Review Article Open Access
Neuronal Carnitine Palmitoyl Transferase1c in the Central Nervous System: Current Visions and Perspectives
Ashraf Virmani1*, Luigi Pinto1, Otto Bauermann1, Saf Zerelli1, Zbigniew Binienda2, Syed Ali3 and Feike R van der Leij4
1Research, Innovation and Development, Sigma-tau Health Science International BV, Utrecht, Netherland and Sigma-tau SpA, Pomezia Rome, Italy
2Neurophysiology Laboratory, National Center for Toxicological Research, FDA, Jefferson, AR 72079, USA
3Neurochemistry Laboratory, Division of Neurotoxicology, National Center for Toxicological Research, FDA, Jefferson, AR 72079, USA
4Van Hall Larenstein and NHL University of Applied Sciences Agora 1, Postbus 1528 8901BV Leeuwarden, The Netherlands
- Corresponding Author:
- Ashraf Virmani
Professor, Research
Innovation and Development Department
Sigma-tau SpA, Via Pontina km 30,400
00040 Pomezia (Roma), Italy
Tel: +39 06 91393804
Fax: +39 06-9116-6912
E-mail: ashraf.virmani@sigma-tau.it
Received date: October 18, 2013; Accepted date: November 16, 2013; Published date: November 21, 2013
Citation: Virmani A, Pinto L, Bauermann O, Zerelli S, Binienda Z, et al. (2013) Neuronal Carnitine Palmitoyl Transferase1c in the Central Nervous System: Current Visions and Perspectives. J Alzheimers Dis Parkinsonism 3:132. doi:10.4172/2161-0460.1000132
Copyright: © 2013 Virmani A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
The Carnitine Palmitoyl Transferase (CPT) system comprises several metabolically important enzymes of the carnitine/choline acyl transferase family. CPTs are transferases that allow the energy-neutral replacement of coenzyme A (CoA) by L-carnitine, and vice versa, when bound to acyl chains as (thio) esters. Together with acyl- CoA synthases and a carnitine/acyl carnitine translocase, the mitochondrial beta-oxidation of long-chain fatty acids is facilitated by distinct CPT proteins. CPT deficiencies manifest as disorders of mitochondrial fatty acid oxidation. Recently a new CPT isoform, CPT1c has been described which is found in entusiasmòc reticulum (ER) of neurons. Unlike CPT1a and CPT1b it has lower palmitoyl transferase activity. It is localised mainly in hypothalamus, amygdala and hippocampus, i.e., brain regions with roles in the control of food intake. It plays a role in energy homeostasis but its exact role in physiology of neurons is still not clear. Studies suggest a biosynthetic rather than catabolic role in long chain acyl carnitine production. The role of CPT1c may extend beyond simply the interchange of CoA and L-carnitine to acyl groups. Studies show that in the CNS the CPT1c affects ceramide levels, endocannabionoids and oxidative processes, and may play an important role in various brain functions such as learning. It is a player in insulin resistance that may occur as a result of oxidative damage and in altered redox status diseases such as metabolic cognitive syndrome, type 2 diabetes, neurodegenerative diseases and cancer. Unhealthy lifestyles, especially high sugar and fat diet in combination with other genetic and environmental risk factors, probably compromise metabolism at various levels. At the cellular level there would be increased ER stress as well as mitochondrial dysfunction leading to impaired oxidative phosphorylation and increased reliance on glucose (Warburg effect). Targeting CPT1c may provide insight on treatment of many metabolically-related diseases as well as pervasive developmental disorders.
Keywords
Adenosine-Triphosphate (ATP); Oxidative phosphorylation; AMP-Activated Protein Kinase (AMPK)
Introduction
Metabolism linked to disease processes such as neurodegeneration and cancer
There are a myriad of metabolic processes within every living cell. Cells survive by utilizing organic molecules, from sugars to lipids and proteins. The metabolic pathways that enable this are interconnected within a single cell but also between cells, tissues, systems, organs all the way to the level of the whole body. The breakdown of energy substrates such as carbohydrates in particular glucose, as well as fatty acids and amino acids, leads to energy mainly in the form of Adenosine- Triphosphate (ATP). Some ATP is formed in the cell cytoplasm by glycolysis but the majority is formed in the mitochondria in the presence of oxygen by the Krebs cycle and oxidative phosphorylation. Of the theoretical 30 to 32 ATP molecules produced by oxidation of glucose, 2 are from glycolysis, 2 from the Krebs cycle, and 26 to 28 from the respiratory or electron transport system (Figure 1). The cellular and plasma levels of the energy substrate glucose are under control of the pancreatic hormone insulin. Insulin is able to modulate cellular glucose uptake via IGF receptor (IGFR) and GLUT transporter expression. Thus impairment of insulin signalling leads to metabolic impairment from cellular-up to body level. The role of anomalous IGFR signalling and subsequent metabolic changes is now recognized in triggering a series of events that lead to the metabolic syndrome and diabetes as well as other disease states such as neurodegeneration and cancer. In this review we discuss the impairment of endoplasmic and mitochondrial function and in particular the role of metabolic dysfunction, increased oxidative stress and inflammation in initiating pathology. We discuss the molecule L-carnitineas a possible target to improve major enzyme function such as CPT which underlies mitochondrial function.
Factors affecting mitochondrial biogenesis
Mitochondrial function diminishes with age and a number of studies suggest strategies that may improve mitochondrial bioenergetics [1-5]. One such strategy is caloric restriction which is a biological phenomenon through which limiting normal caloric intake by about 40% improves the health and lifespan of various. The review by Cantò and Auwerx suggests that AMP-Activated Protein Kinase (AMPK) is the molecular switch in calorie restriction and acts, via Silent Information Regulator 2 type 1 (SIRT1), on mitochondrial health status and its biogenesis by modulating autophagy [3]. Basically SIRT1 is a histone deacetylase that acts on AMPK thereby activating it [6]. This activation affects a number of processes in the context of mitochondrial biogenesis:
• SIRT1 activates Peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1alpha a master regulator of mitochondrial biogenesis)
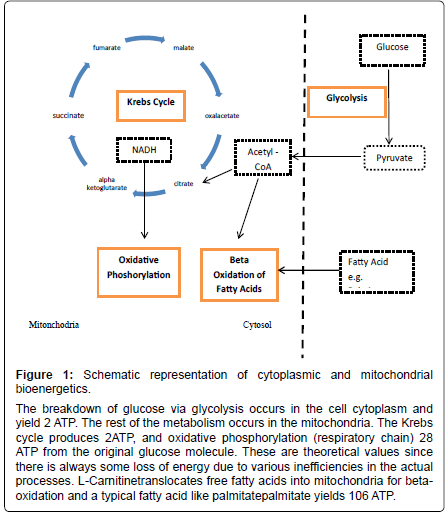
Figure 1: Schematic representation of cytoplasmic and mitochondrial bioenergetics.
The breakdown of glucose via glycolysis occurs in the cell cytoplasm and yield 2 ATP. The rest of the metabolism occurs in the mitochondria. The Krebs cycle produces 2ATP, and oxidative phosphorylation (respiratory chain) 28 ATP from the original glucose molecule. These are theoretical values since there is always some loss of energy due to various inefficiencies in the actual processes. L-Carnitinetranslocates free fatty acids into mitochondria for betaoxidation and a typical fatty acid like palmitatepalmitate yields 106 ATP.
