Neuroprotective Potentials of Ayurvedic Rasayana Desmodium triquetrum on Brain Aging and Chemically Induced Amnesia in Animal Models Relevant to Dementia
Received: 02-Mar-2018 / Accepted Date: 21-Mar-2018 / Published Date: 26-Mar-2018 DOI: 10.4172/2573-4555.1000272
Abstract
Cognitive impairments in dementia is a common feature which disrupts life style of the patients suffering neurodegenerative disorders. Management of cognitive dysfunctions such as dementia and Alzheimer’s disease is very challenging as there is proper medicine with proved effect is available at present. In the current study, neuroprotective potentials of methanol extract of dried roots of Desmodium triquetrum (DT) was investigated in the mouse models relevant to dementia. Exteroceptive behavioral models like Elevated plus maze and Morris water maze were used to assess learning and memory in mice. Scopolamine (0.4 mg/kg, i.p.) induced amnesia and diazepam (1 mg/kg, i.p.) amnesia were the interoceptive behavioral models. DT (50 and 100 mg/kg, p.o.) significantly attenuated amnesic deficits in scopolamine, diazepam and natural aging amnesic models. DT was also useful in reversing aging induced amnesia. DT increased whole brain acetyl cholinesterase inhibition activity in mice. Hence, DT might be used as memory enhancing agent and possibly may be employed in treatment of dementia and other neurodegenerative disorders. The mechanism of action may be due to its potential neuroprotective and acetyl cholinesterase inhibitory properties.
Keywords: Acetyl cholinesterase; Desmodium triquetrum; Dementia; Memory; Scopolamine
Introduction
Memory function is prone to deteriorate due to a number of pathological processes such as neurodegenerative disorders, brain stroke, tumors, head injuries, cerebral ischemia, improver nutrition, attention deficit disorders, depression, anxiety, adverse effects of drugs, and ageing [1]. Alzheimer’s disease (AD) is a type of dementia characterized by neurodegeneration leading to cognitive dysfunction [2]. It is a very chronic, progressive and organic brain disorder with disturbances in various cortical functions, comprising memory impairment, decreased judgment, poor orientation, improper comprehension, reduced learning capability and trouble in language [3]. As the life expectancy among society is increasing the number of patients suffering from cognitive disorders are also increasing, the research for drugs that can possibly decrease or minimize cognitive impairments due to aging is gaining importance. As per estimation in next 50 years, around 30% of the global population shall be aged [4]; by 2025, 70% of world’s aged population shall be living in developing countries [5]. The NIH opines, if the same scenario continues, there may be larger than 8.5 million AD patients population by 2030 in USA itself [6,7]. Nootropic drugs such as piracetam [8], pramiracetam, aniracetam [9] and choline esterase inhibitors like Donepezil®, rivastigmine, galatamine are currently employed for treating dementia and other ailments. But, the adverse reactions due to use of these drugs raising lot of concerns [10] and hence is it necessary to investigate the usefulness of traditional medicines such as Ayurveda in the management of cognitive dysfunctions.
For thousands of years, plants have been used for cognitive impairment in India. Ayurveda, the Indian system of medicine describes the use of medhya rasayana (rejuvenating and intellect promoting) drugs in the management of nervous disorders. The Ayurvedic concept of rasayana consists of specialized class of drugs which prevent ageing, increase longevity, impart immunity, improve mental functions and add vigor and vitality of the body [11].
Desmodium triquetrum belongs to family Leguminosae, Subfamily-Papilionaceae, is an erect or suberect undershrub, distributed throughout central, eastern Himalayas, South India and Sri Lanka. The leaves are used as a substitute for tea by hill tribes in upper Assam. The leaf extracts or pills are used for the treatment of piles [12]. The chloroform and alcohol extracts of DT were reported for their antibacterial activity [13]. The ethanol extract of leaves of this plant has been reported for its wound healing activity. Preliminary phytochemical investigations on the DT revealed the presence of flavonoids, glycosides, steroids, saponins, phenolic compounds, amino acids and fixed oils [14]. It was also reported that the whole plant is boiled and used against the treatment of liver parasite by Lao people [15].
