Novel and Emerging Approach in the Diagnosis and Prognosis of Tuberculosis by Using Biomarkers
Received: 03-Jul-2018 / Accepted Date: 19-Jul-2018 / Published Date: 26-Jul-2018
Keywords: Tuberculosis; Biomarkers; H37Rv pathogenesis; Mycobacterial genetics
Introduction
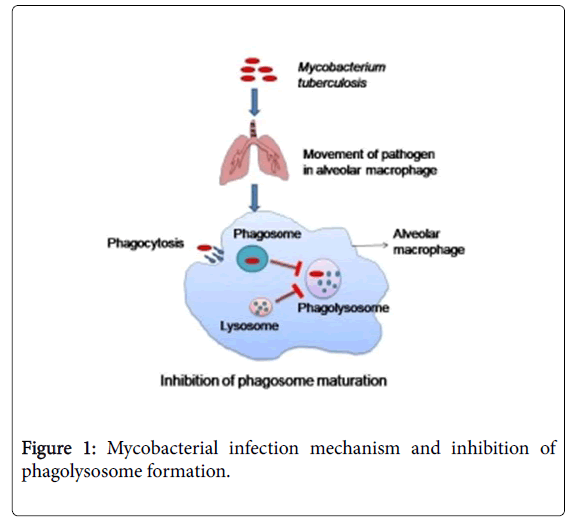
Tuberculosis is one of the major causes of death globally caused by Mycobacterium tuberculosis H37Rv (M. tuberculosis H37Rv). Infection transmits through aerosol particles by coughing and sneezing of an infected person. Generally after inhalation of any bacteria it reaches to lungs and get ingested by alveolar macrophages and gets eliminated but M. tuberculosis H37Rv can persist in macrophages due to its unusual survival strategies such as presence of high lipid content such as lipoarabinomannan (LAM) and mycolic acid, inhibition of phagosome acidification and phagosome-lysosome fusion (Figure 1) [1,2]. Smoking, infection of Human Immunodeficiency Virus (HIV) and poverty is influencing the epidemics of this disease. There were 10.4 million people who fall ill and 3.1 million who die because of tuberculosis (TB) in 2016. The Mortality rate of TB is falling ~3% every year because people are treated successfully if diagnosed earlier. But the rate of successful treatment is low due to the development of the drug-resistant TB like multidrug resistance (MDR), extensively drug resistance (XDR) and total drug resistance (TDR).
Sustainable development goal (SDG) is to finish the TB infection by 2030 [3,4]. By focusing on the low specificity of diagnostic procedures like sputum smear microscopy and sputum cultures etc. we need to develop new strategies or some rapid, accessible and effective diagnostic approaches [5,6]. Interferon-gamma release assay (IGRA) was designed to overcome the low sensitivity of tuberculin skin test (TST) and for better prediction of infection. IGRA measure T-cell responses to M. tuberculosis H37Rv specific antigen (Table 1). Interferon-gamma (type II interferon) is a key signaling molecule which belongs to cytokine proteins. IFN-γ induces the expression of class II major histocompatibility complex (MHCs) on antigen presenting cells (APCs) and promotes immune cells proliferation (Tcells and B-cells), cell adhesion, apoptosis of antigen affected cells and M. tuberculosis H37Rv growth control [7,8]. Observation and quantification of biomarkers are very helpful in the early diagnosis and treatment of infectious diseases [9]. Any characteristics like specific cells, molecule, gene, hormone or enzymes which indicates the emergence of an infection or a disease, can be detected and measured in blood or tissue can act as a biomarker for that disease. Host immunological biomarkers are needed for diagnosis, risk correlation and to generate novel methods for treatment. In this review, the authors explore various immunological biomarkers which may be used as diagnostic tools for M. tuberculosis H37Rv and can help in declining the global epidemic of this disease.
| Test | Accuracy | Reference |
|---|---|---|
| Sputum smear microscopy | The sensitivity is grossly compromised when bacterial load is less than 10,000 organism/ml sample | 37 |
| Also it is difficult to detect extra pulmonary tuberculosis, pediatric tuberculosis and TB co infection with HIV | ||
| Sputum culture | Time consuming procedure takes almost 3-4 weeks to culture the bacteria | 37 |
| Tuberculin skin test (TST) | Time taking job and give accurate result (false positive results) for latent TB | 40-41 |
| Interferon Gamma Release Assays (IGRA) | Blood samples need quick sampling with precautions | 38-39 |
| Only detects latent TB | ||
| Unable to identify TB-HIV co infection |
Table 1: Different tests used for diagnosis of TB with their accuracy.
