Neurocysticercosis and Epilepsy: A Review
Received: 10-Sep-2018 / Accepted Date: 30-Oct-2018 / Published Date: 07-Nov-2018 DOI: 10.4172/2314-7326.1000280
Abstract
Neurocysticercosis is an infection of the central nervous system caused by the larval form of the tapeworm, Taenia solium. It is an endemic disease, especially in developing countries of sub-Saharan Africa, Asia and Latin America. The flow of travelers and immigrants from these places to the developed countries has contributed to increase the prevalence also in Europe and North America. It is known that in addition to genetic and structural causes, infectious and inflammatory causes can also be common causes of epilepsy. Some studies points neurocysticercosis as one of the leading causes of epilepsy in the world. The objective of this review is to highlight the main epidemiological, pathophysiological and clinical aspects that correlate neurocysticercosis with epilepsy.
Keywords: Epilepsy; Neglected diseases; Neurocysticercosis
Introduction
Neurocysticercosis (NCC) is the most common parasitic disease of the central nervous system (CNS), caused by the tapeworm Taenia solium. This worm can cause two types of diseases: Taeniasis and Cysticercosis. The life cycle of this tapeworm begins either with the ingestion of eggs found in the feces of humans contaminated by the worm or through the consumption of undercooked pork containing the larval form of the worm (Cysticerci).
1) If the larvae are ingested, they adhere to the gastrointestinal tract and their maturation occurs for the adult egg-producing form, the tapeworm. In this case the human develops the disease that we call taeniasis and is considered the definitive host of the worm.
2) To development NCC is necessary the ingestion of T. solium eggs, which transforms in the intestine in its larval form, the oncospheres. These hatch in the intestine and then penetrate its wall and disseminate in the bloodstream, spreading through various tissues, with a strong tropism for the CNS. When settled in the tissues, the larva acquires a cystic form denominated cysticerci. Depending on immunological mechanisms of the host, the cysticerci initiates a transitional degenerative form. This form passes through several stages, which are divided into: vesicular stage (without inflammatory signs), colloidal stage, granular stage and calcified nodules [1,2].
NCC affects men and women at any age, being more common in the 20-50 age groups [1]. The clinical manifestations of the disease are variable and depend on the site and number of lesions:
1) Parenchymal form: More frequent the occurrence of seizures, but other manifestations such as headache, cognitive disorders and stroke are also reported.
2) Intraventricular or Subarachnoid: There is a high risk of increased intracranial pressure, with high mortality rates [2,3].
NCC related seizures may be considered either as acute symptomatic seizures or unprovoked seizures. The International League Against Epilepsy (ILAE) definition for epilepsy states: “Two or more epileptic seizures, unprovoked, with a 24-hour interval between them.” Therefore, to define NCC related epilepsy, symptomatic seizures should not be considered [2,4].
Epilepsy has as its main triggering factors genetic and acquired causes, among which brain lesions, stroke, prolonged seizures and infections are the main causes. Considering infectious causes, viral meningitis and parasitic infections are the main one, being neurocysticercosis the most known cause of post infectious epilepsy, especially in Asian and African countries [2].
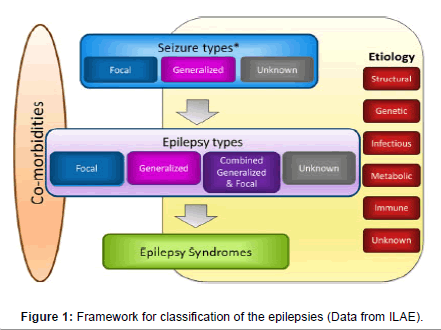
In 2017, the ILAE proposed a new classification of epilepsies, subdividing it into three groups: 1. Seizures type; 2. Epilepsy type; 3. Epilepsy syndromes. The classification incorporates the etiology in each of these stages, emphasizing the need of the etiological definition for a better therapeutic proposal. In Figure 1, we can observe the etiological categories defined by ILAE [4].
Literature Review
This work aims to bring information through the review of recent academic articles on the main aspects of epilepsy related neurocysticercosis, considering the new classification of epilepsy and the new diagnostic criteria of NCC.
We researched on academic articles using the PubMed database (https://www.ncbi.nlm.nih.gov/pubmed/), managed by the US National Institute of Health (NIH). In the research, we opted for selection of recent review articles, with date publication between 2003 to 2017.