In this study, the neuroprotective potential of effects of DT were assessed by using exteroceptive and interoceptive behavioral animal models. Interoceptive behavioural models such as scopolamine, diazepam and natural aging induced amnesia are widely used animal models simulating human dementia in general and dementia of Alzheimer’s type in particular [16].
Material and Methods
Plant materials and extraction
Roots of DT were collected from Chamundi hills, Mysuru, Karnataka, India. The plant was identified by the experts at Dept. of Pharmacognosy, Sarada Vilas College of Pharmacy, Mysuru, Karnataka. Roots were washed under tap water, shade dried, powdered and passed through 10-mesh sieve. The coarsely powdered materials (1000 g) were soaked in distilled water in the ratio of 1:16 (w/v). The extract was filtered, pooled and first concentrated on rotavapour and then freeze dried with high vacuum (yield 14.1% (w/w)). The phytochemical contents of the extract were tested by various methods of qualitative analysis and thin layer chromatography [17], which indicated the presence of alkaloids and flavonoids. Distilled water containing 1% (w/v) carboxy methyl cellulose (CMC) were used for preparation of the suspension.
Drugs and reagents
Scopolamine hydrobromide (Sigma Aldrich, USA), diazepam (Calmpose®, Ranbaxy, India), piracetam (Nootropil, UCB India Pvt. Ltd., India) diluted in saline and injected intra-peritoneally. Volume of administration was 1 ml/100 g. All the drugs were administered in the morning between 8 AM to 9 AM every day. Acetylcholinesterase kits (NS Scientific, India) was used for estimating AChE.
Animals
Swiss mice of either sex with 18 g-20 g (young mice, age 3-4 months) and 30 g and above (aged mice, 12-15 months) were used. The mice were kept for acclimatization for 5 days before subjecting them to behavioral tests. The Institutional Animals Ethics Committee (IAEC) approved the protocol (SVCP/IAEC/2163-2017) and the care was taken as per CPCSEA guidelines, Ministry of Environment, forests and climate change, India. Each group consisted of 6 animals.
Acute toxicity studies
Various doses of DT methanolic extract (10, 100, 500, 1000 and 2000 mg/kg, p.o) was administered to the mice through oral gavage with the help of a specially designed oral needle connected to a polythene tube, at the same timings on every day (i.e. 8 AM-9 AM). The animals were observed for gross behavioral changes for 7 days, during the first four hours after the administration of DT. Hyperactivity, grooming, convulsions, sedation, hypothermia and mortality were recorded. Doses selected were 50 mg/kg and 100 mg/kg per day.
Elevated plus-maze (EPM)
EPM is an exteroceptive behavioral model widely used for evaluating acquisition and retention of memory in mice [16-18]. The EPM (mouse model) has two open arms (16 cm × 5 cm) and two covered arms (16 cm × 5 cm × 12 cm) extended from a central platform (5 cm × 5 cm), and the maze is elevated to height of 25 cm from the floor. On first day, every mouse was placed at the end of the open arm, facing away from central platform. Transfer latency (TL) is defined as the time taken by animal to move from open arm into one of the covered arms with all its four legs. TL was recorded on the first day for each animal. The mouse was permitted to explore the maze for another 2 minutes and then returned to its home cage. Retention of this learned-task was examined 24 h after the first day trial [18].