Mycobacterium invades inside the host and reaches into lungs and recruits alveolar macrophages. Phagocytosis takes place against Mycobacterium tuberculosis to eliminate it, but this bacterium can persist due to its unusual survival strategy, by inhibiting phagosome and lysosome fusion, therefore phagolysosome does not form to degrade the pathogen. So mycobacterium can easily get away from the hydrolytic environment of phagolysosome.
Mycobacterial Genetics and Mechanism of Pathogenesis
Whole genome sequencing of M. tuberculosis H37Rv reveals various gene of this bacterium that plays a significant role in the survival of this bacterium inside host cell phagosome, Later on, a portion of these qualities, the proteins they encode, and additionally newfound ones, ought to give new bacterial focuses on that can be utilized for making antibodies, develop medications and also more specific symptomatic reagents. Genome in the M. tuberculosis H37Rv complex, including the human and creature pathogens M. africanum, M. microti , and M. canetti , and additionally M. tuberculosis and M. bovis were portrayed by DNA sequencing and related strategies. These investigations have demonstrated a more prominent than 99.9% compatability of DNA sequence among the individuals from the M. tuberculosis H37Rv complex; however the presence of uncommon synonymous singlenucleotide polymorphisms (sSNP) permits separation between these firmly related microscopic organisms [10]. M. tuberculosis H37Rv principally remains in macrophages. As opposed to other bacterial pathogens that keep away from phagocytosis as a particular pathogenic procedure, M. tuberculosis H37Rv is unbridled in its utilization of numerous cell surface receptors to pick up passage into macrophages [11]. These receptors incorporate the mannose receptor, supplement receptors and Fc receptors. Once inside the host macrophage, M. tuberculosis H37Rv lives inside a film bound vacuole [12,13].
Unmistakably M. tuberculosis H37Rv alters the development of this phagosomal compartment keeping in mind the end goal to upgrade its own particular intracellular survival [14,15]. This modified phagosomal development is related with modifications in the protein substance of the vacuole including adjusted Rab GTPase structure rejection of the vacuolar proton ATPase with resulting absence of acidification and maintenance of a protein assigned TACO [16]. Uptake of Mycobacteria by macrophages and ensuing maintenance of TACO on the phagosome seems, by all accounts, to be reliant upon the gathering of host cell–inferred cholesterol in the plasma surface of bacterial part [17,19].
Types of Biomarkers and their Application
The biomarker is “a characteristic that is evaluated as a display of any biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention. WHO states that “any physical, chemical or biological characteristic which reflects an interaction between a biological system and a potential hazard” known as a biomarker. The observed response could be functional, physiological, and biochemical at the cellular level [20]. The two major types of biomarkers are “biomarkers of exposure,” helps in risk prediction, and “biomarkers of disease”, used for screening, diagnosis and monitoring the disease [21]. There are some pathogen-specific biomarkers and some host-specific biomarkers. The diagnostic utility of biomarkers revolutionized the field of genomics, proteomics, metabolomics, imaging modalities and neurophysiology [22]. Biomarkers also provide a systemic approach to understand the pathogenesis of disease and its associated risk factors. Serological tests based on biomarkers might be helpful in the diagnosis and prognosis of any disease. An ultimate tuberculosis biomarker should contain the following properties: they must be capable of distinguishing among patients with active tuberculosis and patients having dormant M. tuberculosis H37Rv infection, resuming the standard levels of treatment; expecting clinical effects (for instance, treatment, declining hazard or elimination of M. tuberculosis H37Rv infection) in varied patient populations; and are able to forecast effectiveness of vaccines. There were various exercises have been propelled to target biomarkers in case of tuberculosis. Through increasing dose of drugs, for instance, biomarkers might support for intentional assortment, direct recognition, optimization, representing evidence of perception and selecting drug amalgamation for stabilizing synergistic communication between drugs and immune cells. Even though several immunological biomarkers might be a further proper précising phase of improvement, the sustained use for a solitary marker from preclinical studies during dose selection stage examinations would be an imperative improvement
