the conical flask, placed on hot plate at 40°C mixed gently and titrated with standard potassium hydroxide (0.5M). Values were calculated using the formula,
Free Fatty Acids=25.6× Molarity of KOH × Volume of KOH/mass of sample (4)
Experimental set up for catalytic conversion of palm oil into biodiesel
Experiment was carried out in around bottom flask, equipped with L-shaped tube, connected with a condenser. About 50 ml of pretreated palm oil grade III was mixed with 10 ml of methanol was taken in round bottom flask and 1 g of Co nano catalyst was added. The flask was placed in the furnace at 80°C. The temperature was maintained at 80°C for 1 h. The liquid was condensed in L-shaped tube and collected in a beaker.
Experiment (B)
Conversion of popular wood sawdust into biodiesel by using nanoparticles during the gasification process
Cobalt nanoparticles were used during the gasification process with the sawdust of popular wood.
Sawdust used=30 g;
Cobalt nanoparticles=1 g;
Oil extracted after the completion of gasification=17.5 ml;
Muffle furnace temperature was maintained at 300°C.
Nanoparticles help the gasification to complete at a very low temperature as compared to the gasification done without nano particles. The oil obtained was much pure as compared to the oil extracted without using nano particles.
Experimental set up for catalytic gasification of popular wood sawdust crude oil.
As shown in Figure 1 the catalytic gasification of popular wood sawdust was carried out in around bottom flask, equipped with L-shaped tube, connected with a condenser. About 50 g of pre-treated popular ash was taken in round bottom flask and was mixed with 0.5 g Co nano catalysts. The flask was placed in the furnace and started the reaction. The temperature of the furnace was increased from room temperature to 300°C with a rate of 10°C/ min. The temperature was maintained at 400°C for one hour. Dark brown oil starts vaporized in L-shaped tube which was condensed by condenser and collected in a beaker. This oil product was named as popular wood sawdust oil.
Crude oil after extraction was then treated with Methyl alcohol in the ratio 1:6 respectively. 10 ml oil was taken in the round bottom flask and mixed with 60 ml methanol. First methanol and nano catalyst was mixed and allowed them to stir for 1 h at 45 °C till the methoxide was formed. Placed round bottom flask in Muffle furnace. Condenser was used to condense the gases formed during the process. 10 ml of the crude oil was pour into the round bottom flask already containing the mixture of nanocatalyst. After 2 h heating was stopped and the 2 layers are formed in the round bottom flask. Upper layer is of biodiesel and the lower layer is of soap and glycerin. Solution was then filtered with the help of Whatman filter paper. Biodiesel was separated and was collected for the analysis.
Fourier transforms infrared spectroscopy
FTIR analysis was performed on a BRUKER VECTOR 22 spectrophotometer device. Table 1 shows the functional group obtained from FTIR spectra.
| Frequency (cm-1) | Functional Group Assignment |
|---|---|
| 3383 | Broad symmetrical stretching vibration of hydroxyl OH group |
| 2967 | symmetrical stretching vibration of methylene group |
| 1708 | Stretching vibration of carbonyl and is characteristic of esters group |
| 1371 | Bending vibrations of the CH2 and CH3 aliphatic groups |
| 1288 | Bending vibrations of CH2 groups |
| 1049 | CAO stretching |
Table 1: FTIR results of popular wood sawdust biodiesel.
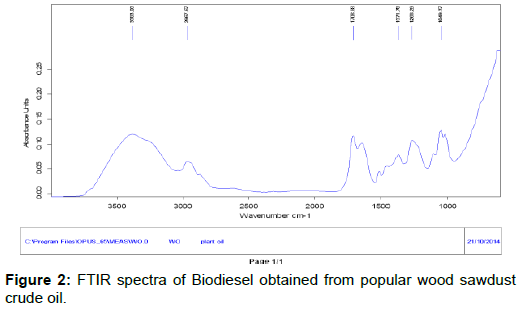
The biodiesel produced from popular wood sawdust crude oil were analyzed using FTIR spectrophotometry. The spectra as shown in Figure 2. The broad band located at approximately 3383 cm-1 are related to O-H vibrations of the hydroxyl groups The band located at approximately 2967 cm-1 are related to C-H vibrations of the methylene groups and to stretching and contraction vibrations of the methyl group. The intense peak located at 1708 cm-1 corresponds to carbonyl radical and is characteristic of esters. The band located at 1371 cm-1 corresponds to the asymmetric stretching of the C-H bond and the asymmetric bending of the functional group. There was a set of bands between 1371 and 1288 cm-1 that were associated with asymmetric vibrations of the C-C (=O)-O and O-C-C bonds. The high intensity peak located at 1009 cm-1 was attributed to the symmetric angular deformation of the C-H bond of olefins.