Morris water maze (MWM)
The MWM test was employed to assess learning and memory of the animals. MWM is a swimming model where the animals learn to escape on to a hidden platform. In the present study the target quadrant was Q4. The mice were subjected to 4 consecutive trials every day with a gap of 5 minutes for 4 e days continuously, during which they were permitted to escape to the hidden platform and to be remained for 20 sec. If the animal was not able to find the hidden platform within 120 seconds, the mouse was gently pushed and guided to the platform and permitted to stay on the platform for further 20 seconds. Escape latency time and to identify the hidden platform in Morris water maze was the index of acquisition (learning) [19]. The starting point on every day to conduct 4 acquisition trials was changed as described below and Q4 was maintained as the target quadrant in all the acquisition trials. The starting point for dropping the mice into water maze on day 1 for four consecutive acquisition trials was in the sequence Q1, Q2, Q3 Q4 and so on. The sequence change in starting point was as follows. Day 1: Q1, Q2, Q3, Q4 Day 2: Q2, Q3, Q4, Q1 Day 3: Q3, Q4, Q1, Q2 Day 4: Q4, Q1, Q2, Q3. Mean escape latency time (ELT) was calculated for each day of the trial. On the 5th day the hidden platform was removed, each mouse was permitted to explore the pool for 120 seconds. The animal was made to take 4 such trials with 5-minute interval time and every trial had a different starting point covering all the 4 quadrants. The mean of time spent by the animal in all 4 quadrants was recorded. The TSTQ (time spent in target quadrant) in Q4 as compared to time spent in other quadrants in locating missing platform was considered as an index of retrieval (memory). Utmost care was employed to ensure that relative location of water maze with respect to any other objects in the laboratory serving as visual clues was not disturbed during the total duration of the study. All the trials were completed between 09:00 and 17:00 hours [20].
Interoceptive behavioral models
Scopolamine-induced amnesia: Scopolamine hydrobromide (0.4 mg/kg, ip) was administered on 8th day and the TL were recorded. Ater 24 h, retention was recorded. DT (50 and 100 mg/kg, po) and piracetam (200 mg/kg) were administered for 8 days successively. On the day 8th, after 45 min of administration of DT, scopolamine was administered, TL was recorded after 45 min. SDL was recorded on 9th day [21].
Diazepam induced amnesia: Diazepam (1mg/kg, i.p) was injected to the young mice and TL was recorded after 45 min of administration on day 8th and after 24 h. DT (50 and 100 mg/kg, p.o.) and standard drug piracetam (200 mg/kg, i.p.) were administered successively for 8 days. 60 min after giving the last dose on day 8th, diazepam (1 mg/kg, ip) was injected. After 45 min of administration of diazepam, TL was noted and again after 24 h. SDL was recorded on 9th day [22].
Collection of brain samples and brain acetyl cholinesterase (AChE) activity
The mice were sacrificed (cervical decapitation) under ether anesthesia on the day 8th that is 90 min after giving the last dose of DT. The whole brain was removed from the skull. For preparing the brain homogenate, the whole brains were weighed and homogenized in ice bath, 10 volumes of 0.9% w/v sodium chloride solution was added. The homogenate was subjected to centrifugation at 3000 rpm for 10 min and the supernatant was used for estimating brain AChE activity spectroscopically using the Ellman method [23].
Locomotor activity
Locomotor activity of all animals was measured using a photoactometer (INCO Ltd., Ambala, India).
Statistical analysis
The results were expressed as mean ± standard error. The data was analyzed using ANOVA followed by Tukey-kramer test using SPSS software.
Results
Acute toxicity
All the doses (5, 50, 250, 500 and 2000 mg/kg, p.o.) of DT did not lead to mortality including the highest dose (2000 mg/kg, p.o.) used. Two submaximal doses (50 and 100 mg/kg, p.o.) were sused for pharmacological and biochemistry studies.
Effects on locomotor functioning
There was no significant change in the locomotor function of animals (232 ± 1.8 and 210 ± 08) treated with DT (50 and 100 mg/kg, p.o.), as compared to control (219.2 ± 09).