Neurocysticercosis and epilepsy
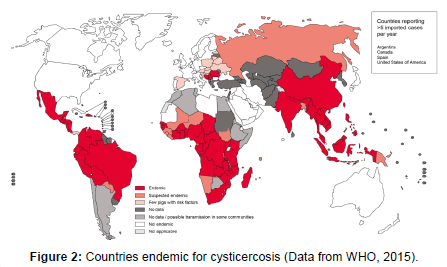
Epidemiology: NCC is considered an endemic disease in some regions of the world, especially in places with basic sanitation problems and exposure of the population to pigs and their feces. Figure 2 illustrates the major risk countries for NCC, according to a recent World Health Organization (WHO) report.
In Brazil, epidemiological data come mainly from regional studies, with little data available nationally. In a literary review, Agapejev et al. estimated, using epidemiological studies, an incidence of cysticercosis of 0.68-6.2% (Md=2.3%), being more frequent in psychiatric hospitals (3.8% to 5%). The national prevalence coefficients are very underestimated, although studies pointed a prevalence of 72: 100,000 and 96: 100,000/inhabitants in country cities of São Paulo State [5,6].
In other countries of the world, prevalence rates are variable, including regional variations such as India, whose Southeast region has an estimated prevalence of 1.28 per 1000 inhabitants, while in the Northeast the prevalence is 3.48 for every 1000 population. Another study in Cameroon showed a prevalence of 2.6 for every 1000 inhabitants. These values contrast with the prevalence in developed countries such as the United States, whose estimated prevalence is 0.2 to 0.6 per 100,000 inhabitants in the general population [7,8].
One of the main difficulties regarding the studies on this subject, refers to the diagnostic criteria of the NCC, since it is based mainly on imaging studies such as computed tomography (CT) and magnetic resonance imaging (MRI), which have limited access in many regions where the disease is endemic. In addition, imaging exams also have their limitations, CT, for example, does not have a good sensitivity for the detection of posterior fossa lesions [2].
Serological tests may also be used for the diagnosis of NCC. Enzyme- Linked Immuno-Electrotranfer Blot (EITB) has a high sensitivity for T. sollium infection, but not exclusive to CNS lesions, which becomes problematic for the diagnosis of NCC. ELISA, which can also be used to detect antibodies against T. sollium, does not have a good sensitivity and specificity profile. Thus, serological tests have a high negative predictive value, but low specificity for the detection of NCC.
Aiming for a better diagnostic standardization, recently Del Brutto in 2017 reviewed the diagnostic criteria of NCC. The classification includes the definition of definitive or probable diagnosis. With a standardization of the diagnosis, the interpretation of research done regarding the theme becomes easier, avoiding biases found in many works, mainly correlating NCC and epilepsy [9,10].
The correlation between NCC and epilepsy has been widely reported [11]. Despite many limitations in available data, a recent metaanalysis by Gripper et al. that considered imaging diagnostic criteria of NCC and clear definition of epilepsy based on guidelines, has estimated an important association between NCC and epilepsy with an odds ratio (OR) of 2.76 (95% CI: 2.19-3.48). Such data translates an increased risk of about 3 times for development of epilepsy in individuals with NCC [7].
When the proportion of NCC cases in epileptic populations was analyzed, a proportion of 31.54% was estimated. These data are in agreement with the literature. In a study carried out in Latin America, using neurological evaluation and video electroencephalogram, NCC was considered the most frequent isolated cause of epilepsy in such areas, and was responsible for more than half of the symptomatic epileptic seizures. In fact, NCC and the presence of perinatal brain lesions appear to be the main conditions associated with a higher incidence of epilepsy in developing countries [1,7].
Physiopathology
The pathophysiological process associated with NCC is largely related to the host’s immune response [2]. In the stage of viable cysts in the parenchyma there is only little or no inflammatory process in the tissues around the lesion, and such stage may remain so for many years. When cysts begin to degenerate, as a result of antigenic exposure and onset of immune-mediated response, involving a balanced response between T helper cells: Th1 and Th2, an intermediate phase of involution of the cyst begins. It is called colloidal and granulomatous stage. At the end there is the formation of a calcified nodule [2,12].