Results And Discussions
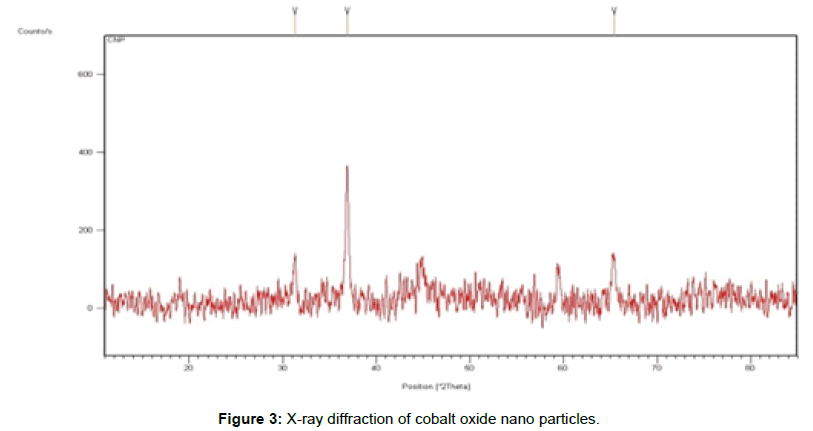
X-ray diffraction analysis: Generally, XRD can be used to characterize the crystallinity of nanoparticles, and it gives the average diameters of the nanoparticles. The precipitated fine particles were characterized by XRD for structural determination and estimation of crystallite size. Further, the formation of oxide could be confirmed from the X-ray diffraction analysis of the samples. Figure 3 indicates the X-ray diffractograms of the cobalt oxide nanoparticles obtained by co-precipitation. Each diffractogram show the peaks characteristic for the cobalt oxide of ~ 31.4°, 36.1°, and 65.7°. The crystallite size of cobalt oxide nanoparticles were 46 nm. The crystallite size determined by Scherer formula.
Scanning electron microscopy-energy dispersive X-ray spectroscopy (SEM-EDX)
Figure 4 Support the microcrystalline nature of the particle after calcinations with least degree of agglomeration. Particles seem to have an irregular shape with chemical homogeneity with uniform morphology and with good porosity due to an inter particle surface connectivity. It was observed as the annealing temperature increases the crystalline nature of the particle changes.
Brunauer-emmett-teller (BET)
The surface area and pore volume was found to be 33.0715 m2/g supporting the presence of limited porosity with a pore size distribution. These values support their industrial applications as a catalyst in organic reactions [26].
Fourier transforms infrared spectroscopy
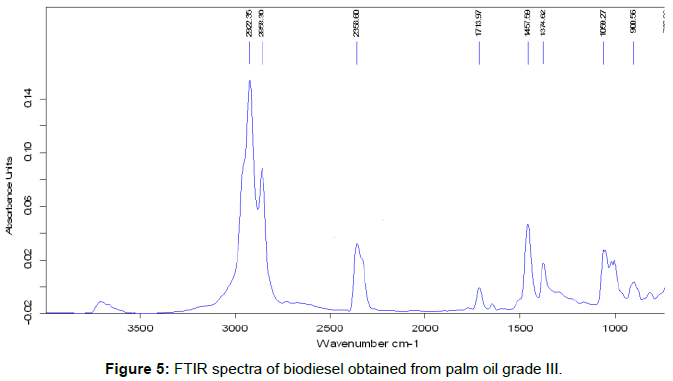
The functional group analysis of pyrolysis oil was carried out by using Fourier Transform Infrared Spectroscopy (FTIR). The FTIR spectra obtained for pyrolysed oil was given in Figure 5. FTIR analysis was performed on a BRUKER VECTOR 22 spectrophotometer device. Table 2 shows the functional group obtained from FTIR spectra.