• SIRT1 also activates Fork head box O (FOXO) transcription factors involved in nutritional stress regulation and mitochondrial biogenesis
• SIRT1, activation of AMPK blocks mechanistic target of rapamycin (mTOR) pathway that is an anabolic and growth signalling pathway
All these pathways are able to regulate and induce autophagy in the context of low caloric intake. At the same time CPT1 (carnitine palmitoyl transferase 1) is usually expressed via mediation through PGC1-alpha and it’s overexpression increases fatty acid oxidation [7].
A review of Schreurs et al. in 2010 shed light on the possibility to target beta-oxidative enzymes for metabolic syndrome, mainly via pharmacological treatment [8]. CPT1c as a key modulator of feeding and may have possible role in neurodegeneration since this isoenzyme form is specifically found in the brain [8-11].
The carnitinepalmitoyl transferase system, mitochondria and bioenergetics
For many biologically important roles, fatty acids are activated to acyl-coenzyme A (acyl-CoA) molecules by several synthases known to exist. Activated fatty acids can be short chain (acetyl-CoA), medium chain (e.g., octanoyl-CoA) or long chain acyl-CoAs such as palmitoyl- CoA. Once activated, these CoA thioesters are unable to cross lipid membranes. The temporal replacement of the CoA moiety by L-carnitine has been nature’s solution to solve the problem of transmembrane transport of activated fatty acids (Figures 2 and 3). The interchange of a CoA group by L-carnitineis reversible and the reactions are energy neutral. In this context, the role of L-carnitine must not be seen only as a shuttling molecule but also as a buffering molecule, enabling the cell to manage the size of acetyl-CoA and free CoA pools.
The CPT system together with its related components is traditionally known to be located in the mitochondria. Biochemical studies pointed at the existence of similar enzyme activities outside mitochondria as well, but for a long time molecular genetic evidence was limited to mitochondrial CPT enzyme localization. Together with a carnitine/acyl carnitine translocase, the mitochondrial beta-oxidation of long-chain fatty acids is facilitated by distinct CPT proteins. CPT1 is active on the outer surface of mitochondria, and serves as a regulatory site for fatty acid oxidation due to its sensitivity for malonyl-CoA (the product of the first step in fatty acid synthesis carried out by acetyl-CoA carboxylase) [8,12]. Two mitochondrial isoforms of CPT1 were identified: CPT1a or L-CPT1 is expressed in the liver and many other tissues, and CPT1b or M-CPT1 is the muscle type isoform. The two genes that encode CPT1a and CPT1b are closely related, but in humans and other mammals localized on different chromosomes [13]. CPT2 is active inside the mitochondrial matrix to regain acyl-CoA from a process generally known as the carnitine shuttle. This protein is expressed in a constitutive way in all cells and tissues. The three-dimensional structures of CPT1 and CPT2 have been fairly well described as they could be deduced from measurements on acetyl transferase (CrAT) and CPT2 itself [14]. However, from a functional point of view the most interesting enzyme is CPT1. This enzyme is tightly bound to the mitochondrial outer membrane by two transmembrane domains, whereas the majority of the proteins, including the active site and the regulated site are positioned on the outer surface facing the cytosol. Mitochondria are responsible for the oxidative metabolism and have their own DNA, tRNA and ribosomes, similar to those of bacteria. They allow cells to produce energy using oxidative metabolism which provides the largest proportion of ATP production, in particular from the utilisation of carbon groups via the beta oxidation pathway from fatty acids (Figure 1). The net amount of energy equivalents to be obtained from a molecule of glucose is about 30ATP whereas the beta oxidation of a fatty acid like palmitate produces 106 ATP. Thus, although L-carnitine is a relatively simple molecule, it can greatly influence cellular energy production by facilitating beta-oxidation.
Role of L-carnitine in mitochondrial metabolism
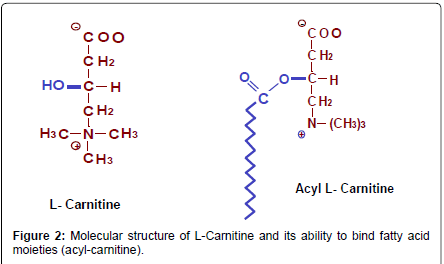
L-carnitine is similar to amino acids in having an amine group and a carboxyl group and it can be manufactured in the body from the essential amino acids lysine and methionine (Figure 2). The special nature of this molecule and its ubiquitous use in nature probably arises for its inherent molecular properties which include:
• Ampiphilic nature (affinities for both polar and non-polar environments)
• It can ionise in water and have positive and negative charge.
• It has osmolar properties and is used to maintain tonicity by cells/bacteria
• It has the alcohol OH group which allows it to bind acyl groups (Figure 2)
L-carnitine is found in many life forms from bacteria to animals and (although in low amounts) plants. Within the cells it plays a special role in the mitochondria but it is also found in the cytoplasm and nucleus of the cell. Further, within the higher organisms it is found at all levels from cells, tissues (muscle, nervous etc.) and organs (brain, liver, heart etc.). The distribution pattern in the cells and organism reflects the various functions of L-carnitine. Its main role is in the metabolism of fatty acids; however a number of secondary roles have also been defined (Table 1).
In the cell the L-carnitine exists only in the L-isomer form, and its acylcarnitine esters include acetyl-L-carnitine, propionyl- L-carnitine, plus other acylcarnitine [15]. A number of specialized proteins have evolved for its synthesis, transport and function within the cell. These include enzymes and transport proteins required for their metabolism and transport, including carnitine acyltransferases, mitochondrial carnitine/acylcarnitine translocases, plasma membrane carnitine importers and the carnitine biosynthesis pathway from lysine and methionine [16]. Thus although the mitochondrion and the carnitine palmitoyl transferase system are the main environments where L-carnitine performs its primary role in the transport of fatty acids from the cytoplasm for beta oxidation, the other enzymes and transport proteins also determine the overall dynamics and function. Therefore there is interplay between L-carnitine, the beta-oxidation pathway, the CPT system and the related cellular machinery, in relation to brain function and physiology. The essential role of L-carnitine in the oxidation of long chain fatty acids by mammalian tissues was discovered by Fritz in 1955 and in the successive years Fritz proposed the presence of a protein pool useful in explaining the molecular aspects of this type of metabolism [17,18]. These were milestones that initiated studies on the CPT system, its molecular structure and biological control function.
The carnitine palmitoyl transferase system
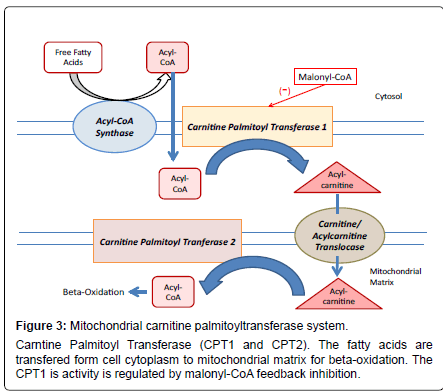
Fatty acids can be oxidized in peroxisomes, endoplasmic reticulum as well as mitochondria. The dysfunction in the metabolism of fatty acids is implicated in particular disease conditions for example; metabolic syndrome, cardiovascular disease, and other diseases such as diabetes type 2 and obesity as well as in its regulation at brain level [19-22]. Fatty acid metabolism is emerging as something more than a simple energy pathway, with important role in energy storage and homeostasis. At the core of all these metabolic pathways lies the CPT System (Figure 3).
| Acetyl-CoA buffering |
| Detoxification of potentially toxic metabolites-short chain acyl groups have toxic/detergent |
| Fatty acid transport - Improving intracellular cell energy production |
| Regulation of the mitochondrial and cytosolic acyl-CoA/CoA ratio - Improving intracellular cell energy production |
| Stabilization of cell membranes - Reducing oxidative stress |
| Other âÂ?Â?Epigenetic regulation (Histone acetylation) |
Table 1: The Essential Role of L-Carnitine.
