Neuroinflammation and Aging: Significance of Declining Circadian Functions and Melatonin
Received: 12-Apr-2018 / Accepted Date: 19-Sep-2018 / Published Date: 28-Sep-2018 DOI: 10.4172/2168-9652.1000243
Keywords: Aging; Circadian; Inflammaging; Melatonin;Neuroinflammation; Sirtuin 1
Introduction
Aging is a highly complex and multifaceted process with considerable inter-individual differences concerning the decay velocities of the various physiological functions. Importantly, aging is much more than just natural wear and tear, although such alterations may contribute to the age-related decline and accelerate functional deterioration. Numerous detrimental and often interrelated cell biological, biochemical and physiological changes are known that impair the health state and can limit lifespan, such as DNA damage, mitochondrial dysfunction, high rates of apoptosis and other forms of cell death, telomere attrition, decreased hormone secretion and immuno-senescence. This complexity indicates a necessity for identifying connections between these processes and, especially, to seek for orchestrating mechanisms and regulators that decline by age. The circadian multioscillator system and melatonin, which is part of the former, display such properties. In the youthful organism, they control countless cellular and physiological functions, which gradually lose rhythmicity and efficacy by age [1-3].
Inflammatory responses play a substantial part in aging processes and strongly contribute to the deteriorations resulting thereof. This connection has been given rise to coining the term of inflammaging [4,5]. Inflammaging has not only to be seen as an aspect of immunosenescence, but its relevance also concerns the inflammation-related oxidative and nitrosative/nitrative stress with its numerous effects of damage to DNA and mitochondria [3-6]. Moreover, the enhanced formation and release of reactive oxygen species and nitric oxide initiate vicious cycles based on the mutual communication between neurons, astrocytes and microglia [3,6,7]. Another specific aspect of inflammaging concerns the Senescence-Associated Secretory Phenotype (SASP), which is apparent in mitotically arrested DNA-damaged cells that release proinflammatory cytokines and chemokines [6,8-10]. Notably, the affected cells are not immune cells in the strict sense, but turn into immunologically relevant players, which attract leukocytes, especially macrophages, and cause persistent local inflammation. In the brain, SASP was observed as a property of DNA-damaged astrocytes, which develop neurotoxic properties and stimulate microglia [11,12]. An additional, recently discovered aspect of inflammaging concerns its relation to the so-called garb-aging, which consists in the contribution of inflammatory responses to altered molecules produced by damaged or dying cells that have become unable to efficiently eliminate them by proteasomal and autophagic processes [13].
Relevance of Low-Grade Neuroinflammation and Neuroinflammaging
The processes of inflammaging, which seem to be involved in the majority of age-related diseases [14] are of particular significance to the brain, because low-grade neuroinflammation strongly contributes to aging and is additionally involved in various neurodegenerative pathologies [2,6,15]. Moreover, detrimental alterations in the central nervous system have countless consequences for peripheral organs, too. Neurodegenerative changes also affect the circadian system, thereby cause sleep disturbances, which, in turn, promote proinflammatory responses [7,16,17]. This role of neuro-inflammation is not only relevant to disease progression, but, importantly, also to disease onset. In Alzheimer’s Disease (AD), inflammaging has been shown to be prodromal to later, more severe manifestations of this pathology [18]. This conclusion conforms with other findings that have identified brain insulin resistance as an early pro-inflammatory change of AD [6,19-23]. Amyloid-β peptides and oligomers further contribute to the inflammatory processes by activating microglia [24,25] and causing neurons to release pro-inflammatory cytokines and chemokines [26].
With regard to the detrimental and aging-promoting actions of lowgrade brain inflammation, counteracting mechanisms and factors are of particular interest. Without wanting to oversimplify the mechanistic networks and options of interventions, this short article shall only focus on two ubiquitously acting regulators, melatonin and Sirtuin 1 (SIRT1). Moreover, these two factors cannot be discussed in a meaningful way without appropriately considering their relationships to the circadian oscillator system [27].