The exact mechanisms related to NCC epileptogenesis have not yet been fully elucidated, although several mechanisms are proposed. In the phases of viable cysts, we can have the induction of reactive crises because of expansive effects in the parenchyma or transient episodes of inflammation. In the colloidal and granulomatous phases, there is also induction of reactive crises related to the inflammatory process generated by the host immune response to the parasite. On the other hand, in the calcified lesions there is a description of the occurrence of perilesional gliosis as well as of late exposure to antigens causing inflammation and edema, this process can cause reactive crises, but in the long term can generate permanent lesions, local or the distance, that become, therefore, epileptogenic foci [3,13,14].
Some case-control studies suggest that there are high rates of calcified cysticerci in epilepsy compared to other stages of cyst evolution. These findings are in agreement with this theory that suggests that the main causes of epilepsy are due to the inflammatory response that occurs in the cyst’s degeneration.
Recently, evidence has also associated the presence of hippocampal sclerosis (EC) in patients with calcified NCC lesions. Publications from a Brazilian center revealed a prevalence of NCC in 35% of patients with temporal lobe epilepsy with hippocampal sclerosis who underwent surgical procedure for drug-resistant epilepsy [15]. In addition, other studies at population levels reported an association between NCC and hippocampal sclerosis. Also, it was reported a better clinical outcome after the resection of both calcified lesion and hippocampus rather than the hippocampus alone. Early precipitating lesions such as traumatic brain injury, neonatal hypoxia, and other infectious causes have been documented as an indirect cause of temporal lobe epilepsy, with damage to the hippocampus and its subsequent sclerosis, and NCC is believed to act in a similar way [7]. The pathophysiological mechanisms proposed for this association are the direct presence of the cysticercus in the hippocampus with consequent inflammatory immune-mediated process or an indirect mechanism of synaptic damage and reorganization in the hippocampus region generated by repeated symptomatic epileptic seizures [7].
Clinical presentation
The clinical presentations of NCC vary according to the location and number of lesions. We can divide the lesions topographically in parenchymal and extra-parenchymal, being the last subdivided into intraventricular, subarachnoid and spinal cord.
Parenchymal lesions have as main manifestation epileptic seizures. In some massive infections with an exacerbated immune response of the host, we may have encephalitis and increased intracranial pressure. Such condition is more frequent in children and young women and is characterized by altered level of consciousness, epileptic seizures, decreased visual acuity, headache, vomiting and papilledema. Psychiatric changes ranging from mild impairment of cognitive function to dementia were also reported in association with NCC [3].
The frequency of types of epileptic seizures in patients with NCC are mostly based on patient reports. Among the few studies with documentation of seizures using video electroencephalography, a frequency of focal seizures with no loss of awareness was reported in 12% of the cases, focal seizures with loss of awareness in 14%, focal seizures with bilateral involvement in 66% and seizures with generalized onset in 6% of cases [1].
Other data from a prospective cohort study, focal not evolving to bilateral tonic clonic seizures were the most common, accounting for 90-95% of patients. Such statistical difference between the analyzes may result from previous therapy with anticonvulsive drugs and treatment with antiparasitic drugs for the viable cysts [1].
When the crisis semiology is taken into account, motor crises are considered the most frequent, accounting for 60-90% of focal onset crises. Crises with somatosensory and visual symptoms are also common although there is a great statistical variation between the studies.
In an Indian study, Mehta et al. performed an electroencephalographic study in patients with epilepsy related to cysticercosis and described the patients’ seizures profile. Among the patients studied with focal seizures, the most frequent were the clonic seizures. Among the generalized seizures, tonic-clonic seizures were the most common. Focal onset with impaired awareness represented only 2% of the cases. Status epilepticus was reported as the onset of adult seizures in only 2% of cases.
However, it is important the knowledge that seizures can change either with the natural evolution of the disease or with the administration of antiepileptic drugs and/or anti-helmitic drugs. In extraparenchymal forms, the main manifestations consist of hydrocephalus due to mechanical or non-communicating obstruction, headache, visual changes and cranial nerve deficits. Spinal cord manifestations range from spinal arachnoiditis with radicular pain and subacute motor and/or sensory deficits with level depending on the presence of cysts in the medullary parenchyma.
Image aspects
Central nervous system images are essential in the diagnosis and follow-up of NCC. MRI is superior to CT in the visualization of cerebral structures and cystic formations, although CT is superior in the detection of calcifications [16]. Vesicular cysts are visualized on CT and MRI as small cystic lesions rounded and well delimited in the parenchyma, without edema or contrast uptake. In T2-weighted sequence on MRI, the cystic content has a hyperintense signal similar to that of CSF. In some of the lesions it is possible to see a hyperdense nodule corresponding to the scolex [1,3].