Interrelation between Host Immunological Biomarkers and M. tuberculosis H37Rv Pathogenesis
Various pathogen-specific biomarkers were identified for TB but only the antigen 85 complex (Ag85), secretory antigenic target 6 (ESAT6) and the lipoglycan lipoarabinomannan (LAM) are useful [23]. In the pathology of tuberculosis infection, one key virulence factor is LAM present in the cell wall of M. tuberculosis H37Rv is amphiphilic in nature and associated with host lipid carrier molecules such as high-density lipid (HDL) and is detected in low concentrations in serum and in higher concentration in urine of TB infected person [24,25]. Presence of LAM in urine sample confirmed the infection of TB in HIV-positive population also [26, 27]. LAM is also a potential indicator of bovine tuberculosis (bTB) infection [28]. Ag85 is a major secretory protein with fibronectin binding capacity and mycolyltransferase activity and this antigen involved in receptormediated diagnosis of M. tuberculosis H37Rv [29]. Tuberculosisassociated meningitis or extrapulmonary TB can be diagnosed by the detection of Ag85 in the blood or urine sample [30]. ESAT6 helps M. tuberculosis H37Rv in translocation from phagolysosomes to host cytosol, so it can be considered as a virulent factor. Intracellular presence of ESAT6 can be used as a biomarker because it is related to metabolically active M. tuberculosis H37Rv [31]. Culture filtrate protein (CFP-10) forms a heterodimeric complex with ESAT6. ESAT6/ CFP10 complex is secreted by the ESX-1 secretion system which is used by M. tuberculosis H37Rv to deliver virulence factors into host macrophages. This complex is a basis of infection, and can be detected by the interferon-γ release assays [32]. The microbial adhesions may also act as determinants at the time of host-pathogen interactions because their interaction leads to an induce immune response which is required for host defense such as 19 kDa lipoprotein antigen (Rv3763), malate synthase, M. tuberculosis H37Rv pili, and heparin binding haemagglutinin adhesion (HbhA) [33].
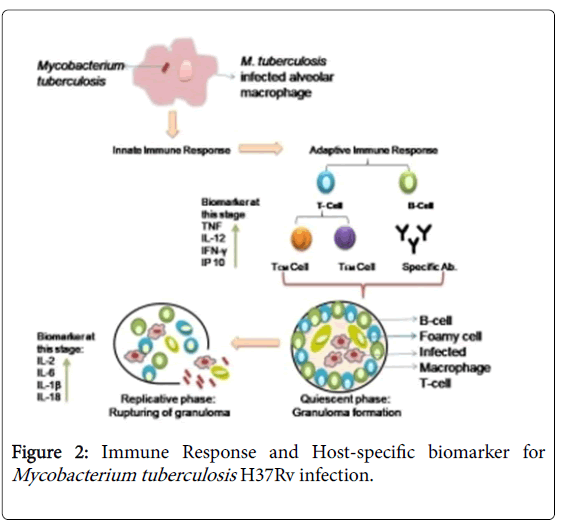
There are some host regulatory factors which up and downregulates during exposure of TB infection. There is an enhancement in the secretion of M. tuberculosis H37Rv specific antibodies and binding of any antigen with T-cell receptor stimulates secretion of certain proinflammatory cytokines and chemokines such as IL-2, IL-1β, IL-6 and tumour necrosis factor (TNF) and influence T cell response and TH cell balance [34]. IP-10 IFN-γ inducible protein, a chemokineinduced in various cells in response to IFN-γ and lipopolysaccharide (LPS) is an alternative marker to IFN-γ (Figure 2) [7]. Some acute phase proteins like serum amyloid A1 (SAA1); pentraxin (PTX-3); propionate CoA transferase (PCT); C-reactive protein (CRP) produced by the liver and promotes phagocytosis may also act as marker and factor associated with tissue reorganization matrix metallopeptidase 8 (MMP-8) can also provide blood-based targets to check the severity of infection and to monitor the disease during treatment [35]. Use of Biomarkers might be a basic mechanism for treatment of any disease. These are fundamentally based on epidemiological, therapeutic, pathophysiological shreds of evidences, therefore they are a primary candidate in drug discovery accelerate dose selection in an early phase of clinical research, and shortening the time to licensing of new drugs and vaccines [36-41].
Host Alveolar macrophages upon activation by M. tuberculosis H37Rv generate an innate immune response which on further activation by secreted innate immunity compounds convert into an adaptive immune response. In adaptive immune response T cells and B cells are generated. Upon activation, T cell divided into TEM (effectors memory) which help in generating B cell and TCM (central memory cell) provide antigen recognizing property to host immune system. These cells also release various chemokine like TNF-α, IL-12, IFN-γ and IP-10 etc. which help in the elimination of foreign particle. In case of M. tuberculosis H37Rv, this bacterium is capable of persisting in the macrophage which is termed as the quiescent phase. In this phase, a bacterium is surrounded by B cell, T cell, NK cell, dendritic cell and foamy macrophages etc. which forms granuloma-like structure, which provides bacterial cell with a protected environment to replicate itself. In the immune compromise stage, granuloma ruptures and releases bacilli in the surrounding environment and secretes various chemokine-like IL-1β, IL-2, IL-6 and IL-18 have been secreted which is a sign of an active disease [28,29].