The CPT system is a multiprotein complex with catalytic activity localized with a core represented by CPT1 and CPT2 into the outer and inner membrane of the mitochondria respectively. Acyl-CoA synthases and a translocase are also part of the CPT system. The structures of CPT1 and CPT2 has been described and the CPT2 is a protein of 71 KDa (658 amino acids), formed as a precursor containing an NH2 terminal sequence that is cleaved during the translocation into the mitochondrial inner membrane.CPT1 has two principal isoforms called ‘L’ and ‘M’ that are localised to hepatic and muscular tissues respectively. Both are involved in the long-chain fatty acid metabolism and are of interest as therapeutic target for the treatment of principal metabolic diseases such as diabetes. These isoforms are encoded by CPT1a and CPT1b genes and have different biochemical properties. They display different Km values for L-carnitine and a strong difference in sensitivity for malonyl-CoA [23,24]. Both have a theoretical molecular weight of 88 KDa (hepatic CPT1a has 773 amino acids while the muscular CPT1b has 772 amino acids). In terms of protein and gene sequence the CPT1a and CPT1b have strong similarities. The CPT1 displays a large N-terminal region of 170 amino acid residues, consisting of two hydrophobic regions named H1 and H2 (both spanning the membrane), a substrate binding site, an inhibitor binding site and a catalytic site.
Recently, a third isoform of CPT was described: CPT1c [10]. This isoform was discovered in 2002, and its mRNA is very similar to that of CPT1a and CPT1b. Its gene in humans is localized on chromosome 19 and the amino acid sequences of CPT1a, CPT1b and CPT1c are equally similar (there has been a double duplication of genes, very close in time in evolution, probably when organ differentiation started to emerge in animals) [10]. It is localized in the endoplasmic reticulum and possibly in mitochondria [25]. The CPT1c protein has palmitoyl-carnitine transferase activity but lower than the hepatic and muscular isoforms. Immunohistochemical studies have detected its presence in particular brain regions such as hypothalamus, amygdala and hippocampus, regions that are strongly related to the control of food intake [26]. In particular studies by Gao et al. showed that in CPT1c knock-out mice induction of obesity by a high-fat diet led to the animals becoming insulin-resistant [27]. Hence a role for CPT1c has been hypothesized in energy homeostasis and in the hypothalamic control of food intake (orexigenic action) [11,28].
As mentioned before a key molecule into the regulation of CPT1a, CPT1b and also CPT1c is the metabolite, malonyl CoA. The CPT1 enzymes have a specific site allowing for their physiological regulation through malonyl-CoA, which therefore forms part of the body’s control mechanism involved in the regulation of food intake. This control center is localized in the hypothalamic region of the brain and is able to modulate the functionality of CPT1c. Malonyl-CoA is the allosteric inhibitor of fatty acid oxidation by suppressing the translocation of fatty acids into mitochondria catalysed by all the CPT1 forms [29]. The production of malonyl-CoA is catalysed by the enzyme acetyl-CoA carboxylase via AMPK. This latter enzyme is regulated by anorexigenic hormones such as leptin [30]. Studies show that an increase in the concentration of malonyl-CoA is needed in the regulatory pathway that leads to decrease the food intake and to weight loss.
It is also important to highlight the biosynthetic role of CPT1c and its localization in ER membrane since by modulation of the fatty acid, palmitate, which is a strong modulator of ER-stress a number of cellular functions, can be affected. ER-stress is a typical cellular and molecular condition that allows for metabolic diseases such as the metabolic syndrome and diabetes type 2. Thus a possible role of CPT1c and AMP-activated Kinase in the modulation of ER-stress and subsequent decrease of insulin resistance via the control region in hypothalamus has been suggested [31]. Furthermore, studies suggest a direct role of CPT1c as target of the gastric hormone ghrelin and recent evidence suggests involvement of the biosynthetic action of CPT1c [21,32].
A role for CPT and fatty acid metabolism in the CNS
It is widely recognized that free fatty acids do not readily gain access to the CNS and that the CNS does not use fatty acids as a major fuel source. Rather, glucose is used almost exclusively in the fed state, whereas ketones (derived from fatty acids) are a major fuel source in the CNS during prolonged fasting. Why, then, are fatty acids in the CNS so intimately involved in energy homeostasis? Furthermore, blood levels of fatty acids are elevated during times of fasting, the inverse of what would be expected for an appetite-suppressing molecule. Clearly, further studies on the metabolism/role of fatty acids in the CNS on energy homeostasis are needed to understand how these energy-rich molecules affect how the brain senses and responds to peripheral energy sources.
The increase in L-C content of brain coincides with adaptive changes promoting fatty acid uptake and metabolism. Indeed, fatty acid oxidation is highest 7-10 days after birth as reflected by the increased activity of CPT enzyme and ability of cultured rat cortical neurones to take up L-carnitine [33]. We examined the endogenous carnitine levels, total CPT activity (CPT1 and CPT2) and lipid composition in cultured neurones at different ages of development and found that:
• Levels of endogenous L-carnitine in primary rat cortical neurones increase with age (upto day 14) and on exposure to exogenous L-carnitine and acetyl-L-carnitine.
• Level of CPT increases correspondingly.
• Level of CPT is affected differently by exogenous levels of L-carnitine and acetyl-L-carnitine (Table 2).
This data is important for the various theories regarding neuroprotection since there could be a correlation between development of the ability for fatty oxidation and neuronal damage under ischemic or hypoxic conditions as well as in lipid turnover in the membranes.
CPT activity is expressed as nmoles/min/mg protein. The values represent the mean ± SD (n=4). The effect of L-C or ALC treatment is significantly different (p<0.001) from controls at same age. DIV=days in vitro. This data was presented in before in abstract form [34].
| Neurone Age | 1 | 7 | 14 | 21 DIV |
|---|---|---|---|---|
| Control | 1.17 ± 0.10 | 5.93 ± 0.19 | 8.96 ± 0.08 | 3.38 ± 0.30 |
| L-Carnitine treated | 0.44 ± 0.10 | 1.94 ± 0.06 | 1.51 ± 0.05 | 7.42 ± 0.37 |
| Acetyl-L-carnitine treated | 1.63 ± 0.10 | 9.86 ± 0.09 | 6.24 ± 0.10 | 10.65 ± 0.29 |
Table 2: Modulation of carnitinepalmitoyltransferase enzyme system by carnitine in cultured rat cortical neurones at different ages of development.