Melatonin in the Brain
Melatonin is usually known as the hormone of the pineal gland, which is, however, only the main source of circulating melatonin, but not the main site of overall synthesis, since quantities in extrapineal sources exceed by orders of magnitude those in the pineal gland [1,17]. From the pineal gland, melatonin is released both into the circulation and, via the pineal recess, into the third ventricle of the brain [28-30]. As melatonin is synthesized and released by the pineal gland preferentially at night, the chronobiological information of high melatonin is delivered via the circulation primarily to the peripheral tissues. Although melatonin can also cross the blood-brain barrier and is taken up via the choroid plexus, the direct release into the third ventricle has been recently judged to be more important with regard to the influence on the hypothalamic circadian master clock, the Suprachiasmatic Nucleus (SCN) [29,30]. Additional routes of melatonin delivery to the brain are possible via the aqueduct of the midbrain into the fourth ventricle, from there via the medial foramen of Magendie and the two lateral foramina of Luschka to the subarachnoid space [29]. However, in quantitative terms, highest concentrations are found in the third ventricle, from where the adjacent SCN pair is easily reached [29,30]. Moreover, melatonin is formed in some parts of the central nervous system [1,31]. A recent study demonstrated enhanced melatonin synthesis in response to inflammation in the cerebellum, however, without substantial release to other parts of the brain [32].
In mammals, melatonin is mutually interconnected to the SCN, in terms of being both an output and an input factor of the master clock [33]. The light/dark information that the SCN receives from melanopsincontaining retinal ganglion cells is transmitted via a neuronal pathway to the pineal gland, where melatonin synthesis is mainly stimulated by norepinephrine from postganglionic sympathetic fibers, with some modulation by other neuronal connections [34]. On the other hand, the SCN receives information from melatonin by virtue of a high density of melatonin receptors present in this place [1]. Melatonin can phase shift circadian rhythms generated in the SCN [1,35], but there is additional evidence that it also influences semi-autonomous and almost autonomous peripheral and other central oscillators [27,36]. Although the mammalian pineal gland also harbors an endogenous clock [37], this is sensitive to the input by norepinephrine, and melatonin synthesis strongly declines when this input is reduced. Therefore, a functional weakening of the SCN, e.g., by reduced light transmission or by neurodegeneration, and likewise by degenerative impairments of the neural transduction pathway to the pineal gland, also lead to a flattening of the melatonin rhythm in the pineal gland, in the circulation and, expectably, in the third ventricle [2,27]. In fact, aging is typically associated with functional losses of the circadian system. This concerns the rhythm amplitudes in both the SCN [38] and numerous peripheral clocks [39]. In some oscillators, amplitudes are reduced, in others shifted and, thus, more poorly coupled, whereas some are only moderately affected. In other peripheral clocks, overt rhythmicity appears to be completely lost, but can be reactivated by appropriate stimuli [39]. Reductions of nocturnal melatonin levels are typically observed during aging, but also occur in numerous diseases and disorders of different etiologies [2,40]. The decrease in pineal and circulating melatonin levels is particularly obvious in neurodegenerative diseases and, in these cases, clearly associated with SCN dysfunction. In AD, melatonin levels are not only reduced, but the remaining small maxima also dysphased and temporally strongly scattered [41]. In post-mortem pineals of AD patients, the melatonin rhythm seemed to be completely lost, whereas this rhythmicity was clearly preserved in age-matched controls [42]. As a consequence, age- or diseaserelated reductions of melatonin signify the loss of an important orchestrating regulator molecule that displays numerous beneficial actions. These concern antioxidant, antiexcitatory, anti-inflammatory, and antifibrillogenic effects [1-3,6,27,43-46], in addition to the losses in coordinative functions within the circadian system [1]. With regard to neuroinflammation, the antiexcitatory, mitochondria-protective and activation of microglia suppressing effects are of particular importance. Moreover, recent findings concerning an increase of α-secretase activity in cells overexpressing human β-Amyloid Precursor Protein (βAPP) to generate the non-amyloidogenic and neuroprotective fragment sAPPα [47] and the inhibition of the amyloidogenic β- and γ-secretases [48] indicate additional neuroprotective properties. Generally, neuroprotection belongs to the most amply documented actions of melatonin, which have been studied under various conditions and multiply reviewed, e.g., in refs. [1,6,31,43,44,49-53].
Relationship of Melatonin to Sirtuin 1 and Consequences to Circadian Amplitudes
Investigators have mostly regarded the beneficial effects of melatonin from a non-dynamic point of view, which would, however, be important with regard to its role in the circadian system. Circadian rhythmicity itself contributes to protection against damage by free radicals and mitochondrial malfunction [54]. Nevertheless, melatonin can act both directly on cellular processes that are susceptible to melatonergic signaling and indirectly via modulation of circadian oscillators [36]. Changes in the expression of circadian core oscillator components by melatonin have been repeatedly observed [36] and melatonin-deficient mice exhibited flattened, almost undetectable variations of such components, contrary to well-pronounced rhythms in melatonin-proficient strains [55,56]. These findings strongly indicate that melatonin represents an amplitude-enhancing regulator in the circadian system [36].