Conclusion
Tuberculosis is a global health hazard so there is a need to develop new strategies for early diagnosis and successful treatment. Any specific characteristic present in tissue, cell and fluid of body, which gives the specific response to any infection and help in risk prediction, diagnosis and prognosis of the disease is known as a biomarker. Biomarkers provide a systematic approach for diagnosing a disease and evaluation of new drug therapies but there is lack of specific validated biomarkers for tuberculosis up till now. There are some pathogenspecific and some host-specific markers secreting during infection which can be detected in blood and urine sample of the patient which might be useful and require further studies. Treatment and diagnosis of tuberculosis by using appropriate biomarker may be helpful for future perspective.
Acknowledgement
The authors acknowledge financial support from the Department of Science and Technology-SERB, Council of Scientific and Industrial Research-Institute of Genomics and Integrative Biology under the research project GAP0145.
Conflict of Interest
There is no conflict of interest.
References
- Meena LS, Rajni (2010) Survival mechanisms of pathogenic Mycobacterium tuberculosis H37Rv. FEBS J 277: 2416-2427.
- Rajni, Meena LS (2011) Unique Characteristic Features of Mycobacterium Tuberculosis in Relation to Immune System. Am J Immunol 7: 1-8
- WHO (2016) global tuberculosis report, World Health Organization, Geneva.
- WHO (2017) global tuberculosis report, World Health Organization, Geneva.
- Riccardi N, Giannini B, Borghesi ML, Taramasso L, Cattaneo E, et.al. (2018) Time to change the single-centre approach to management of patients with tuberculosis: a novel network platform with automatic data import and data sharing. ERJ Open Research 4: 00108-2017.
- Swanepoel C, Snyders C, Isaacs S, Abayomi A, Grewal R (2015) Biomarker discovery for diagnosis and treatment of tuberculosis: a role for biobanking?. Dovepress 3: 47-56.
- Ruhwald M, Aabye MG, Ravn P (2012) IP-10 release assays in the diagnosis of tuberculosis infection: current status and future directions. Expert Rev Mol Diagn 12: 175-187.
- Chegou NN, Heyckendorf J, Walzl G, Lange C, Ruhwald M (2014) Beyond the IFN-c horizon: biomarkers for immunodiagnosis of infection with Mycobacterium tuberculosis. Eur Respir J 43: 1472-1486.
- Strimbu K, Tavel JA (2010) What are biomarkers. Curr Opin HIV AIDS 5: 463-466.
- Smith I (2003) Mycobacterium tuberculosis Pathogenesis and Molecular Determinants of Virulence. Clin Microbio Rev 16:463-496.
- Ernst JD (1998) Macrophage receptors for Mycobacterium tuberculosis. Infect Immun 66: 1277–1281.
- Aderem A, Underhill DM (1999) Mechanisms of phagocytosis in macrophages. Annu Rev Immunol 17: 593–623.
- Armstrong JA, Hart PD (1971) Response of cultured macrophages to Mycobacterium tuberculosis with observation on fusion of lysosomes with phagosomes. J Exp Med 134: 713-740.
- Clemens D, Horwitz M (1995) Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J Exp Med. 181: 257–270.
- Via LE, Deretic D, Ulmer RJ, Deretic V (1997) Arrest of mycobacterial phagosome maturation is caused by a block in vesicle fusion between stages controlled by rab5 and rab7. J Biol Chem. 272: 13326-13331.
- Clemens DL, Lee BY, Horwitz MA (2000) Deviant expression of Rab5 on phagosomes containing the intracellular pathogens Mycobacterium tuberculosis and Legionella pneumophila is associated with altered phagosomal fate. Infect Immun 68: 2671-2684.
- Sturgill-Koszycki S, Schlesinger PH, Chakraborty P, Haddix PL, Collins HL, et al. (1994) Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science 263: 678-681.
- Ferrari G, Langen H, Naito M, Pieters J (1999) A coat protein on phagosomes involved in the intracellular survival of mycobacteria. Cell 97:435–447.
- Gatfield J, Pieters J (2000) Essential role for cholesterol in entry of mycobacteria into macrophages. Science 288: 1647–1650.
- Mayeux R (2004) Biomarkers: Potential Uses and Limitations. NeuroRx 1: 182-188.
- Ganesalingam J, Bowser R (2010) The application of biomarkers in clinical trials for motor neuron disease. Biomark Med 4: 281-297.