We have shown earlier that in neurones CPT is important in phospholipid and triglyceride fatty acid remodelling [35]. A deficiency of L-carnitine at a critical period of brain development is known to occur in premature infants receiving parenteral nutrition [36]. The development and expression of CPT activity in neonatal cortical neurones was dependent on the exposure to L-carnitine and acetyl- L-carnitine, more importantly, in different ways to each compound. The possible mechanism(s) of action of L-carnitine and acetyl-Lcarnitine on the induction, expression and activity of CPT and whether the malonyl-CoA sensitive (CPT1) or insensitive activity (CPT2) or both affected are currently under study. Such an approach includes effects via changes in the levels of the intracellular acyl CoA/CoA ratio, or effects on the activity of other enzymes e.g. protein kinases, in a similar way as reported for palmitoyl carnitine or phenothiazines (e.g., chlorpromazine). These have been shown to inhibit a number of calmodulin-sensitive Ca2+-dependent (phospholipid sensitive) enzymes. Acetyl-L-carnitine also stimulates the incorporation of stearic acid into neutral lipids i.e. phosphatidic acid and phosphatidyl choline, by shifting the equilibrium towards acetyl-CoA formation and consequently lipid synthesis [37]. Thus whatever the mechanism(s), both L-carnitine and acetyl-L-carnitine demonstrate multifactorial properties and interact at the biochemical, neurotransmitter and membrane level [15].
CPT deficiencies and disorders in the CNS
Carnitine palmitoyl transferase deficiencies present themselves as disorders of mitochondrial fatty acid oxidation. CPT1a deficiency is inherited in an autosomal recessive manner and is relatively rare [37,38]. CPT1a deficiency causes recurrent attacks of fasting hypoketotic hypoglycaemia, hepatomegaly, seizures, renal tubular acidosis and hyper ammonemia. Signs and symptoms usually begin around eight to eighteen months of age and may be life-threatening. In particular treatment of hypoglycaemia is important to prevent eventual neurologic injury seen as developmental delay, seizures, coma, and death. Avoidance of fasting and administration of mediumchain triglycerides with a low fat diet also helps limit the metabolic consequences. In contrast CPT1b and CPT1c deficiencies have not been well identified to date [38].
The CPT2 deficiency is more common and is also inherited in an autosomal recessive manner. Clinically it presents asthree main types, a lethal neonatal (perinatal) form, a severe infantile (hepatocardiomuscular) form, and a mild myopathic form [38]. The myopathic form predominates and manifests from infancy to adulthood. While the former two are severe forms characterized by liver failure with hypoketotic hypoglycemia, cardiomyopathy, seizures, and early death. The myopathic form is the most common disorder of lipid metabolism characterized by episodes of rhabdomyolysis triggered by prolonged exercise, fasting, or febrile illness [39]. The infantile-type occurs between 6 to 24 months of age and presents as severe bouts of hypoketotic hypoglycemia, and at times cardiac damage which underlies sudden death before 1 year of age. A report on possible role of CPT2 in CNS disorders was made based on two male infants with central nervous system disorders, i.e. infantile spasms in one and athetotic quadriplegia in the other [40]. The CPT1 activity in biopsied muscle was normal in both cases but CPT2 was decreased to 37% and 25% of the control, respectively. In animal experiments they found CPT activity in almost all brain regions, especially in the brainstem, cerebellum and spinal cord. This study suggested that another symptomatology of CPT2 deficiency with CNS involvement could be a possibility. The neonatal form is the least common clinical presentation and is almost invariably fatal. Onset has been documented just hours after birth to within 4 days of life [38]. In this form developmental issues are present in brain and kidney and these are visible even during prenatal ultrasound. Neuronal migration defects have also been documented [41].
Treatment for CPT2 deficiency is based upon avoidance of fasting and/or exercise, a low fat diet enriched with medium chain triglyceridessuch as triheptanoinand carnitine supplementation [42,43].
CPT1c in neurological diseases-possible links
The CPT1 ‘a’ and ‘b’ enzymes have undisputed metabolic roles. However, the role of the isoform CPT1c, found mainly in neurones is not clear although a role in energy homeostasis has been postulated [10,25,26,29]. Studies also suggest a biosynthetic role via the entry of long chain acyl-CoA into the endoplasmic reticulum (ER) and in the production of sphingolipids and ceramides for signal transduction [32,44-46].
The over-expression of CPT1c in the brain of developing transgenic mice has been shown to cause microencephaly [47]. Whereas the group of Carrasco et al., 2012 reported that CPT1c knock-out (KO) mice demonstrated a marked reduction ìn spatial learning ability [45]. This group also demonstrated that CPT1c is found in hippocampal pyramidal neurons and the enzyme is located in the endoplasmic reticulum throughout the neuron including the dendritic spines [45,46]. These studies also showed that CPT1c played a role in ceramide metabolism. In primary hippocampal cultured neurons the overexpression of CPT1c led to increased ceramide levels and in CPT1C-deficient neurons the ceramide levels were reduced. Reduced ceramide levels were also found in the hippocampus of CPT1c KO mice. The altered dendritic spine morphology was dependent on altered ceramide since the treatment of the cultured neurons with exogenous ceramide reverted the KO phenotype, as did ectopic over expression of CPT1c [45]. The CPT1c regulation of spine maturation via ceramide indicates its possible physiological role during brain development and function. Thus CPT1c is necessary for the correct maturation of dendritic spines and for proper spatial learning and its deficiency impairs spatial learning [45]. These studies also suggest that CPT1c mutations may underlie some human cognition disabilities of unknown etiology.
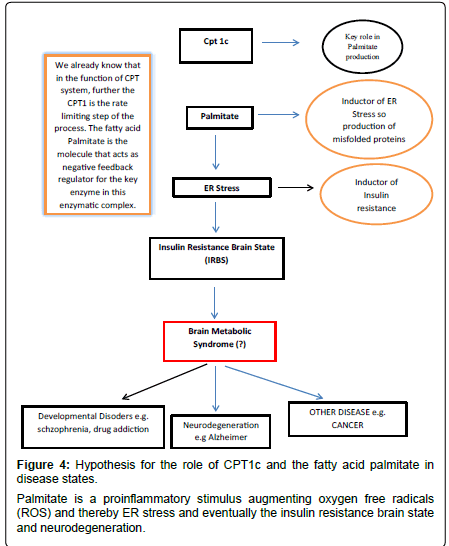
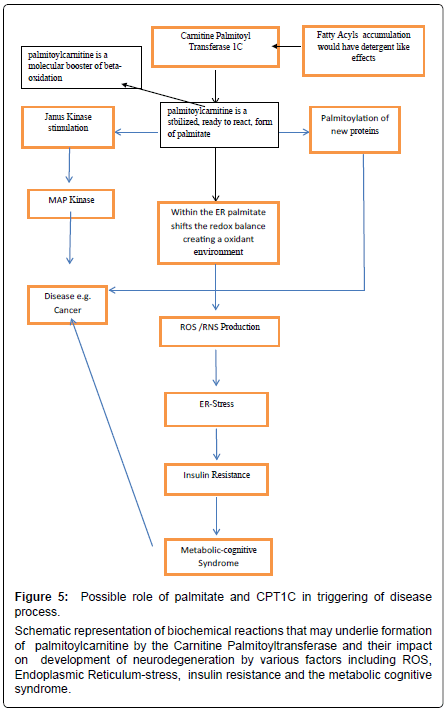
From this we may start to deduce some possible hypothetical links between CPT1c and neurological diseases. Further, since CPT1c has palmitoyl transferase activity and is localized in ER of neurons it is conceivable a boost in stabilization of the acyl fatty acid by its complex with L-carnitine thereby preventing deleterious effects by the acyl molecules such as provoking inflammation (Figure 4). The acyl carnitine may also be used for beta-oxidation, possibly via the close physical connections between mitochondria and ER [48,49].