The modes by which melatonin exerts these amplitude-enhancing effects on oscillators has remained for quite some time rather unclear. Although one of the melatonergic signaling pathways, that of PKCdependent ERK1/2 activation [57], has been shown to be decisive for phase shifting of circadian oscillations [58], this may not yet explain the increases of rhythm amplitudes. Recent data on the relationship between melatonin and SIRT1 may provide a link to this problem. Initially, this connection was largely overlooked, because studies in cancer cells or tissue revealed strong reductions of SIRT1 expression by melatonin. However, melatonin behaves entirely differently in nontumor cells. Especially in the context of aging, melatonin was shown to upregulate SIRT1 expression in various models, as recently summarized [27]. This discrepancy is explained by tumor-cell specific epigenetic silencing of core oscillator components that display tumor suppressor properties, whereas these components undergo normal cycling in non-tumor cells [27].
SIRT1 has been shown to play an important role in circadian oscillators. The profound chronobiological actions of the protein deacetylase SIRT1 were discovered in the group of P. Sassone-Corsi [59- 61]. In brief, SIRT1 was identified as an accessory oscillator component. Basis of its cycling activity is the binding of the core oscillator components BMAL1 and CLOCK to an E-box in the promoter of the nicotinamide phosphoribosyltransferase (Nampt) gene. The resulting cyclicity in NAMPT protein expression leads to a rhythm in NAD+ concentration, which drives the activities of various sirtuins which use NAD+ as a substrate and activator. Notably, rhythmic expression of SIRT1 is not required, because the decisive parameter is SIRT1 activity rather than protein concentration.
An important and, in the beginning, surprising property of SIRT1 is its capability of enhancing circadian oscillation amplitudes. This has been explained in two different ways, (1) By physical interaction of SIRT1 with the BMAL1/CLOCK heterodimer; and (2) By SIRT1- dependent deacetylation of PGC-1α (peroxisome proliferator-activated receptor-γ coactivator-1α), binding of deacetylated PGC-1α to RORα (retinoic acid receptor-related orphan receptor-α), an activator at the ROR response elements in the promoters of the Bmal1 and Clock genes [38]. Regarding these two possibilities, differences may exist between the various cellular oscillators in central and peripheral tissues. An important aspect of aging is the observed senescence-associated decline of SIRT1 expression [17,27,38]. The above-mentioned upregulation of SIRT1 expression by melatonin in the context of aging [27], thus, indicates a mode by which exogenous melatonin might increase circadian amplitudes indirectly by re-initiating enhanced SIRT1 expression. Whether melatonin also upregulates other sirtuin subforms, in particular, the mitochondrially located SIRT3 and the constitutively chromatin-associated SIRT6, would be of great interest, but would still require a broader experimental basis. Both SIRT3 and SIRT6 are driven by the NAD+ cycle and transmit circadian information [62,63], but do not seem to feed back to the core oscillator components.