| Frequency(cm-1) | Functional Group Assignment |
|---|---|
| 2924 and 2852 | Asymmetrical and symmetrical stretching vibration of methylene group |
| 1713 | Stretching vibration of carbonyl and is characteristic of esters group |
| 1457 | Bending vibrations of the CH2 and CH3 aliphatic groups |
| 1374 | Bending vibrations of CH2 groups |
| 1009 | CAO stretching |
| 900 | =CH2 wagging |
Table 2: FTIR results of Palm oil grade III biodiesel.
The biodiesel produced from palm oil were analyzed using FTIR spectrophotometry. The spectra as shown in Figure 5. The band located at approximately 2924 and 2852 cm-1 are related to C-H vibrations of the methylene groups and to stretching and contraction vibrations of the methyl group. The intense peaks located at 1713 cm-1 correspond to carbonyl radical and is characteristic of esters. The band located at 1457 cm-1 corresponds to the asymmetric stretching of the C-H bond and the asymmetric bending of the functional group. There was a set of bands between 1009 and 1374 cm-1 that were associated with asymmetric vibrations of the C-C (=O)-O and O-C-C bonds. The high intensity peak located at 900 cm-1 correspond to deflections out of the plane of the C-O group and the one located at 1009 cm-1 was attributed to the symmetric angular deformation of the C-H bond of olefins.
Gas chromatography-mass spectrometry (GC-MS)
The chemical composition of the prepared biodiesel was determined by Gas chromatography. For this purposeGC-6890N model coupled with mass spectrometer; model MS-5973 MSD (mass selective detector) was used. A DB-5MS capillary column (30 × 0.2 m2) was used to separate the different compounds present in the diesel. Helium was used as a carrier gas. The temperature of capillary column was raised from 120-300°C at a fixed rate of 10°C/min. Biodiesel in chloroform (0.1 μL) was injected as a sample using split mode with split ratio of 1:10. The scan rate of mass spectrometer was set from 50-550 m/z. Electron Impact (EI) mode of ionization was used.
Fatty Acid Composition of Palm Oil Methyl Ester (POME) was determined using gas chromatography. The study of chemical properties of oils before the experimental work plays an essential role in understanding the behavior and the structure of materials. This study focused on using the Palm Oil Grade III (POG-III) as a renewable and sustainable raw material for biodiesel production. Usually, Palm Oil Grade III (POG-III) is traded based on the FFA content, moisture and impurities. Table 3 Illustrates fatty acid composition of Palm Oil Grade III (POG-III). The results showed that the prevalent fatty acids available were oleic, palmitic, lauric and stearic acid. Saturated fatty acids in palm oil grade III (POG-III) were 44.02 weight % while unsaturated fatty acids were 55.96 weight %. Due to its high percentage of saturated fatty acids and free fatty acids, Palm Oil Grade III (POG-III) exists in semi-solid phase at room temperature (28 ± 2°C). Hence, Palm Oil Grade III (POG-III) has higher pour and cloud points as compared to normal palm oil. Higher saturated fatty acids in oils give a higher cetane number, and the oil is less prone to oxidation.
| Fatty Acid | Structure | Formula | Molecular Mass (wt%) | Fatty acids |
|---|---|---|---|---|
| Lauric | 12:00 | C12H24O2 | 200 | 0.209 |
| Myristic | 14:00 | C14H28O2 | 228 | 1.497 |
| Palmitic | 16:00 | C16H32O2 | 256 | 34.638 |
| Palmitioleic | 16:01 | C16H30O2 | 254 | 0.314 |
| Margaric | 17:00 | C17H34O2 | 270 | 0.106 |
| Stearic | 18:00 | C18H36O2 | 284 | 6.908 |
| Oleic | 18:01 | C18H34O2 | 282 | 41.662 |
| Arachidic | 20:00 | C20H40O2 | 312 | 0.575 |
| Eicosenoic | 20:01 | C20H38O2 | 310 | 0.209 |
Table 3: Fatty acid composition of palm oil by GC.