The CPT1c is highly homologous with its isoforms CPT1a and CPT1b but its role in neuronal mitochondrial fatty acid oxidation is not yet clear [25,45,46,50]. It has low CPT1 activity and binds the CPT1 physiological inhibitor malonyl-CoA with similar affinity as CPT1a [25,26]. Studies using CPT1c-transfected cells showed that CPT1c had carnitine palmitoyl transferase activity and that palmitoyl-CoA was the substrate [25]. Thus although the Km values were similar to those of CPT1a the Vmax values were 66 times lower than those of CPT1a [25,51]. This group suggested that role of CPT1c was different from the CPT1a or CPT1b and not related to import of long chain fatty acid into mitochondria or peroxisomes for β-oxidation but rather CPT1c played arole in biosynthetic pathways and control of the equilibrium between acyl-CoA pools in the cytosol and the ER lumen [25].
Further clues as to the role of CPT1c come from studies in cancer cells where it has been shown to protect transformed tumour cells from death induced by glucose deprivation or hypoxia [50,52]. These studies showed that the cancer cells compensated by metabolic transformationand instead of the Warburg effect where they upregulate glycolysis in the absence of glucose they compensate by activation of fatty-acid oxidation [53,54]. The increase in ATP production confers resistance to glucose deprivation or hypoxia to these cancer cells. It has been suggested that protection from CPT1c might derive from unidentified fatty acid-derived products that would be beneficial for cell survival under metabolic stress [45].
Indeed the depletion of CPT1c has been shown to cause an increased sensitivity to oxidative stress and may be a key mediator of oxidative stress [55-57]. The metabolomic profiling studies in CPT1c KO mouse brains showed that the loss of CPT1c results in elevated oxidative demands in the brain [55]. This study found that beta-oxidation was unchanged but the redox homeostasis was affected with greater than 2-fold increase in oxidized glutathione. Loss of CPT1c also resulted in decreased levels of endogenous endocannabinoids and other fatty acid derived metabolites which may explain its effect on food intake. They concluded that the exact role of CPT1c in relation to oxidative stress was still unclear however CPT1c could play an alternative role in neuronal oxidative metabolism.
High fat intake has been associated with the metabolic syndrome and chronic inflammatory status via effects on oxidative stress, lipid peroxidation, DNA damage, mitochondrial damage and the worsening of the metabolic condition of the cell [58]. The fatty acid palmitate induces ER-stress and promotes the onset of misfolded proteins that provoke inflammation [59]. Misfolded proteins are associated with damage and loss of neurons in various neurodegenerative conditions. Thus CPT1c, by producing palmitoyl carnitine and enhancing betaoxidation, would reduce the ER stress condition and protect against damage [60].
The list of neurodegenerative and pervasive developmental disorders (PDD) having possible involvement of the CPT1 enzyme is huge. Disease states such as Parkinson’s disease (PD), Alzheimer disease (AD), Multiple sclerosis (MS), autism, attention deficit disorder, depression and schizophrenia etc. have a high social impact and for which no cure has been found to date.
PD is a particularly debilitating progressive degenerative process that involves the dopaminergic neurons of the substantia nigra region in the brain that controls movement and the result is brady kinesia, akinesia and cognitive and language problems, related to loss of the dopaminergic neurotransmission.
Although role of CPT1c in PD is not yet recognized, it is possible to theorise one since a strong link between this pathology and Diabetes Mellitus Type 2 exists [61]. In fact, CPT1 plays a key role in improving of insulin resistance and insulin resistance is a peculiar feature of type 2 Diabetes Mellitus, in turn this latter is associated with an increased risk of PD [62,63]. Thus it is possible hypothesize that CPT1 is involved in PD due to its recognized role in energy homeostasis and particularly since insulin resistance is able to impair the functionality of substantia nigra [64]. This is highlighted in the study by Lefort 2010 where associated with the condition of insulin resistance, was an increase in ROS production, lowering of mitochondrial Complex 1 and lowering of CPT1b in the mitochondria extracted from the skeletal muscle cells [65]. Loss or alteration of Complex 1 is strongly associated with the onset of Parkinson’s disease as shown by Hauser and Hastings (2013), and L-carnitine derivative acetyl-L-carnitine was able to reduce toxicity in vitro caused by blocking complex 1 with N-methyl-4-phenyl-1,2,3,6- tetrahydropyridine (MPP+) [66,67]. It is interesting to note that palmitate modulates the insulin resistance at the hypothalamic region [68].
Another key disease where a role by CPT1c is conceivable is AD. This is the most common form of age-related dementia with typical clinic events such as loss of memory and cognition and the presence of typical histological features such as extracellular beta-amyloid (Abeta) plaques and intracellular hyper-phosphorylated microtubuleassociated protein tau accumulation [69]. AD is the most important of the so called taupathies and the presence in brain of beta-amyloid plaques is a strong marker of AD. This protein abnormality is possibly due to alteration of normal production, folding and functionality of beta-amyloid protein or to alterations in the genetic sequence coding for this protein. The role exerted by metabolism in the onset and development of AD is still not clear, however, links between insulin resistance and onset of AD have been shown [69,70]. Recent studies suggest a strong connection between AD and metabolic syndrome and also between AD and diabetes mellitus type 2 [71-73].
A possible role of CPT1c is also conceivable in multiple sclerosis. This kind of pathology is due to an autoimmune process directed against the myelin sheath that surrounds neurons by an overactive immune system. In multiple sclerosis, as in neuro inflammation and neurodegeneration in general, there is an underlying condition of insulin resistance and possible fatty acid metabolism dysfunction. Indeed, a study in mice using the model of Experimental Autoimmune Encephalomyelitis, which mimics multiple sclerosis, showed that inhibition of fatty acid metabolism with a synthetic malonyl-CoA agonist (Etomoxir) was useful in ameliorating the disease condition [73,74].
Schizophrenia is another disabling disease linked to genetic and environmental causes that bring about severe dementia and psychiatric manifestation that compromise the individual. The pharmacologic treatment is generally aimed at modulation of the main neurotransmitters affected in this disease. From a pathological point of view, schizophrenia could be due to a neuroinflammation processes, mostly by alterations of the kynurenic acid (a tryptophan metabolite) pathway [75,76]. In schizophrenic patients kynurenic acid is not degraded and there is an increase in its concentration which leads to an imbalance in the glutamate neurotransmission. Kynurenic acid increases Ca2+ levels and insulin secretion via Ca2+ channels in the brain [77]. This insulin resistant brain state may trigger the neuroinflammatory condition. In our opinion, insulin resistance together with free fatty acids, such as palmitate, could be key mediators in many diseases due to their ability to modulate apoptosis and inflammation, making these diseases difficult to treat (Figure 5).
Could carnitines be useful in this context?