Conclusion
A concept of jointly increasing melatonin and SIRT1 levels is highly attractive in gerontological terms, especially with regard to neuroinflammation. Melatonin, which is very short-lived in the circulation because of a half-life mostly in the range of 20-30 min, may induce more persistent effects by upregulating SIRT1, which, as a protein, should have a considerably longer half-life. In cultured glomerular mesangial cells, the half-life of SIRT1 was about 8 h [64]. Under certain conditions and in certain cells, this may be shortened by stimuli that enhance SIRT1 ubiquitinylation, followed by proteasomal degradation [64]. Corresponding data in brain tissue would be required for a definite judgment, but the half-life of SIRT1 in neurons will be, with some likelihood, in the range of several hours and, therefore, much longer than that of melatonin. Independently of the rather moderate effects of melatonin on sleep maintenance [2], daily repeated and appropriately timed application of this hormone may improve, via SIRT1, circadian rhythms in elderly patients, as far as the decline in the circadian system has not been caused by irreversible neurodegeneration. Moreover, the antioxidant, mitochondria-protective and anti-inflammatory actions of melatonin might be complemented and enhanced by corresponding actions of SIRT1, which displays beneficial effects in the same fields and may, according to recent data, partially mediate actions by melatonin [65-74] (Figure 1). Anti-inflammatory effects of SIRT1 deserve further attention and extension towards studies on levels of proinflammatory cytokines in the brain would be required, especially concerning TNF-α, IL-2 and IL-6. To date, most pertinent information has been based on the application of powerful proinflammatory agents such as LPS (bacterial lipopolysaccharide) in combination with sirtuin activators and inhibitors, whereas investigations on upregulation of SIRT1 in the otherwise non-compromised aging brain, along with measurements of cytokine levels, are urgently desired. Nevertheless, the already available data on neuroprotection and anti-inflammatory properties of SIRT1 are encouraging [75]. The enhancement of melatonin, SIRT1 activity and, thereby, circadian amplitudes seems to be a worth-while aim for reducing low-grade neuro-inflammation and for improving health and life quality in elderly subjects. As a consequence, the hypothesis should be experimentally examined that, in aging mammals, exogenous melatonin not only elevates the levels of SIRT1, but that this upregulation also increases circadian amplitudes, which may also influence the rhythmicity of endogenous melatonin. With regard to the anti-inflammatory actions of both melatonin and SIRT1 and to the circadian control of several immunological functions, concomitant improvements of the three orchestrating regulators, melatonin, SIRT1 and the circadian system, may reduce aging-related inflammation and enhance physiological functioning.
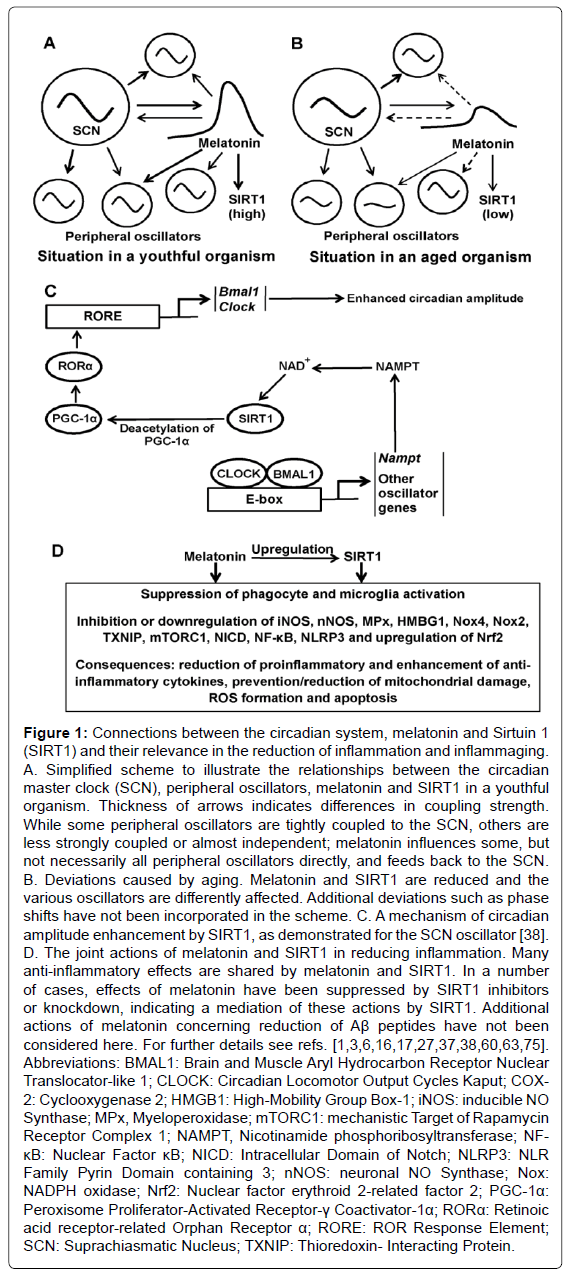
Figure 1: Connections between the circadian system, melatonin and Sirtuin 1 (SIRT1) and their relevance in the reduction of inflammation and inflammaging. A. Simplified scheme to illustrate the relationships between the circadian master clock (SCN), peripheral oscillators, melatonin and SIRT1 in a youthful organism. Thickness of arrows indicates differences in coupling strength. While some peripheral oscillators are tightly coupled to the SCN, others are less strongly coupled or almost independent; melatonin influences some, but not necessarily all peripheral oscillators directly, and feeds back to the SCN. B. Deviations caused by aging. Melatonin and SIRT1 are