Effect on transfer latency (TL)
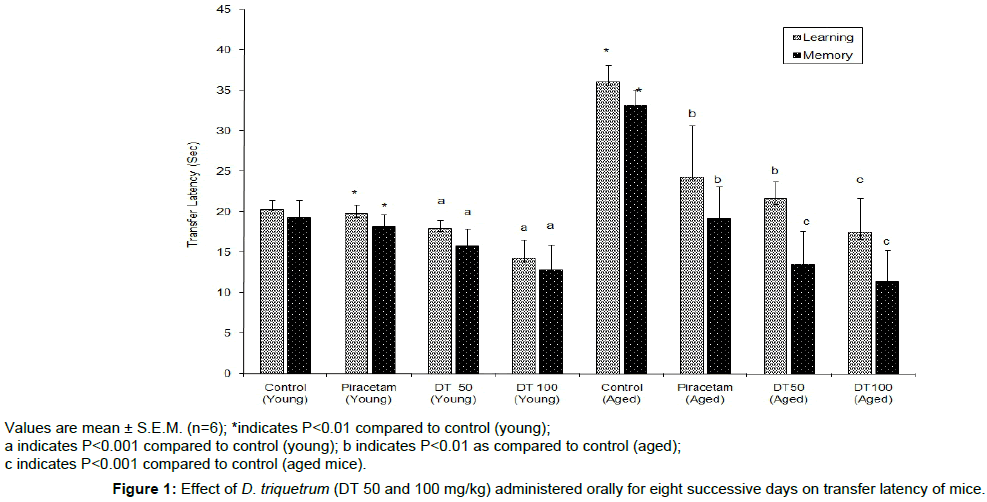
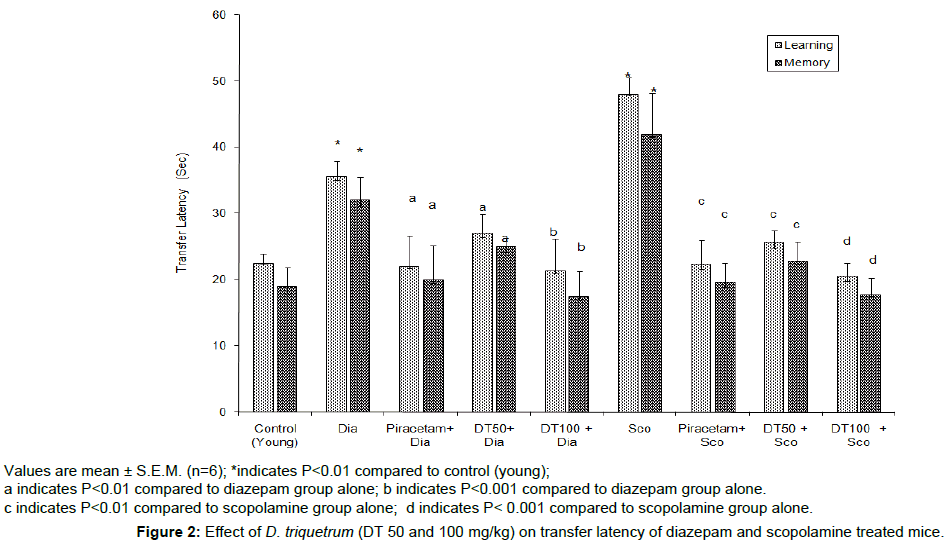
DT (50 and 100 mg/kg, p.o.) showed dose-dependent decrease in TL on day 8th and day 9th, indicating remarkable improvement in learning ability and memory of the mice (young and aged) when compared to their respective control groups (Figure 1). Diazepam (1 mg/kg, i.p.) and scopolamine (0.4 mg/kg, i.p.) significantly increased (P<0.01) the TL of day 9th which indicated memory impairment. DT (50 and 100 mg/kg, p.o.) successfully (P<0.001) attenuated the memory impairment due to diazepam and scopolamine (Figure 2).
Values are mean ± S.E.M. (n=6); *indicates P<0.01 compared to control (young);
a indicates P<0.001 compared to control (young); b indicates P<0.01 as compared to control (aged);
c indicates P<0.001 compared to control (aged mice).
Figure 1: Effect of D. triquetrum (DT 50 and 100 mg/kg) administered orally for eight successive days on transfer latency of mice.
Values are mean ± S.E.M. (n=6); *indicates P<0.01 compared to control (young);
a indicates P<0.01 compared to diazepam group alone; b indicates P<0.001 compared to diazepam group alone.
c indicates P<0.01 compared to scopolamine group alone; d indicates P< 0.001 compared to scopolamine group alone.