There are many studies suggesting a positive role for the acetylated form of the L-carnitine, acetyl-L-carnitine. Studies suggest that acetyl-L-carnitine has the ability to modulate and improve the brain’s cognition status in impairment conditions such as AD and PD [78,79]. As mentioned before, both these diseases are characterized by a general condition of insulin resistance in the brain [64,69,70]. Some forms of mental impairment such as vascular dementia are also strongly influenced by acetyl-L-carnitine [78]. L-carnitine and acetyl-L-carnitineis“energizers” with the ability to improve cognitive functions and are generally prescribed in elderly [80]. In this context acetyl-L-carnitine has epigenetic modulation properties by donating acetyl group for histone acetylation. This epigenetic mechanism is diminished in aging cells [81]. L-carnitine is also utilized by CPT1c for the formation of long chain acyl carnitines.
All the disease states mentioned before have a common link and that is metabolic dysfunction. This is also an important aspect in relation to cancer [82]. As discussed, the alteration of metabolism brings about the spectrum of metabolic diseases from diabetes to cancer [83,84]. There are several studies suggesting role of CPT1c in onset and evolution of neoplastic phenomena [50,85]. As is seen for CPT1a in other cancer types increased CPT1 enzyme activity may contribute to fueling the high energy demands of neoplastic cells. Furthermore the acetylation and deacetylation of histones may be directly affected [86,87].
Conclusion
CPT1c is localized mainly in ER of neurons and has palmitoyl transferase activity that leads to palmitoyl carnitine production. It plays a role in energy homeostasis but its exact role in physiology of neurons is still not clear. Studies suggest a biosynthetic rather than catabolic role in long chain acyl carnitine production. The role of CPT1c may extend beyond simply the interchange of CoA and L-carnitine to acyl groups. Studies have shown that in the CNS the CPT1c effects ceramide levels, endocannabionoids and oxidative processes and may play an important role in various brain functions. It is a player in insulin resistance that may occur as a result of oxidative damage and in altered redox status diseases such as metabolic cognitive syndrome, diabetes type 2, neurodegenerative diseases and cancer. Unhealthy lifestyles, especially high sugar and fat diet in combination with other genetic and environmental risk factors, probably compromise metabolism at various levels. At the cellular level there would be increased ER stress as well as mitochondrial dysfunction leading to impaired oxidative phosphorylation and increased reliance on glucose (the “Warburg effect”). The CPT1c may provide insight on treatment of many diseases however further experimental studies are needed in this field since it offers a great potential to target various disease states.
Figure 5: Possible role of palmitate and CPT1C in triggering of disease process.
Schematic representation of biochemical reactions that may underlie formation of palmitoylcarnitine by the Carnitine Palmitoyltransferase and their impact on development of neurodegeneration by various factors including ROS, Endoplasmic Reticulum-stress, insulin resistance and the metabolic cognitive syndrome.
References
- Virmani MA, Bisselli R, Spadoni A, Rossi S, Corsico N, et al. (1995) Protective actions of L-carnitine and acetyl-L-carnitine on the neurotoxicity evoked by mitochondrial uncoupling or inhibitors. Pharmacol Res 32: 383-389.
- Virmani A, Gaetani F, Binienda Z (2005) Effects of metabolic modifiers such as carnitines, coenzyme Q10, and PUFAs against different forms of neurotoxic insults: metabolic inhibitors, MPTP, and methamphetamine. Ann N Y Acad Sci 1053: 183-191.
- Cantó C, Auwerx J (2011) Calorierestriction: is AMPK a keysensor and effector? Physiology (Bethesda) 26: 214-224.
- Palacios HH, Yendluri BB, Parvathaneni K, Shadlinski VB, Obrenovich ME, et al. (2011) Mitochondrion-specific antioxidants as drug treatments for Alzheimer disease. CNS Neurol Disord Drug Targets 10: 149-162.
- Hernández-Aguilera A, Rull A, Rodríguez-Gallego E, Riera-Borrull M, Luciano-Mateo F, et al. (2013) Mitochondrial dysfunction: a basic mechanism in inflammation-related non-communicable diseases and therapeutic opportunities. Mediators Inflamm 2013: 135698.
- Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, et al. (2012) SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab 15: 675-690.
- Song S, Zhang Y, Ma K, Jackson-Hayes L, Lavrentyev EN, et al. (2004) Peroxisomal proliferator activated receptor gamma coactivator (PGC-1alpha) stimulates carnitine palmitoyltransferase I (CPT-Ialpha) through the first intron. Biochim Biophys Acta 1679: 164-173.
- Schreurs M, Kuipers F, van der Leij FR (2010) Regulatory enzymes of mitochondrial beta-oxidation as targets for treatment of the metabolic syndrome. Obes Rev 11: 380-388.
- van der Leij FR, Cox KB, Jackson VN, Huijkman NC, Bartelds B, et al. (2002) Structural and functional genomics of the CPT1B gene for muscle-type carnitine palmitoyltransferase I in mammals. J Biol Chem 277: 26994-27005.
- Price N, van der Leij F, Jackson V, Corstorphine C, Thomson R, et al. (2002) A novel brain-expressed protein related to carnitine palmitoyltransferase I. Genomics 80: 433-442.
- Wolfgang MJ, Kurama T, Dai Y, Suwa A, Asaumi M, et al. (2006) The brain-specific carnitine palmitoyltransferase-1c regulates energy homeostasis. Proc Natl Acad Sci USA 103: 7282-7287.
- McGarry JD, Brown NF (1997) The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur J Biochem 244: 1-14.
- van der Leij FR, Huijkman NC, Boomsma C, Kuipers JR, Bartelds B (2000) Genomics of the human carnitine acyltransferase genes. Mol Genet Metab 71: 139-153.
- Woldegiorgis G, Dai J, Arvidson D (2005) "Structure-Function Studies with the Mitochondrial Carnitine Palmitoyltransferases I and II". Monatshefte fur Chemie 136: 1325-1340.
- Virmani A, Binienda Z (2004) Role of carnitine esters in brain neuropathology. Mol Aspects Med 25: 533-549.
- Virmani MA, Rossi S, Conti R, Spadoni A, Arrigoni-Martelli E, et al. (1996) Structural, metabolic and ionic requirements for the uptake of L-carnitine by primary rat cortical cells. Pharmacol Res 33: 19-27.
- Fritz I (1955) The effect of muscle extracts on the oxidation of palmitic acid by liver slices and homogenates. Acta Physiol Scand 34: 367-385.
- fritz IB, Yue KT (1963) Long-Chain Carnitine Acyltransferase and the Role of Acylcarnitine Derivatives in the Catalytic Increase of Fatty Acid Oxidation Induced by Carnitine. J Lipid Res 4: 279-288.
- Frohnert BI, Jacobs DR Jr, Steinberger J, Moran A, Steffen LM, et al. (2013) Relation between serum free fatty acids and adiposity, insulin resistance, and cardiovascular risk factors from adolescence to adulthood. Diabetes 62: 3163-3169.
- Cascio G, Schiera G, Di Liegro I (2012) Dietary fatty acids in metabolic syndrome, diabetes and cardiovascular diseases. Curr Diabetes Rev 8: 2-17.
- López M, Lage R, Saha AK, Pérez-Tilve D, Vázquez MJ, et al. (2008) Hypothalamic fatty acid metabolism mediates the orexigenic action of ghrelin. Cell Metab 7: 389-399.
- López M, Lelliott CJ, Vidal-Puig A (2007) Hypothalamic fatty acid metabolism: a housekeeping pathway that regulates food intake. Bioessays 29: 248-261.