The fatty acid composition of biodiesel from the Palm oil grade III biodiesel is shown in Table 4.
| Systematic Name of FAME | Carbon chain | Type of fatty acid | FAME content/% |
|---|---|---|---|
| Dodecanoic acid methyl ester | C12:0 | Saturated | 0.19 ± 0.01 |
| Tetradecanoic acid methyl ester | C14:0 | Saturated | 0.90 ± 0.03 |
| Hexadecanoic acid methyl ester | C16:0 | Saturated | 44.00 ± 2.00 |
| Hexadecenoic acid methyl ester | C16:1 | Unsaturated | 0.285 ± 0.003 |
| Octadecanoic acid methyl ester | C18:0 | Saturated | 4.35 ± 0.04 |
| cis-9-Octadecenoic acid methyl ester | C18:1 | Unsaturated | 40.40 ± 2.20 |
| All-cis-9,12-Octadecadienoic acid methyl ester | C18:2 | Unsaturated | 9.44 ± 0.05 |
| All-cis-9,12,15-Octadecatrienoic acid methyl ester | C18:3 | Unsaturated | 0.28 ± 0.02 |
| Eicosanoic acid methyl ester | C20:0 | Saturated | 0.301 ± 0.003 |
Table 4: Fatty acid composition of biodiesel from POG III; results are the average of three replicates ± standard deviation.
Fuel Properties Of Biodiesel
Biodiesel fuel characterization is done by the following parameterscetane number, cloud point, flash point, sulfur contents, density and acid value, pour point, ash contents, kinematics viscosity. The mentioned parameters are discussed below in detail [27].
Flash point
Flash point shows the flammability hazards of the fuel while in the air presence. Flash point is important in a sense that it is useful for storage, handling and safety purposes. Flash point of the prepared biodiesel was 179.8°. It is a higher value than the ordinary petroleum fuels which shows that the prepared biodiesel is less volatile and it is for the storage and transportation purposes.
Kinematic viscosity
The flow rate of fuel is determined by the kinematic viscosity. Kinematic viscosity also controls the atomization of fuel. Viscosity is a factor that hinders the flow of fuel and the engine life is affected by the high levels of viscosity as it causes knocking and poor fuel atomization. Low levels of viscosity in the fuel are good because fuel will be easily propelled and fine droplets are formed. High level of viscosity results in the blockage of fuel injector as the fuel does not burn fully and deposits are formed. Prepared biodiesel has viscosity within the limits.
Sulphur content
Presence of sulphur in the biodiesel should also be known because if the sulphur is more in the diesel its burning will be effecting the environment. In the prepared biodiesel the sulfur content is much less as compared with the standard values hence the biodiesel made from the palm oil is environment friendly.
Cloud point and pour
The temperature at which wax crystal clouds are formed in the diesel fuel when it is cooled is said as cloud point and the temperature at which the fuel flows is called as pour point. Cloud and pour point of the prepared biodiesel are in the range of standards so the diesel can be used both in moderate and high temperature climates.
Density
Limit which is favorable: While designing a fuel injection system density is very important. Higher density causes the greater mass delivery of the fuel and fuel is consumed in a large quantity which is not good. Density of the prepared biodiesel is much less than the given standard values and it is favorable for use.
Characteristics of Biodiesel
Table 5 presents the biodiesel specifications following EN 14214 standard [28-30].