Figure 2: Effect of D. triquetrum (DT 50 and 100 mg/kg) on transfer latency of diazepam and scopolamine treated mice.
Effect on escape latency time (ELT) using Morris water maze
In MWM extreroceptive model, there was a profound fall in ELT of standard drug and DT (50 and 100 mg/kg, p.o.) treated groups (24 days) when compared with control group which infers the increase in acquisition. Further there was a significant rise in TSTQ of standard drug and DT (100 mg/kg, p.o.) on 25th day compared to TSTQ of normal control group. Even though there was a profound decrease in ELT and increase in TSTQ of DT (50 mg/kg, p.o.) was not significant as compared to normal control group (Table 1).
| Drug | ELT (21st day) | ELT (24th day) | TSTQ (25th day) |
|---|---|---|---|
| Control (10 mg/kg, P.O.) | 91.06 ± 0.57 | 49.13 ± 0.91 | 59.05 ± 0.4 |
| Piracetam (200 mg/kg, i.p.) | 73.10 ± 1.9* | 34.05 ± 1.8* | 70.69 ± 1.1* |
| DT (50 mg/kg) | 79.13 ± 02a | 43.05 ± 0.2a | 51.12 ± 0.2a |
| DT (100 mg/kg, P.O.) | 73.11 ± 0.7a | 41.16 ± 0.8a | 39.19 ± 0.8a |
Table 1: Effect of D. triquetrum (DT 50 and 100 mg/kg) on Escape Latency time (ELT) and Time Spent in Target Quadrant (TSTQ) in young mice.
Effect on AChE activity
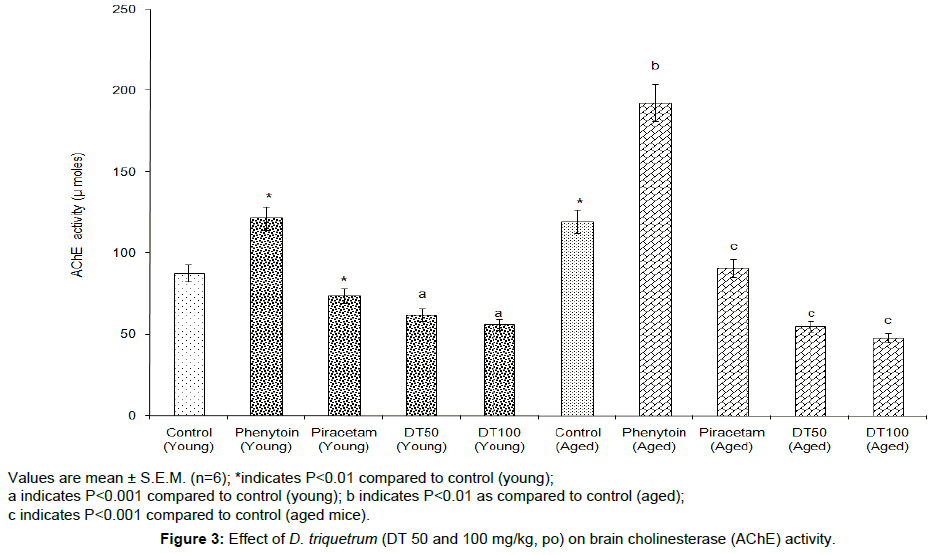
DT (50 and 100 mg/kg, p.o.) showed a remarkable reduction in the whole brain AChE activity in both young and aged mice, when compared with their control groups. Whereas, phenytoin (12 mg/kg, p.o.) significantly (P<0.01) increased the AChE activity (Figure 3).
Values are mean ± S.E.M. (n=6); *indicates P<0.01 compared to control (young);
a indicates P<0.001 compared to control (young); b indicates P<0.01 as compared to control (aged);
c indicates P<0.001 compared to control (aged mice).
Figure 3: Effect of D. triquetrum (DT 50 and 100 mg/kg, po) on brain cholinesterase (AChE) activity.