- Kerner J, Hoppel C (2000) Fatty acid import into mitochondria. Biochim Biophys Acta 1486: 1-17.
- Steiber A, Kerner J, Hoppel CL (2004) Carnitine: a nutritional, biosynthetic, and functional perspective. Mol Aspects Med 25: 455-473.
- Sierra AY, Gratacós E, Carrasco P, Clotet J, Ureña J, et al. (2008) CPT1c is localized in endoplasmic reticulum of neurons and has carnitine palmitoyltransferase activity. J Biol Chem 283: 6878-6885.
- Dai Y, Wolfgang MJ, Cha SH, Lane MD (2007) Localization and effect of ectopic expression of CPT1c in CNS feeding centers. Biochem Biophys Res Commun 359: 469-474.
- Gao XF, Chen W, Kong XP, Xu AM, Wang ZG (2009) Enhanced susceptibility of Cpt1c knockout mice to glucose intolerance induced by a high-fat diet involves elevated hepatic gluconeogenesis and decreased skeletal muscle glucose uptake. Diabetologia 52: 912-920.
- Wolfgang MJ, Cha SH, Millington DS, Cline G, Shulman GI, et al. (2008) Brain-specific carnitine palmitoyl-transferase-1c: role in CNS fatty acid metabolism, food intake, and body weight. J Neurochem 105: 1550-1559.
- Wolfgang MJ, Lane MD (2006) The role of hypothalamic malonyl-CoA in energy homeostasis. J Biol Chem 281: 37265-37269.
- Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, et al. (2004) AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 428: 569-574.
- Mayer CM, Belsham DD (2010) Palmitate attenuates insulin signaling and induces endoplasmic reticulum stress and apoptosis in hypothalamic neurons: rescue of resistance and apoptosis through adenosine 5' monophosphate-activated protein kinase activation. Endocrinology 151: 576-585.
- Ramírez S, Martins L, Jacas J, Carrasco P, Pozo M (2013) Hypothalamic ceramide levels regulated by CPT1C mediate the orexigenic effect of ghrelin. Diabetes 62: 2329-2337.
- Virmani MA, Conti R, Spadoni A, Rossi S, Arrigoni-Martelli E (1994) L-carnitine uptake into primary rat cortical cultures: interaction with GABA. Brain Res Mol Brain Res 25: 105-112.
- Virmani A, Koverech A (2010) Modulation of carnitine palmitoyltransferase enzyme system by carnitine in cultured rat cortical neurones at different ages of development. Neuroprotective Agents, 10th International Conference on Neuroprotective Agents, Asilomar, California, USA.
- Arduini A, Denisova N, Virmani A, Avrova N, Federici G, et al. (1994) Evidence for the involvement of carnitine-dependent long-chain acyltransferases in neuronal triglyceride and phospholipid fatty acid turnover. J Neurochem 62: 1530-1538.
- Penn D, Schmidt-Sommerfeld E, Wolf H (1980) Carnitine deficiency in premature infants receiving total parenteral nutrition. Early Hum Dev 4: 23-34.
- Schulze A, Lindner M, Kohlmüller D, Olgemöller K, Mayatepek E, et al. (2003) Expanded newborn screening for inborn errors of metabolism by electrospray ionization-tandem mass spectrometry: results, outcome, and implications. Pediatrics 111: 1399-1406.
- Bonnefont JP, Djouadi F, Prip-Buus C, Gobin S, Munnich A, et al. (2004) Carnitine palmitoyltransferases 1 and 2: biochemical, molecular and medical aspects. Mol Aspects Med 25: 495-520.
- Thuillier L, Rostane H, Droin V, Demaugre F, Brivet M, et al. (2003) Correlation between genotype, metabolic data, and clinical presentation in carnitine palmitoyltransferase 2 (CPT2) deficiency. Hum Mutat 21: 493-501.
- Ohtani Y, Tomoda A, Miike T, Matsukura M, Miyatake M, et al. (1994) Central nervous system disorders and possible brain type carnitine palmitoyltransferase II deficiency. Brain Dev 16: 139-145.
- Longo N, Amat di San Filippo C, Pasquali M (2006) Disorders of carnitine transport and the carnitine cycle. Am J Med Genet C Semin Med Genet 142C: 77-85.
- de Almeida Rabello Oliveira M, da Rocha Ataíde T, de Oliveira SL, de Melo Lucena AL, de Lira CE, et al. (2008) Effects of short-term and long-term treatment with medium- and long-chain triglycerides ketogenic diet on cortical spreading depression in young rats. Neurosci Lett 434: 66-70.
- Hori T, Fukao T, Kobayashi H, Teramoto T, Takayanagi M, et al. (2010) Carnitine palmitoyltransferase 2 deficiency: the time-course of blood and urinary acylcarnitine levels during initial L-carnitine supplementation. Tohoku J Exp Med 221: 191-195.
- Washington L, Cook GA, Mansbach CM 2nd (2003) Inhibition of carnitine palmitoyltransferase in the rat small intestine reduces export of triacylglycerol into the lymph. J Lipid Res 44: 1395-1403.
- Carrasco P, Sahun I, McDonald J, Ramirez S, Jacas J, et al. (2012) Ceramide levels regulated by carnitine palmitoyltransferase 1C control dendritic spine maturation and cognition. J Biol Chem 287: 21224-21232.
- Gao S, Zhu G, Gao X, Wu D, Carrasco P, et al. (2011) Important roles of brain-specific carnitine palmitoyltransferase and ceramide metabolism in leptin hypothalamic control of feeding. Proc Natl Acad Sci U S A 108: 9691-9696.
- Reamy AA, Wolfgang MJ (2011) Carnitine palmitoyltransferase-1c gain-of-function in the brain results in postnatal microencephaly. J Neurochem 118: 388-398.
- Wang X, Mick GJ, Maser E, McCormick K (2011) Manifold effects of palmitoylcarnitine on endoplasmic reticulum metabolism: 11β-hydroxysteroid dehydrogenase 1, flux through hexose-6-phosphate dehydrogenase and NADPH concentration. Biochem J 437: 109-115.
- Rowland AA, Voeltz GK (2012) Endoplasmic reticulum-mitochondria contacts: function of the junction. Nat Rev Mol Cell Biol 13: 607-625.
- Zaugg K, Yao Y, Reilly PT, Kannan K, Kiarash R, et al. (2011) Carnitine palmitoyltransferase 1C promotes cell survival and tumor growth under conditions of metabolic stress. Genes Dev 25: 1041-1051.
- Morillas M, Go’mez-Puertas P, Bentebibel A, Selle’s E, Casals N, et al. (2003) Identification of conserved amino acid residues in rat liver carnitine palmitoyltransferase I critical for malonyl-CoA inhibition. Mutation of methionine 593 abolishes malonyl-CoA inhibition. J Biol Chem 278: 9058-9063.
- Barger JF, Gallo CA, Tandon P, Liu H, Sullivan A, et al. (2013) S6K1 determines the metabolic requirements for BCR-ABL survival. Oncogene 32: 453-461.
- Warburg O, Wind F, Negelein E (1927) THE METABOLISM OF TUMORS IN THE BODY. J Gen Physiol 8: 519-530.
- Warburg O (1956) On the origin of cancer cells. Science 123: 309-314.