reduced and the various oscillators are differently affected. Additional deviations such as phase shifts have not been incorporated in the scheme. C. A mechanism of circadian amplitude enhancement by SIRT1, as demonstrated for the SCN oscillator [38]. D. The joint actions of melatonin and SIRT1 in reducing inflammation. Many anti-inflammatory effects are shared by melatonin and SIRT1. In a number of cases, effects of melatonin have been suppressed by SIRT1 inhibitors or knockdown, indicating a mediation of these actions by SIRT1. Additional actions of melatonin concerning reduction of Aβ peptides have not been considered here. For further details see refs. [1,3,6,16,17,27,37,38,60,63,75]. Abbreviations: BMAL1: Brain and Muscle Aryl Hydrocarbon Receptor Nuclear Translocator-like 1; CLOCK: Circadian Locomotor Output Cycles Kaput; COX- 2: Cyclooxygenase 2; HMGB1: High-Mobility Group Box-1; iNOS: inducible NO Synthase; MPx, Myeloperoxidase; mTORC1: mechanistic Target of Rapamycin Receptor Complex 1; NAMPT, Nicotinamide phosphoribosyltransferase; NF- κB: Nuclear Factor κB; NICD: Intracellular Domain of Notch; NLRP3: NLR Family Pyrin Domain containing 3; nNOS: neuronal NO Synthase; Nox: NADPH oxidase; Nrf2: Nuclear factor erythroid 2-related factor 2; PGC-1α: Peroxisome Proliferator-Activated Receptor-γ Coactivator-1α; RORα: Retinoic acid receptor-related Orphan Receptor α; RORE: ROR Response Element; SCN: Suprachiasmatic Nucleus; TXNIP: Thioredoxin- Interacting Protein.
References
- Hardeland R, Cardinali DP, Srinivasan V, Spence DW, Brown GM, et al. (2011) Melatonin – A pleiotropic, orchestrating regulator molecule. Prog Neurobiol 93: 350-384.
- Hardeland R (2012) Melatonin in aging and disease -Multiple consequences of reduced secretion, options and limits of treatment. Aging Dis 3: 194-225.
- Hardeland R (2013) Melatonin and the theories of aging: A critical appraisal of melatonin’s role in antiaging mechanisms. J Pineal Res 55: 325-356.
- Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, et al. (2000) Inflamm-aging. An evolutionary perspective in immunosenescence. Ann NY Acad Sci 908: 244-254.
- Monti D, Ostan R, Borelli V, Castellani G, Franceschi C (2017) Inflammaging and human longevity in the omics era. Mech Ageing Dev 165(Pt B):129-138.
- Hardeland R, Cardinali DP, Brown GM, Pandi-Perumal SR (2015) Melatonin and brain inflammaging. Prog Neurobiol 127-128: 46-63.
- Hardeland R (2018) Circadian disruption, sleep loss, and low-grade inflammation. Res Rev Healthcare Open Access J 1: 000109.
- Coppé JP, Patil CK, Rodier F, Sun Y, Muñoz DP, et al. (2008) Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and p53 tumor suppressor. PloS Biol 6: 2853-2868.
- Young ARJ, Narita M (2009) SASP reflects senescence. EMBO Rep 10: 228-230.
- Coppé JP, Desprez PY, Krtolica A, Campisi J (2010) The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu Rev Pathol 5: 99-118.
- Salminen A, Ojala J, Kaamiranta K, Haapasalo A, Hiltunen M, et al. (2011) Astrocytes in the aging brain express characteristics of senescence-associated secretory phenotype. Eur J Neurosci 34: 3-11.
- Turnquist C, Horikawa I, Foran E, Major EO, Vojtesek B, et al. (2016) p53 isoforms regulate astrocyte-mediated neuroprotection and neurodegeneration. Cell Death Differ 23: 1515-1528.
- Franceschi C, Garagnani P, Vitale G, Capri M, Salvioli S (2017) Inflammaging and 'garb-aging'. Trends Endocrinol Metab 28: 199-212.
- Franceschi C, Campisi J (2014) Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci 69: S4-S9.
- Hardeland R (2016) Melatonin and synthetic melatoninergic agonists in psychiatric and age-associated disorders: Successful and unsuccessful approaches. Curr Pharm Des 22: 1086-1101.