| Properties | Biodiesel from PO | Test methoda | Limit |
|---|---|---|---|
| Ester content/(mol mol−1) | 94% | EN 14103 | min 96.5% |
| Monoacylglycerols content/(mol mol−1) | 0.06% | EN 14105 | max 0.80% |
| Diacylglycerols content/(mol mol−1) | 0.03% | EN 14105 | max 0.20% |
| Triacylglycerols content/(mol mol−1) | <0.02% | EN 14105 | max 0.20% |
| Free glycerol content/(mol mol−1) | <0.02% | EN 14105 | max 0.02% |
| Total glycerol content/(mol mol−1) | 0.05% | EN 14105 | max 0.25% |
| Water content/(mg kg−1) | 412 | EN ISO 12937 | max 500 |
| K content/(mg kg−1) | max 1 | EN 14108 | max 5.0 |
| P content/(mg kg−1) | max 7.3 | EN 14107 | max 10.0 |
| Density (15°C)/(kg m−3) | 858.32 | EN ISO 3675 | 860–900 |
| Flash-point/°C | 179.8 | EN ISO 3679 | min 120 |
| Cloud-point/°C | 14.4 | – | – |
| Sulphated ash/(mol mol−1) | <0.004% | ISO 3987 | max 0.02% |
| Total contamination/(mg kg−1) | 0.0075 | EN 12662 | max 24 |
| Acid value/(mg g−1) | 0.07 | EN 14104 | max 0.50 |
| Iodine value/(g g−1) | 60.20% | EN 14111 | max 120% |
| Copper-strip corrosion (3 h at 50°C) | Class 1 | EN ISO 2160 | Class 1 rating |
Table 5: Specifications of biodiesel.
Conclusion
The findings achieved in this work showed that the biodiesel produced from the Palm Oil Grade III (POG-III)met the European standard specifications but the ester content of the treated Palm Oil Grade III (POG-III) was slightly lower than the international standard limit. This can be rectified by optimising the transesterification reaction. The phosphorus value represents the phosphatide (gum) content in the fuel and it was found at a very low concentration. The flash-point and copper-strip corrosion tests were found to be highly suitable as operational safety properties for biodiesel compared with petroleum diesel. In general, the biodiesel produced achieved favourable scores with reference to the EN 14214 standard [28].
The results of this study revealed that palm oil grade III (POG-III) has the potential for use as a renewable and industrial feedstock for biodiesel production (Figure 6).
References
- Utlu Z, Kocak MS (2008) The effect of biodiesel fuel obtained from waste frying oil on direct injection diesel engine performance and exhaust emissions. Renew Energy 33: 1936-1941.
- Demirbas A (2002) Diesel fuel from vegetable oil via transesterification and soap pyrolysis. Energy Sources 24: 835-841.
- Fuentes MJ, Font R, Gómez-Rico MF, MartÃn-Gullón I (2007) Pyrolysis and combustion of waste lubricant oil from diesel cars: Decomposition and pollutants. J Anal Appl Pyrol 79: 215-226.
- Ampaitepin S, Tetsuo T (2010) The waste-to-energy framework for integrated multi-waste utilization: Waste cooking oil, waste lubricating oil, and waste plastics. Energy 35: 2544-2551.
- El-Fadel M, Khoury (2001) Strategies for vehicle waste-oil management: a case study. Resour Conserv and Recycling 33: 75-91.
- Bhaskar N, Hosokawa M, Miyashita K (2004) Growth inhibition of human pro-myelocytic leukemia (HL-60) cells by lipid extracts of marine alga Sargassummarginatum (Fucales, Phaeophyta) harvested off Goa (west coast of India) with special reference to fatty acid composition. Indian J Mar Sci 33: 355-360.
- NerÃn C, Asensio E, Fernández C, Batlle R (2000) Supercritical fluid extraction of additives and degradation products from both virgin and recycled PET. Quim Anal 19: 205-212.
- Sharaf J, Mishra B, Sharma RB (2013) Production of gasoline-like fuel obtained from waste lubrication oil and its physicochemical properties. Int J Eng Res Appl 3: 113-118.
- Shukla VK, Singh RP, Pandey AC (2010) Black pepper assisted biomimetic synthesis of silver nanoparticles. J Alloy Compd 507: 13-16.
- Hernandez-MartÃn E, Otero C (2008) Different enzyme requirements for the synthesis of biodiesel: Novozym-435 and Lipozyme TL IM. Bioresour Technol 99: 277-286.
- Jitputti J, Kitiyanan B, Rangsunvigit P, Bunyakiat K, Attanatho L, et al. (2006) Transesterification of crude palm kernel oil and crude coconut oil by different solid catalysts. Chem Eng J 116: 61-66.