Discussion
The complications associated with dementia are due to neurotransmission impairments and neurodegeneration in neuronal circuits in the affected areas of brain [24]. Deterioration of learning and memory in AD patients might be due to severe loss of cholinergic neurons leading to decreased levels of acetylcholine (ACh) in the brain, mainly in the temporal areas, parietal neocortex and hippocampus of brain [25]. These indicate cholinergic function impairments majorly contribute to the various signs and symptoms of dementia. Cholinergic replacement therapy (CPT) can be useful for dementia patients. Acetylcholine is a very important neurotransmitter controlling and regulating the memory functions. There are evidences establishing a good linkage between central cholinergic system and memory [26]. Memory impairments have been found to be associated with decreased transmission of cholinergic neurons and the agents which can facilitates the activity of central cholinergic transmission leading to cognition enhancement [27]. Loss of cholinergic neurons and reduced in cholinacetyl transferase has been reported to be major indications of senile dementia of the Alzheimer’s type [28]. Blocking of cholinesterase induced hydrolysis of ACh leading to possible increase in concentration of ACh in synapses and potentiating of cholinergic function provides the some relief to the patients which can be observed in AD patients who are on pharmacotherapy with cholinesterase inhibitors [29-32]. In this study D. triquetrum significantly improve learning and memory in both exteroceptive and interoceptive behavioral models in mice. It also increased the acetyl cholinesterase activity indicating profound improvement of acquisition and retention in mice.
Conclusion
D. triquetrum an Ayurvedic rasayana (rejuvenating herb) may be a useful memory restorative agent for the preliminary management of various pre symptoms of memory dysfunctions such as Alzheimer’s disease and dementia. Further investigations using human volunteers are warranted for confirming the neuroprotective efficacy.
Acknowledgements
The authors are thankful to the Rajiv Gandhi University of Health Sciences, Karnataka, Bengaluru for the research grant. Thanks are also due to UCB India Pvt. Ltd., Vapi, Gujarat, India for gift sample of Piracetam.
References
- Rowan M (2012) Information selectivity of beta-amyloid pathology in an associative memory model. Front Comput Neurosci 6: 2-16.
- Drngenberg HC (2000) Alzheimer’s disease: More than a cholinergic disorder evidence that cholinergic-monoaminergic interactions contribute to EEG slowing and dementia. Behav Brain Res 115: 235-239.
- Robert K (1998) Risk factors for alzheimer’s disease. Neuro Sci News 1: 27-44.
- Youdim KA, Joseph JA (2001) A possible emerging role of phytochemicals in improving age-related neurological dysfunctions: A multiplicity of effects. Free Radic Biol Med 30: 583-594.
- Kalache A, Aboderin I, Hoskins I (2002) Compression of morbidity and active ageing: Key priorities for public health policy in the 21st century. Bulletin of the World Health Organization 80: 243-244.
- Doody RS, Stevens JC, Beck RN, Dubinsky RM, Koye JA, et al. (2001) Practice parameters: Management of dementia (an evidence based review): Report of the quality standards subcommittee of the American Academy of Neurology. Neurology 56: 1154-1161.
- Herbert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA (2003) Alzheimer disease in the U.S. Population: Prevalence estimates using the 2000 Census. Arch Neurol 160: 1119-1122.
- Schever K, Rostock A, Bartsch P, Muller WE (1999) Piracetam improved cognitive performance by restoring neurochemical deficits of the aged rat brain. Pharmacopsychiatry32: 10.
- Cumin R, Bandle EF, Gamzu E, Haefely WE (1982) Effects of the novel compound aniracetam (Ro-13-5057) upon impaired learning and memory in rodents. Psychopharmacol 78: 104-111.
- Rogers EJ, Milhalik S, Ortiz D, Shea TB (2004) Apple juice prevents oxidative stress and impaired cognitive performance caused by genetic and dietary deficiencies in mice. J Nutr Health Aging 8: 92-97.
- Sharma PV, Dravya G (1982) Chaukambha Bharati Academy. 2nd edn. Varanasi.