- Brown GM, McIntyre RS, Rosenblat J, Hardeland R (2018) Depressive disorders: Processes leading to neurodegeneration and potential novel treatments. Prog Neuropsychopharmacol Biol Psychiatry. (Pt C): 189-204.
- Hardeland R (2018) Brain inflammaging: Roles of melatonin, circadian clocks and sirtuins. J Clin Cell Immunol 9: 543.
- Giunta B, Fernandez F, Nikolic WV, Obregon D, Rrapo E, et al. (2008) Inflammaging as a prodrome to Alzheimer’s disease. J Neuroinflamm 5: 51.
- Clark IA, Vissel B (2013) Treatment implications of the altered cytokine-insulin axis in neurodegenerative disease. Biochem Pharmacol 86: 862-871.
- De Felice FG, Lourenco MV, Ferreira ST (2014) How does brain insulin resistance develop in Alzheimer’s disease? Alzheimers Dement 10: S26-S32.
- de la Monte SM, Tong M (2014) Brain metabolic dysfunction at the core of Alzheimer’s disease. Biochem Pharmacol 88: 548-559.
- Diehl T, Mullins R, Kapogiannis D (2017) Insulin resistance in Alzheimer's disease. Transl Res 183: 26-40.
- Gasiorowski K, Brokos B, Leszek J, Tarasov VV, Ashraf GM, et al. (2017) Insulin Resistance in Alzheimer disease: p53 and microRNAs as important players. Curr Top Med Chem 17: 1429-1437.
- Tan B, Choi RH, Chin TJ, Kaur C, Ling EA (2012) Manipulation of microglial activity as a therapy for Alzheimer’s disease. Front Biosci 4: 1402-1412.
- McLarnon JG (2014) Correlated inflammatory responses and neurodegeneration in peptide-injected animal models of Alzheimer’s disease. Biomed Res Int: 923670.
- Hanzel CE, Pichet-Binette A, Pomentel LS, Iulita MF, Allard S, et al. (2014) Neuronal driven pre-plaque inflammation in a transgenic rat model of Alzheimer’s disease. Neurobiol Aging 35: 2249-2262.
- Hardeland R (2017) Melatonin and the pathologies of weakened or dysregulated circadian oscillators. J Pineal Res 62: e12377.
- Tricoire H, Locatelli A, Chemineau P, Malpaux B (2002) Melatonin enters the cerebrospinal fluid through the pineal recess. Endocrinology 143: 84-90.
- Reiter RJ, Tan DX, Kim SJ, Cruz MH (2014) Delivery of pineal melatonin to the brain and SCN: Role of canaliculi, cerebrospinal fluid, tanycytes and Virchow-Robin perivascular spaces. Brain Struct Funct 219: 1873-1887.
- Tan DX, Manchester LC, Reiter RJ (2016) CSF generation by pineal gland results in a robust melatonin circadian rhythm in the third ventricle as an unique light/dark signal. Med Hypotheses 86: 3-9.
- Hardeland R, Poeggeler B (2012) Melatonin and synthetic melatonergic agonists: Actions and metabolism in the central nervous system. Cent Nerv Syst Agents Med Chem 12: 189-216.
- Pinato L, da Silveira Cruz-Machado S, Franco DG, Campos LM, Cecon E, et al. (2015)Â Selective protection of the cerebellum against intracerebroventricular LPS is mediated by local melatonin synthesis. Brain Struct Funct 220: 827-840.
- Stehle JH, von Gall C, Korf HW (2003) Melatonin: A clock-output, a clock-input. J Neuroendocrinol 15: 383-389.
- Hardeland R (2008) Melatonin, hormone of darkness and more-occurrence, control mechanisms, actions and bioactive metabolites. Cell Mol Life Sci 65: 2001-2018.
- Dawson D, Armstrong SM (1996) Chronobiotics-drugs that shift rhythms. Pharmacol Ther 69: 15-36.
- Hardeland R, Madrid JA, Tan DX, Reiter RJ (2012) Melatonin, the circadian multioscillator system and health: the need for detailed analyses of peripheral melatonin signaling. J Pineal Res 52: 139-166.
- Wongchitrat P, Felder-Schmittbuhl MP, Govitrapong P, Phansuwan-Pujito P, Simonneaux V (2011) A noradrenergic sensitive endogenous clock is present in the rat pineal gland. Neuroendocrinology 94: 75-83.