- Lee J, Wolfgang MJ (2012) Metabolomic profiling reveals a role for CPT1c in neuronal oxidative metabolism. BMC Biochem 13: 23.
- Sanvicens N, Cotter TG (2006) Ceramide is the key mediator of oxidative stress-induced apoptosis in retinal photoreceptor cells. J Neurochem 98: 1432-1444.
- Andrieu-Abadie N, Gouazé V, Salvayre R, Levade T (2001) Ceramide in apoptosis signaling: relationship with oxidative stress. Free Radic Biol Med 31: 717-728.
- Grishko V, Rachek L, Musiyenko S, Ledoux SP, Wilson GL (2005) Involvement of mtDNA damage in free fatty acid-induced apoptosis. Free Radic Biol Med 38: 755-762.
- Mondal AK, Das SK, Varma V, Nolen GT, McGehee RE, et al. (2012) Effect of endoplasmic reticulum stress on inflammation and adiponectin regulation in human adipocytes. Metab Syndr Relat Disord 10: 297-306.
- Virmani A, Pinto L, Binienda Z, Ali S (2013) Food, nutrigenomics, and neurodegeneration--neuroprotection by what you eat! Mol Neurobiol 48: 353-362.
- Sun Y, Chang YH, Chen HF, Su YH, Su HF, et al. (2012) Risk of Parkinson disease onset in patients with diabetes: a 9-year population-based cohort study with age and sex stratifications. Diabetes Care 35: 1047-1049.
- Bruce CR, Hoy AJ, Turner N, Watt MJ, Allen TL, et al. (2009) Overexpression of carnitine palmitoyltransferase-1 in skeletal muscle is sufficient to enhance fatty acid oxidation and improve high-fat diet-induced insulin resistance. Diabetes 58: 550-558.
- Hu G, Jousilahti P, Bidel S, Antikainen R, Tuomilehto J (2007) Type 2 diabetes and the risk of Parkinson's disease. Diabetes Care 30: 842-847.
- Morris JK, Bomhoff GL, Gorres BK, Davis VA, Kim J, et al. (2011) Insulin resistance impairs nigrostriatal dopamine function. Exp Neurol 231: 171-180.
- Lefort N, Glancy B, Bowen B, Willis WT, Bailowitz Z, et al. (2010) Increased reactive oxygen species production and lower abundance of complex I subunits and carnitine palmitoyltransferase 1B protein despite normal mitochondrial respiration in insulin-resistant human skeletal muscle. Diabetes 59: 2444-2452.
- Hauser DN, Hastings TG (2013) Mitochondrial dysfunction and oxidative stress in Parkinson's disease and monogenic parkinsonism. Neurobiol Dis 51: 35-42.
- Virmani A, Gaetani F, Binienda Z, Xu A, Duhart H, et al. (2004) Role of mitochondrial dysfunction in neurotoxicity of MPP+: partial protection of PC12 cells by acetyl-L-carnitine. Ann N Y Acad Sci 1025: 267-273.
- Benoit SC, Kemp CJ, Elias CF, Abplanalp W, Herman JP, et al. (2009) Palmitic acid mediates hypothalamic insulin resistance by altering PKC-theta subcellular localization in rodents. J Clin Invest 119: 2577-2589.
- Rhein V, Eckert A (2007) Effects of Alzheimer's amyloid-beta and tau protein on mitochondrial function -- role of glucose metabolism and insulin signalling. Arch Physiol Biochem 113: 131-141.
- Rönnemaa E, Zethelius B, Sundelöf J, Sundström J, Degerman-Gunnarsson M, et al. (2008) Impaired insulin secretion increases the risk of Alzheimer disease. Neurology 71: 1065-1071.
- Solfrizzi V, Scafato E, Capurso C, D'Introno A, Colacicco AM, et al. (2010) Italian Longitudinal Study on Ageing Working Group. Metabolic syndrome and the risk of vascular dementia: the Italian Longitudinal Study on Ageing. J Neurol Neurosurg Psychiatry 81: 433-440.
- Raffaitin C, Gin H, Empana JP, Helmer C, Berr C, et al. (2009) Metabolic syndrome and risk for incident Alzheimer's disease or vascular dementia: the Three-City Study. Diabetes Care 32: 169-174.
- Profenno LA, Porsteinsson AP, Faraone SV (2010) Meta-analysis of Alzheimer's disease risk with obesity, diabetes, and related disorders. Biol Psychiatry 67: 505-512.
- Shriver LP, Manchester M (2011) Inhibition of fatty acid metabolism ameliorates disease activity in an animal model of multiple sclerosis. Sci Rep 1: 79.
- Müller N, Myint AM, Schwarz MJ (2011) Kynurenine pathway in schizophrenia: pathophysiological and therapeutic aspects. Curr Pharm Des 17: 130-136.
- Erhardt S, Schwieler L, Nilsson L, Linderholm K, Engberg G (2007) The kynurenic acid hypothesis of schizophrenia. Physiol Behav 92: 203-209.
- Thibault O, Anderson KL, Demoll C, Brewer LD, Landfield PW, et al. (2013) Hippocampal calcium dysregulation at the nexus of diabetes and brain aging. Eur J Pharmacol.
- Gavrilova SI, Kalyn IaB, Kolykhalov IV, Roshchina IF, Selezneva ND (2011) [Acetyl-L-carnitine (carnicetine) in the treatment of early stages of Alzheimer's disease and vascular dementia]. Zh Nevrol Psikhiatr Im S S Korsakova 111: 16-22.
- Malaguarnera M, Gargante MP, Cristaldi E, Colonna V, Messano M, et al. (2008) Acetyl L-carnitine (ALC) treatment in elderly patients with fatigue. Arch Gerontol Geriatr 46: 181-190.
- Passeri M, Cucinotta D, Bonati PA, Iannuccelli M, Parnetti L, et al. (1990) Acetyl-L-carnitine in the treatment of mildly demented elderly patients. Int J Clin Pharmacol Res 10: 75-79.
- Sanders YY, Liu H, Zhang X, Hecker L, Bernard K, et al. (2013) Histone modifications in senescence-associated resistance to apoptosis by oxidative stress. Redox Biol 1: 8-16.
- Zhang Y, Yang JM (2013) Altered energy metabolism in cancer: a unique opportunity for therapeutic intervention. Cancer Biol Ther 14: 81-89.
- Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R (2009) Diabetes and cancer. Endocr Relat Cancer 16: 1103-1123.
- Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, et al. (2010) Diabetes and cancer: a consensus report. Diabetes Care 33: 1674-1685.
- Reilly PT, Mak TW (2012) Molecular pathways: tumor cells Co-opt the brain-specific metabolism gene CPT1C to promote survival. Clin Cancer Res 18: 5850-5855.
- Linher-Melville K, Zantinge S, Sanli T, Gerstein H, Tsakiridis T, et al. (2011) Establishing a relationship between prolactin and altered fatty acid Î2-oxidation via carnitine palmitoyl transferase 1 in breast cancer cells. BMC Cancer 11: 56.
- Mazzarelli P, Pucci S, Bonanno E, Sesti F, Calvani M, et al. (2007) Carnitine palmitoyltransferase I in human carcinomas: a novel role in histone deacetylation? Cancer Biol Ther 6: 1606-1613.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 16907
- [From(publication date):
February-2014 - Aug 18, 2025] - Breakdown by view type
- HTML page views : 12259
- PDF downloads : 4648