- Mukundan H, Kumar S (2011) Rapid Detection of Mycobacterium tuberculosis Biomarkers using a Waveguide-based Biosensor. Tubercul (Edinb) 92: 407-416.
- Rao N, Meena LS (2011) Biosynthesis and virulent behavior of lipids produced by Mycobacterium tuberculosis: LAM and cord factor: an overview. Biotechnol Res Int 4: 2746-2793.
- Sakamuri RM, Price DN, Lee M, Cho SN, Barry CE, et al. (2013) Association of lipoarabinomannan with high density lipoprotein in blood: Implications for diagnostics. Tuberculosis (Edinb) 93: 301-307.
- Shah M, Variava E, Holmes CB, Coppin A, Golub JE, et al. (2009) Diagnostic accuracy of a urine lipoarabinomannan test for tuberculosis in hospitalized patients in a High HIV prevalence setting. J Acquir Immune Defic Syndr 52: 145-151.
- Drain PK, Losina E, Coleman SM, Giddy J, Ross D, et al. (2017) Clinic-Based Urinary Lipoarabinomannan as a Biomarker of Clinical Disease Severity and Mortality Among Antiretroviral Therapy-Naive Human Immunodeficiency Virus-Infected Adults in South Africa. Open forum infect Dis 4: ofx167.
- Â Lamont EA, Lima JR, Waters WR, Thacker T, Sreevatsan S (2014) Mannosylated lipoarabinomannan in serum as a biomarker candidate for subclinical bovine tuberculosis. BMC Research Notes 20147: 559.
- Belisle JT, Vissa VD, Sievert T, Takayama K, Brennan PJ, et al. (1997) Role of the Major Antigen of Mycobacterium tuberculosis in Cell Wall Biogenesis. Science 276: 1420-1422.
- Goletti D, Petruccioli E, Joosten SA, Ottenhoff THM (2016) Tuberculosis Biomarkers: From Diagnosis to Protection. Infect Dis Rep 8: 6568.
-  Poulakis N, Gritzapis AD, Ploussi M, Leventopoulos M, Papageorgiou CV (2014) Intracellular ESATâ€6: A new biomarker for Mycobacterium tuberculosis infection. Cytometry B Clin Cytom 90: 312-314.
- Â Zwerling A, van den Hof S, Scholten J, Cobelens F, Menzies D, et al. (2012) Interferon-gamma release assays for tuberculosis screening of healthcare workers: a systematic review. Thorax 67: 62-70.
- Govender VS, Ramsugit S, Pillay M (2014) Mycobacterium tuberculosis adhesins: potential biomarkers as anti-tuberculosis therapeutic and diagnostic targets. Microbiology 160: 1821-1831.
- Â Walzl G, Ronacher K, Hanekom W, Scriba TJ, Zumla A (2011) Immunological biomarkers of tuberculosis. Nat Rev Immunol 11: 343-354.
- Â Sigal GB, Segal MR, Mathew A, Jarlsberg L, Wang M (2017) Biomarkers of Tuberculosis Severity and Treatment Effect: A Directed Screen of 70 Host Markers in a Randomized Clinical Trial. EBioMedicine 25: 112-121.
- Shivangi, Meena LS (2018) A Novel Approach in Treatment of Tuberculosis by Targeting Drugs to Infected Macrophages Using Biodegradable Nanoparticles, Appl Biochem Biotechnol 185: 815-821.
- Aggarwal AN, Agarwal R, Gupta D, Dhooria S, Behera D (2015) Interferon Gamma Release Assays for Diagnosis of Pleural Tuberculosis: a Systematic Review and Meta-Analysis. J Clin Microbiol 53(8): 2451-2459.
- Mazurek GH1, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K, et al. (2010) Updated Guidelines for Using Interferon Gamma Release Assays to Detect Mycobacterium tuberculosis Infection. MMWR Recomm Rep 59: 1-25.
- Wenli P, Lyness M, Lesley W, Hawkridge T, Hanekom W, et al. (2009) Comparison of Mantoux and Tine Tuberculin Skin Tests in BCG-Vaccinated Children Investigated for Tuberculosis. PLoS One. 4: e8085.
- KY Loh (2011) Role of Mantoux Test in the Diagnosis of Tuberculosis. Malays Fam Physician 6: 85–86.
Citation: Meena S, Shivangi, Meena LS (2018) Novel and Emerging Approach in the Diagnosis and Prognosis of Tuberculosis by Using Biomarkers. J Tuberc Ther 3: 119.
Copyright: © 2018 Meena LS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 5130
- [From(publication date): 0-2018 - Dec 06, 2025]
- Breakdown by view type
- HTML page views: 4176
- PDF downloads: 954