In the colloidal and granulomatous phase, the fluid content becomes dense and the walls of the cyst enhance the contrast, around the lesion we have the presence of edema. In calcified lesions, better visualized on CT, we have the presence of hyperdense lesions, with a solid aspect, usually with a diameter of less than 10 mm, with a random distribution through the parenchyma. Sometimes such lesions may present perilesional edema [17]. In patients with subarachnoid presentation the most common imaging findings are hydrocephalus related to inflammation and occlusion of the foramina of Luschka and Magendie [3].
NCC lesions may be similar to lesions of tumors or other central nervous system infections and, in such cases, advances in MR protocols are useful in differentiating lesions. The DWI (diffusion-weighted imaging) sequence, for example, differentiates NCC lesions from brain abscesses; since cystic lesions show no signal restriction and their contents have signal intensity like CSF [1]. Other techniques such as resonance with spectroscopy are also useful in differentiating lesions, especially tumors. Elevated levels of lactate, succinate and alanine are identified in the cystic lesions of NCC, whereas in their degenerative forms we have high peaks of N-acetylaspartate (NAA) and creatine. Tuberculoma, an important differential diagnostic of NCC, shows a high peak of lipids, differing the pattern of degenerating NCC lesions.
Seizures evolution after anti-parasitary therapy
The first descriptions of the use of antiparasitic drugs in the treatment of neurocysticercosis were made in 1979 with the introduction of praziquantel and later in 1987 with the use of albendazole. Thereafter, the use of antiparasitic drugs for the treatment of NCC has been subject of intense debate [18].
The first descriptions of the use of antiparasitic drugs in the treatment of neurocysticercosis were made in 1979 with the introduction of praziquantel and later in 1987 with the use of albendazole. Thereafter, the use of antiparasitic drugs for the treatment of NCC has been subject of intense debate [18].
Overall, recommendations for the treatment of NCC vary according to clinical topography and number of lesions. Thus, we have, for the parenchymal form, the following recommendations [20]:
Viable cysts
Mild (1 to 5 cysts): May opt for antiparasitic treatment associated with corticosteroids; antiparasitic treatment alone with corticosteroids association only side effects; or no treatment and neuroimaging follow-up.
Moderate (More than 5 cysts) and severe (More than 100 cysts): It is recommended treatment with antiparasitic associated with high doses of corticosteroids, or long-term corticosteroids therapy and neuroimaging follow-up.
Degenerating cysts
Mild or Moderate: May opt for no treatment and neuroimaging follow-up; antiparasitic and corticoid treatment; or antiparasitic alone and corticoid treatment only if side effects.
Severe: No anti-parasitic treatment; high doses of corticoid and osmotic diuretic.
Calcified lesion: Independent of number of lesions not recommended anti-parasitic treatment.
Discussion
Another controversy on the subject is related to the benefits of antiparasitic treatment in the control of epilepsy. In his double-blind, randomized study, Garcia et al. showed a reduction in the frequency of generalized seizures, but not in all types of seizures, in the group treated with albendazole compared to the placebo group. Another recent Cochrane review of 21 randomized controlled trials of anthelmintic treatment concluded that the use of albendazole in adults with viable cysts was associated with a reduction in the number of cysts but not a decrease in the recurrence of epileptic seizures.
One of the main criticisms regarding studies about antiparasitic treatment in the parenchymal forms is the lake of definition of seizures control. Rather than a comparison of total reduction in the number of seizures in the control and placebo groups, the comparison between patients who became seizure free could be a more specific and reliable parameter for a comparative analysis of treatment effectiveness. Another criticism is that most patients are on antiepileptic drug use, but there is no homogenization in relation to drugs and monitoring of the serum level. Finally, studies evaluating the outcome of seizures generally have a short duration. A long-term evaluation could provide a better response to the prognosis of recurrence of seizures [18,21].