- Chang HC, Guarente L (2013) SIRT1 mediates central circadian control in the SCN by a mechanism that decays with aging. Cell 153:1448-1460.
- Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, et al. (2002) Effects of aging on central and peripheral mammalian clocks. Proc Natl Acad Sci USA 99: 10801-10806.
- Hardeland R (2012) Neurobiology, pathophysiology, and treatment of melatonin deficiency and dysfunction. Scientific World Journal 2012: 640389.
- Mishima K, Tozawa T, Satoh K, Matsumoto Y, Hishikawa Y, et al. (1999) Melatonin secretion rhythm disorders in patients with senile dementia of Alzheimer's type with disturbed sleep-waking. Biol Psychiatry 45: 417-421.
- Skene DJ, Vivien-Roels B, Sparks DL, Hunsaker JC, Pévet P, et al. (1990) Daily variation in the concentration of melatonin and 5-methoxytryptophol in the human pineal gland: effect of age and Alzheimer's disease. Brain Res 528: 170-174.
- Srinivasan V, Pandi-Perumal SR, Maestroni GJM, Esquifino AI, Hardeland R, et al. (2005) Role of melatonin in neurodegenerative diseases. Neurotox Res 7: 293-318.
- Srinivasan V, Pandi-Perumal SR, Cardinali DP, Poeggeler B, Hardeland R (2006) Melatonin in Alzheimer’s disease and other neurodegenerative disorders. Behav Brain Funct 2: 15.
- Pandi-Perumal SR, Srinivasan V, Maestroni GJM, Cardinali DP, Poeggeler B, et al. (2006) Melatonin-Nature’s most versatile biological signal? FEBS J 273: 2813-2838.
- Pandi-Perumal SR, BaHammam AS, Brown GM, Spence DW, Bharti VK, et al. (2013): Melatonin oxidative defense: Therapeutical implications for aging and neurodegenerative processes. Neurotox Res 23: 267-300.
- Shukla M, Htoo HH, Wintachai P, Hernandez JF, Dubois C, et al. (2010) Melatonin stimulates the nonamyloidogenic processing of βAPP through the positive transcriptional regulation of ADAM10 and ADAM17. J Pineal Res 58: 151-165.
- Panmanee J, Nopparat C, Chavanich N, Shukla M, Mukda S, et al. (2015) Melatonin regulates the transcription of βAPP-cleaving secretases mediated through melatonin receptors in human neuroblastoma SH-SY5Y cells. J Pineal Res 58: 151-165.
- Reiter RJ (1998) Oxidative damage in the central nervous system: protection by melatonin. Prog Neurobiol 56: 359-384.
- Muñoz-Hoyos A, Sánchez-Forte M, Molina-Carballo A, Escames G, Martin-Medina E, et al. (1998) Melatonin's role as an anticonvulsant and neuronal protector: experimental and clinical evidence. J Child Neurol 13: 501-509.
- Reiter RJ, Cabrera J, Sainz RM, Mayo JC, Manchester LC, et al. (1999) Melatonin as a pharmacological agent against neuronal loss in experimental models of Huntington's disease, Alzheimer's disease and parkinsonism. Ann NY Acad Sci 890: 471-485.
- Cuzzocrea S, Reiter RJ (2001) Pharmacological action of melatonin in shock, inflammation and ischemia/reperfusion injury. Eur J Pharmacol 426: 1-10.
- Reiter RJ, Manchester LC, Tan DX (2010) Neurotoxins: free radical mechanisms and melatonin protection. Curr Neuropharmacol 8: 194-210.
- Hardeland R Coto-Montes A, Poeggeler B (2003) Circadian rhythms, oxidative stress, and antioxidative defense mechanisms. Chronobiol Int 20: 921-962.
- Torres-Farfan C, Serón-Ferré M, Dinet V, Korf HW (2006) Immunocytochemical demonstration of day/night changes of clock gene protein levels in the murine adrenal gland: differences between melatonin-proficient (C3H) and melatonin-deficient (C57BL) mice. J Pineal Res 40: 64-70.
- Dinet V, Ansari N, Torres-Farfan C, Korf HW (2007) Clock gene expression in the retina of melatonin-proficient (C3H) and melatonin-deficient (C57BL) mice. J Pineal Res 42: 83-91.