- Arzamendi G, Campo I, Arguinarena E, Sanchez M, Montes M, et al. (2007) Synthesis of biodiesel with heterogeneous NaOH/alumina catalysts: Comparison with homogeneous NaOH. Chem Eng J 134: 123-130
- Abreu FR, Lima DG, Hamu EH, Wolf C, Suarez PAZ (2004) Utilization of metal complexes as catalysts in the transesterification of Brazilian vegetable oils with different alcohols. J Mol Catal A Chem 209: 29-33.
- Liu Y, Lotero E, Goodwin J, James G, Xuahua M (2007) Transesterification of poultry fat with methanol using Mg-Al-hydrotalcite derived catalysts. Appl Catal 331: 138-148.
- Cordeiro CS, Arizaga GGC, Ramos LP, Wypych F (2008) A new zinc hydroxide nitrate heterogeneous catalyst for the sterfication of free fatty acids and the transesterfication of vegetable oils. Catal Commun 9: 2140-2143.
- Leclercq E, Finiels A, Moreau C (2001) Transesterification of rapeseed oil in the presence of basic zeolites and related solid catalysts. J Am Oil Chem Soc 78: 1161-1165.
- Lopez DE, Goodwin JG, Bruce DA (2007) Transesterification of triacetin with methanol on Nafion acid resins. J Catal 245: 381-391.
- Narasimharao K, Lee A, Wilson K (2007) Catalysts in production of biodiesel: a review. J Biobased Mater Bioenergy 1: 19-30.
- Kiss FE, Jovanovic M, Boskovic GC (2010) Economic and ecological aspects of biodiesel production over homogeneous and heterogeneous catalysts. Fuel Process Technol 91: 1316-1320.
- Xia X, Jin R, He Y, Deng J, Li H (2000) Surface properties and catalytic behaviors of WO3/SiO2 in selective oxidation of cyclopentene to glutaraldehyde. Appl Surf Sci 165: 255-259.
- Mondala A, Liang K, Toghiani H, Hernandez R, French T (2009) Biodiesel production by in situ transesterification ofmunicipal primary and secondary sludges. Bioresour Technol 100: 1203-1210.
- Watkins RS, Lee AF, Wilson K (2004) Li-CaOcatalysed tri-glyceride transesterification for biodiesel applications. Green Chem 6: 335-340.
- Hanh HD, Dong NT, Okitsu K, Nishimura R, Maeda Y (2009) Biodiesel production through transesterification of triolein with various alcoholsin an ultrasonic field. Renew Energy 34: 766-768.
- Jayed MH, Masjuki HH, Saidur R, Kalam MA, Jahirul MI (2009) Environmental aspects and challenges of oilseed produced biodiesel in Southeast Asia. Renew Sustain Energy 13: 2452-2462.
- Bako T, Umogbai VI, Obetta SE (2014) Extraction and characterization of mackery (Scomberscombrus) oil for industrial use. Researcher 6: 80-85.
- Sing K (2001) The use of nitrogen adsorption for the characterisation of porous materials. Physicochem Eng Aspects.
- Naz A, Ullah M, Danish S, Mahmood T, Siddiq M (2014) Nano Catalytic Conversion of used Lubricating Oil into Diesel Fuel. IJERSTE 3: 438-446.
- Demirbas A (2009) Progress and recent trends in biodiesel fuels. Energ Convers Manage 50: 14-34.
- Hayyan A, Alam MdZ, Mirghani MES, Kabbashi NA, Hakimi NINM (2010) Sludge palm oil as a renewable raw material for biodiesel production by two-step processes. Bioresour Technol 101: 7804-7811.
Citation: Mahmood T, Malik M, Bano A, Umer J, Shaheen A (2017) Nanocatalytic Conversion of Waste Palm Oil Grade III and Poplar Plant’s Wood Sawdust into Fuel. Innov Ener Res 6: 170. DOI: 10.4172/2576-1463.1000170
Copyright: ©2017 Mahmood T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2906
- [From(publication date): 0-2017 - Jul 01, 2025]
- Breakdown by view type
- HTML page views: 2061
- PDF downloads: 845