- Anonymous (1952) The wealth of India. Raw materials Council of Scientific and Industrial Research. 42-43.
- Shirwaikar A, Jahagirdar S, Udupa AL (2003) Wound healing activity of Desmodium triquetrum leaves. Indian J Pharm Sci 65: 461-464.
- Mujumdar AM, Dhuley JN, Deshmukh VK, Raman H, et al. (1990) Anti-inflammatory activity of piperin. Jpn J Med Sci Biol 43: 95-100.
- Itoh J, Nabeshima T, Kameyama T (1990) Utility of an elevated plus maze for the evaluation of nootropics, scopolamine and electro convulsive shock. Psychopharmacol 101: 27-33.
- Parle M, Dhingra D (2003) Ascorbic acid: A promising memory enhancer in mice. J Pharmacol Sci 93: 129-135.
- Biradar SM, Joshi H, Chheda TK (2012) Neuropharmacological effect of mangiferin on brain cholinesterase and brain biogenic amines in the management of Alzheimer’s disease. Eur J Pharmacol 683: 140-147.
- Joshi H, Mittal B (2014) Evaluation of freeze dried extract of Mentha piperita in management of cognitive dysfunctions in mice. Alzheimers Dement 10: 461-462.
- Morris R (1984) Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11: 47-60.
- Hooge DR, De Deyn PP (2001) Application of the Morris water maze in the study of learning and memory. Brain Res Rev 36: 60-90.
- Joshi H, Parle M (2006) Evaluation of nootropic potential of Ocimum sanctum linn. in mice. Indian J Exp Biol 44: 133-136.
- Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colourimetric determination of acetylcholinestrase activity. Biochem Pharmacol7: 88-95.
- Ellis JM (2005) Cholinesterase inhibitors in the treatment of dementia. J Am Osteopath Assoc 3: 145-158.
- Ghelardini C, Galeotti N, Barboloni A, Furukawa S (1998) Memory facilitation and stimulation of endogenous nerve growth factor synthesis by the acetylcholine releaser PG-9. Jpn J Pharmacol 78: 245-251.
- Joshi H, Parle M (2006) Antiamnesic effects of Desmodium gangeticum in mice. Yakugaku Zasshi 126: 795-804.
- Joshi H, Parle M (2007) Antiamnesic potentials of Foeniculum vulgare linn. in mice. Orient Pharm Exp Med 7: 182-190.
- Agnolli A, Martucci N, Manna V, Conti L (1983) Effect of cholinergic and anticholinergic drugs on short term memory in electroencephalographic study. Clin Neuropharmacol 6: 311-323.
- Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, et al. (1982) Alzheimer’s disease and senile dementia: Loss of neurons in the basal forebrain. Science 215: 1237-1239.
- Collinge J (2016) Mammalian prions and their wider relevance in neurodegenerative diseases. Nature 539: 217-226.
- Joshi H, Parle M (2006) Zingiber officinale: Evaluation of its nootropic effect in mice. Afr J Trad Compl Alt Med 3: 64-74.
- Lu T, Aron L, Zullo J, Pan Y, Kim H, et al. (2016) Addendum: REST and stress resistance in ageing and Alzheimer’s disease. Nature 507: 470.
- Joshi H, Majed AA, Charan CS (2017) Ameliorative effects of roots of Asparagus adscendens roxb. on cognitive impairments and brain aging induced by scopolamine and diazepam in animal models relevant to Alzheimer’s disease. J of Pharm Res 16: 199-207.
Citation: Joshi H, Charan CS, Alkanad MA (2018) Neuroprotective Potentials of Ayurvedic Rasayana Desmodium triquetrum on Brain Aging and Chemically Induced Amnesia in Animal Models Relevant to Dementia. J Tradit Med Clin Natur 7: 272. DOI: 10.4172/2573-4555.1000272
Copyright: © 2018 Joshi H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6882
- [From(publication date): 0-2018 - Dec 08, 2025]
- Breakdown by view type
- HTML page views: 5885
- PDF downloads: 997