Conclusion
Although it is a subject of growing interest in the world literature, there are still many limitations in research on the association of NCC and epilepsy. With the new definitions of Epilepsy by the ILAE and the recent review of the diagnostic criteria for NCC by Del Brutto in 2017, further studies that take into account such changes are also needed. As we can see from the review carried out, many topics of the NCC and epilepsy relationship still need to be better addressed, especially regarding the treatment of NCC and outcome involving the clinical response to the number and pattern of seizures, improvement of non-epileptic symptoms (headache, cognition), changes in the pattern of brain image, reduction of antiepileptic medication used in better control of seizures and absence of response to anti-parasitic treatment.
References
- Duque KR, Burneo JG (2017) Clinical presentation of neurocysticercosis-related epilepsy. Epilepsy & Behav 76: 151-157.
- Reddy DS, Volkmer R (2017) Neurocysticercosis as an infectious acquired epilepsy worldwide. Seizure 52: 176-181.
- Del Brutto H (2012) Neurocysticercosis: A review. The Scientific World Journal 2012: 1-8.
- Fisher R, Cross JH, Jacqueline AF, Norimichi H, Edouard H, et al. (2017) Operacional classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 58: 522-530.
- Agapejev S (2003) Aspectos clÃnico-epidemiológicos da neurocisticercose no Brasil. Arq Neuropsiquiatr 61: 822-828.
- Carpio A, Romo M (2014) The relationship between neurocysticercosis and epilepsy: An endless debate. Arq Neuropsiquiatr 72: 383-390.
- Gripper LB, Welburn SC (2017) The causal relationship between neurocysticercosis infection and the development of epilepsy - A systematic review. Infectious Diseases of Poverty 6: 31.
- Tellez-Zenteno JF, Hernandez-Ronquillo L (2017) Epidemiology of neurocysticercosis and epilepsy, is everything described? Epilepsy Behav 76: 146-150.
- Del Brutto H (2013) Neurocysticercosis: New thoughts on controversial issues. Curr Opin Neurol 26: 289-294.
- Del Brutto H, Nash T, White AC, Rajshekhar V, Wilkins PP, et al. (2017) Revised diagnostic criteria for neurocysticercosis. Journal of the Neurological Sciences 372: 202-210.
- Burneo JG (2017) Neurocysticercosis-related epilepsy. Epilepsy Behav 76: 145.
- Nash TE, Manhanty S (2015) Neurocysticercosis: A natural human model of epileptogenesis. Epilepsia 56: 177-183.
- Garcia HH, Del Brutto OH (2017) Antiparasitic treatment of neurocysticercosis -The effect of cyst destruction in seizure evolution. Epilepsy Behav 76: 158-162.
- Singh AK, Garg R (2017) Clinical and neuroimaging predictors of seizure recurrence in solitary calcified neurocysticercosis: A prospective observational study. Epilepsy Research 137: 78-83.
- Del Brutto H, Engel J, Dawn SE, Hector HG (2015) Update on cysticercosis epileptogenesis: The role of the hippocampus. Curr Neurol Neurosci Rep 16: 1.
- Melo-Souza SE (2013) Neurocisticercose In: Tratamento das doenças neurologicas. (3rd edn). Rio de Janeiro: Guanabara, Koogan.
- Leon A, Saito E (2015) Calcified parenchymal central nervous system cysticercosis and clinical outcomes in epilepsy. Epilepsy & Behavior 43: 77-80.
- Singh G, Sharma R (2017) Controversies in the treatment of seizures associated with neurocysticercosis. Epilepsy Behav 76: 163-167.
- Mahale R, Mehta A, Rangasetty S (2015) Extraparenchymal (Racemose) neurocysticercosis and its multitude manifestations: A comprehensive review. J Clini Neurol 11: 203-211.
- Nash TE, Garcia HH (2011) Diagnosis and treatment of neurocysticercosis. Nat Rev Neurol 7: 584-594.
- Bustos J, GarcÃa H, Del Brutto H (2016) Antiepileptic drug therapy and recommendations for withdrawal in patients with seizures and epilepsy due to neurocysticercosis. Expert Review of Neurotherapeutics 16: 1079-1085.
Citation: Machado LMS, De Oliveira FTM, De Oliveira ACP (2018) Neurocysticercosis and Epilepsy: A Review J Neuroinfect Dis 9: 280. DOI: 10.4172/2314-7326.1000280
Copyright: © 2018 Machado LMS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4572
- [From(publication date): 0-2018 - Dec 09, 2025]
- Breakdown by view type
- HTML page views: 3687
- PDF downloads: 885