- Hardeland R (2009) Melatonin: Signaling mechanisms of a pleiotropic agent. BioFactors 35: 183-192.
- Hunt AE, Al-Ghoul WM, Gillette MU, Dubocovich ML (2001) Activation of MT2 melatonin receptors in rat suprachiasmatic nucleus phase advances the circadian clock. Am J Physiol 280: C110-C118.
- Grimaldi B, Nakahata Y, Kaluzova M, Masubuchi S, Sassone-Corsi P (2009) Chromatin remodeling, metabolism and circadian clocks: the interplay of CLOCK and SIRT1. Int J Biochem Cell Biol 41: 81-86.
- Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P (2009) Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science 324: 654-657.
- Sahar S, Sassone-Corsi P (2013) The epigenetic language of circadian clocks. Handb Exp Pharmacol 217: 29-44.
- Peek CB, Affinati AH, Ramsey KM, Kuo HY, Yu W, et al. (2013) Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science 342: 1243417.
- Masri S (2015) Sirtuin-dependent clock control: New advances in metabolism, aging and cancer. Curr Opin Clin Nutr Metab Care 18: 521-527.
- Huang KP, Chen C, Hao J, Huang JY, Liu PQ, et al. (2015) AGEs-RAGE system down-regulates Sirt1 through the ubiquitin-proteasome pathway to promote FN and TGF-β1 expression in male rat glomerular mesangial cells. Endocrinology 156: 268-279.
- Hernández-Jiménez M, Hurtado O, Cuartero MI, Ballesteros I, Moraga A, et al. (2013) Silent information regulator 1 protects the brain against cerebral ischemic damage. Stroke 44: 2333-2337.
- Petegnief V, Planas AM (2013) SIRT1 regulation modulates stroke outcome. Transl Stroke Res 4: 663-671.
- Brenmoehl J, Hoeflich A (2013) Dual control of mitochondrial biogenesis by sirtuin 1 and sirtuin 3. Mitochondrion 13: 755-761.
- Yang Y, Jiang S, Dong Y, Fan C, Zhao L, et al. (2015) Melatonin prevents cell death and mitochondrial dysfunction via a SIRT1-dependent mechanism during ischemic-stroke in mice. J Pineal Res 58: 61-70.
- Ma Y, Nie H, Chen H, Li J, Hong Y, et al. (2015) NADâº/NADH metabolism and NADâº-dependent enzymes in cell death and ischemic brain injury: current advances and therapeutic implications. Curr Med Chem 22: 1239-1247.
- Zhao L, An R, Yang Y, Yang X, Liu H, et al. (2015) Melatonin alleviates brain injury in mice subjected to cecal ligation and puncture via attenuating inflammation, apoptosis, and oxidative stress: the role of SIRT1 signaling. J Pineal Res 59: 230-239.
- Carloni S, Favrais G, Saliba E, Albertini MC, Chalon S, et al. (2016) Melatonin modulates neonatal brain inflammation through endoplasmic reticulum stress, autophagy, and miR-34a/silent information regulator 1 pathway. J Pineal Res 61: 370-380.
- Zhao L, Liu H, Yue L, Zhang J, Li X, et al. (2017) Melatonin attenuates early brain injury via the melatonin receptor/Sirt1/NF-κB signaling pathway following subarachnoid hemorrhage in mice. Mol Neurobiol 54: 1612-1621.
- Yang H, Gu ZT, Li L, Maegele M, Zhou BY, et al. (2017) SIRT1 plays a neuroprotective role in traumatic brain injury in rats via inhibiting the p38 MAPK pathway. Acta Pharmacol Sin 38: 168-181.
- Meng X, Tan J, Li M, Song S, Miao Y, et al. (2017) Sirt1: Role under the condition of ischemia/hypoxia. Cell Mol Neurobiol 37: 17-28.
- Hardeland R (2018) Recent findings in melatonin research and their relevance to the CNS. Cent Nerv Syst Agents Med Chem 18: 102-114
Citation: Hardeland R (2018) Neuroinflammation and Aging: Significance of Declining Circadian Functions and Melatonin. Biochem Physiol 7: 243. DOI: 10.4172/2168-9652.1000243
Copyright: © 2018 Hardeland R. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4220
- [From(publication date): 0-2018 - Dec 15, 2025]
- Breakdown by view type
- HTML page views: 3310
- PDF downloads: 910